Increased muscle echointensity correlates with clinical disability and muscle strength in chronic inflammatory demyelinating polyneuropathy
Abstract
Background and purpose
We evaluated muscle echointensity as a marker for secondary axonal damage in patients with chronic inflammatory demyelinating polyneuropathy (CIDP) using ultrasonography. Findings were correlated with clinical disability and muscular strength.
Methods
Eighty patients with CIDP (40 with typical and 40 with atypical CIDP) were examined clinically, including assessment of Medical Research Council (MRC) sum score and Inflammatory Neuropathy Cause and Treatment Overall Disability Sum Score (INCAT-ODSS). Echointensity in eight proximal and distal muscles of the arms and legs was evaluated by muscle ultrasonography using the Heckmatt scale.
Results
Alterations of echointensity occurred most frequently in the distal leg muscles, with a median (range) Heckmatt score of 1.5 (1–4). There were no differences between typical and atypical CIDP patients with regard to Heckmatt score. Alterations of echointensity correlated to disability and muscle strength. The arm score of the INCAT-ODSS correlated to Heckmatt score for the distal arm muscles (r = 0.23, p = 0.046) and the leg score of the INCAT-ODSS correlated to Heckmatt scores for the proximal (r = 0.34, p = 0.002) and distal leg muscles (r = 0.33, p = 0.004). MRC sum score, as well as individual MRC scores for arm and leg muscles, correlated to Heckmatt scores of the corresponding muscle groups (r = −0.25, p = 0.02 for MRC sum score).
Conclusion
Increased muscle echointensity, reflecting fibrosis and fatty infiltration due to secondary axonal damage, correlated to muscular strength and disability in a large cohort of CIDP patients. Alterations of echointensity occur in both typical and atypical CIDP patients and are pronounced in the distal leg muscles.
Abbreviations
-
- ADM
-
- abductor digiti minimi
-
- APB
-
- abductor pollicis brevis
-
- BB
-
- biceps brachii
-
- CIDP
-
- chronic inflammatory demyelinating polyneuropathy
-
- DADS
-
- distal acquired demyelinating symmetric
-
- ECR
-
- extensor carpi radialis longus
-
- EFNS
-
- European Federation of Neurological Societies
-
- EMG
-
- electromyography
-
- GM
-
- gastrocnemius muscle
-
- INCAT-ODSS
-
- Inflammatory Neuropathy Cause and Treatment Overall Disability Sum Score
-
- MADSAM
-
- multifocal acquired demyelinating sensory and motor neuropathy
-
- MRC
-
- Medical Research Council
-
- MUS
-
- muscle utrasonography
-
- PNS
-
- Peripheral Nerve Society
-
- RF
-
- rectus femoris
-
- TA
-
- tibial anterior muscle
-
- TB
-
- triceps brachii
INTRODUCTION
Chronic inflammatory demyelinating polyneuropathy (CIDP) may have a chronic, relapsing-remitting or a (self)-limiting disease course [1]. Disease characteristics in cases with a chronic course may be different from those of early CIDP, as demyelination is superimposed by secondary axonal damage. Chronic axonal damage leads to structural muscular alterations in CIDP patients, as reported in biopsy or magnetic resonance imaging studies [2]. Structural muscular alterations can be assessed by echointensity in muscle ultrasonography (MUS) [3], which is already used in other neuromuscular diseases, such as myositis and amyotrophic lateral sclerosis [3]. As axonal damage is a predictor of poor outcome [4], assessment of axonal damage using MUS may be a useful non-invasive marker. To date, only one study has focused on MUS in CIDP; that study showed high echointensity and reduced muscle thickness of hand muscles in 16 CIDP patients, suggesting secondary axonal degeneration [5]. However, it is still not known whether increased muscle echointensity in MUS correlates with clinical impairment. The aim of the present study was to evaluate muscle echointensity in CIDP patients and to correlate ultrasonography findings with clinical disability and muscular strength.
METHODS
Patients and clinical examination
A total of 80 patients with CIDP treated in St Josef-Hospital, University Hospital Bochum, Germany between June 2019 and April 2020 were included in the present study. The ethics committee of the Ruhr University Bochum approved the study (prospective Immunmediated Neuropathies Biobank [INHIBIT; vote-no. 18-6534-BR]). Diagnosis of CIDP was made according to the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) criteria [1]. For diagnosis of atypical CIDP, including distal acquired demyelinating symmetric (DADS) neuropathy and multifocal acquired demyelinating sensory and motor neuropathy (MADSAM), the criteria reported by Doneddu et al. [6] were used in addition to EFNS/PNS criteria.
Clinical examination included neurological examination and assessment of Medical Research Council (MRC) score [7] for the following muscles: deltoid, biceps, wrist extensor, finger abductor, iliopsoas, quadriceps femoris, tibialis anterior, and big toe extensor. MRC sum score was calculated from those muscles described by the MRC [7]. Disability was recorded using the Inflammatory Neuropathy Cause and Treatment Overall Disability Sum Score (INCAT-ODSS) [8], with separate recording of arm and leg score as well as sum score.
Muscle ultrasonography
Muscle ultrasonography was performed using an Affinity® 70G ultrasound system (Philips, Hamburg, Germany) with an 18-MHz linear array transducer. Ultrasound settings (e.g., contrast), excluding depth and focus, were kept constant during all examinations. The following muscles were examined on both sides: the abductor pollicis brevis (APB; between carpometacarpal joint and metacarpophalangeal joint, longitudinal section); abductor digiti minimi (ADM; between wrist and finger root, longitudinal section); extensor carpi radialis longus (ECR; at level of elbow joint, cross section), biceps brachii (BB; distal third to middle of the upper arm, cross section), triceps brachii (TB; middle of the upper arm, cross section), rectus femoris (RF; distal third of thigh, cross section); tibial anterior muscle (TA; proximal third of the lower leg, cross section); and medial head of gastrocnemius muscle (GM; proximal third of the lower leg, cross section). Muscle echointensity was graded using the Heckmatt scale, which ranges from 1 (normal echointensity) to 4 (increased echointensity), according to Heckmatt et al.[9]
Additionally, we counted the number of fasciculations detected by MUS in 30 s. For some analyses muscles were arranged into the following groups: proximal arm muscles: BB, TB; distal arm muscles: APB, ADM, ECR; proximal leg muscle: RF; and distal leg muscles: TA, GM.
In a few patients, not all muscles from both sides could be examined (due to peripheral venous catheter), so that the number of patients was reduced to approximately 75 patients, depending on analysis. The numbers of patients included in each analysis are provided in the results and tables accordingly.
Intra- and interrater reliability of echointensity analysis using the Heckmatt scale
Ultrasonography was performed by three examiners (A.F., S.F., Z.S.). To ensure comparability of echointensity measures, we analysed the intra- and interrater reliability of the Heckmatt analysis. For this, the BB, APB, RF and TA of five patients were examined by all three examiners, three times each on different days. Cronbach's alpha was used for the intrarater reliability analysis for each examiner and intraclass correlation coefficients were used to analyse the interrater reliability among all three examiners. Cronbach's alpha values of >0.9 were considered “excellent”, and intraclass correlation coefficient values between 0.75 and 0.9 were considered “good”.
Electromyography
To validate the suitability of MUS to detect fasciculations in CIDP, we compared fasciculations detected by ultrasonography with fasciculations detected by electromyography (EMG), the "gold standard" for detecting fasciculations in neuromuscular disease.
Needle EMG was performed by a blinded examiner with a Dantec™ Keypoint® G4 four-channel EMG device (Natus Europe GmbH, Planegg, Germany) and a corresponding disposable concentric monopolar needle electrode 50 × 0.46 mm, with a 0.07-mm2 recording area (Value Line DCN, Natus, Ireland) as described by Stöhr et al. [10]. The number of fasciculations were sampled in the TA in 10 different needle locations, each observed for 10 s. EMG was performed independently of this study. After power analysis using G*Power (version 3.1.9.6; Heinrich-Heine-Universität Düsseldorf, Germany) determining that 13 patients would be needed to depict a correlation with r = 0.7, p ≤ 0.05 and a power of 0.8, we analysed the EMG results for 15 patients.
Statistics
Clinical and ultrasound data were compared between patients with typical and atypical CIDP using the t-test (for parametric variables) or the Mann–Whitney U-test (for non-parametric variables) and the chi-squared test as applicable. Correlation analyses were performed using Spearman correlation. Statistical analysis was performed using IBM® SPSS Statistics (version 26.0.0.0; IBM Deutschland GmbH, Ehningen, Germany). For all analyses, the statistically significant threshold was set at p <0.05.
RESULTS
Characteristics of patients
Of the 80 CIDP patients, 40 had typical and 40 had atypical CIDP. Of atypical CIDP, 20 patients had DADS neuropathy, 10 had MADSAM and 10 had other atypical CIDP-like pure sensory, pure motor or acute-onset CIDP. Diagnosis according to EFNS/PNS criteria was definite in 66 (83%), probable in five (6%) and possible in nine patients (11%).
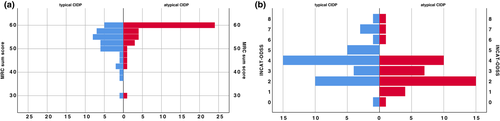
Table 1 shows the baseline characteristics of all patients and typical and atypical CIDP. Disease duration from first symptoms and from diagnosis to examination did not differ between the groups, but time between first symptoms to diagnosis was longer in atypical CIDP patients. Pure sensory symptoms without motor impairment at diagnosis was more frequent in atypical CIDP. At the time of examination, atypical CIDP patients were less disabled, according to INCAT-ODSS and MRC sum score, than typical CIDP patients (Figure 1).
| All patients (n = 80) | Typical CIDP (n = 40) | Atypical CIDP (n = 40) | p value | ||||
|---|---|---|---|---|---|---|---|
| n | n | n | |||||
| Gender, male/female | 60/20 | 80 | 25/15 | 40 | 35/5 | 40 | 0.01 |
| Mean ± SD age at examination, years | 59±13 | 80 | 58.8±15.4 | 40 | 58.5±10.7 | 40 | n.s. |
| Median (range) disease duration from diagnosis until examination, months | 28 (0–261) | 80 | 43 (0–261) | 40 | 21 (0–188) | 40 | n.s. |
| Median (range) disease duration from first symptoms until examination, months | 66.5 (0–266) | 80 | 66.5 (0–266) | 40 | 64 (6–237) | 40 | n.s. |
| Median (range) INCAT-ODSS at time of examination | 3 (0–8) | 80 | 4 (0–8) | 40 | 2.5 (0–8) | 40 | 0.02 |
| Median (range) MRC score at time of examination | 56.5 (30–60) | 79 | 54 (31–60) | 39 | 59.75 (30–60) | 40 | <0.01 |
| Median (range) time between first symptoms and diagnosis, months | 22 (0–180) | 80 | 12 (0–48) | 40 | 24 (0–180) | 40 | 0.03 |
| Median (range) INCAT-ODSS at diagnosis | 2 (0–6) | 42 | 2 (0–6) | 24 | 1 (1–6) | 18 | n.s. |
| Symptoms at diagnosis | |||||||
| Sensory symptoms without motor symptoms | 22 | 59 | 5 | 26 | 17 | 33 | 0.01 |
| Sensorimotor symptoms | 37 | 59 | 21 | 26 | 16 | 33 | 0.01 |
| Pain | 25 | 72 | 11 | 35 | 14 | 37 | n.s. |
| Affection of cranial nerves | 3 | 70 | 1 | 33 | 2 | 37 | n.s. |
| First symptoms | |||||||
| Sensory symptoms without motor Symptoms | 38 | 60 | 17 | 28 | 21 | 32 | n.s. |
| Sensorimotor symptoms | 22 | 60 | 11 | 28 | 11 | 32 | n.s. |
| Pain | 24 | 76 | 11 | 38 | 13 | 38 | n.s. |
| Affection of cranial nerves | 3 | 76 | 1 | 37 | 2 | 39 | n.s. |
| Symptoms at timepoint of ultrasound examination | |||||||
| Sensory symptoms without motor symptoms | 21 | 80 | 33 | 40 | 26 | 40 | n.s. |
| Sensorimotor symptoms | 59 | 80 | 7 | 40 | 14 | 40 | n.s. |
| Pain | 31 | 80 | 16 | 40 | 15 | 40 | n.s. |
| Affection of cranial nerves | 5 | 80 | 2 | 40 | 3 | 40 | n.s. |
| Treatment at time point of ultrasound examination | |||||||
| Corticosteroids | 21 | 80 | 13 | 40 | 8 | 40 | n.s. |
| Immunoglobulins | 55 | 80 | 29 | 40 | 26 | 40 | n.s. |
| Other immunotherapy | 39 | 80 | 24 | 40 | 15 | 40 | n.s. |
| No treatment | 2 | 80 | 2 | 40 | 0 | 40 | n.s. |
- Abbreviations: CIDP, chronic inflammatory demyelinating polyneuropathy; INCAT-ODSS, Inflammatory Neuropathy Cause and Treatment Overall Disability Sum Score; MRC, Medical Research Council; n.s., nonsignificant.
Of the 80 patients, 78 received immunotherapy. Two patients had no treatment as their disease was stable after initial treatment and immunotherapy could be stopped. First-line therapy with corticosteroids or immunoglobulins was applied in 62 of 80 patients. Plasma exchange was not performed. Details on treatments are given in Table 1. Applied treatments were not statistically different between the typical and atypical CIDP groups.
Distribution of alterations of echointensity
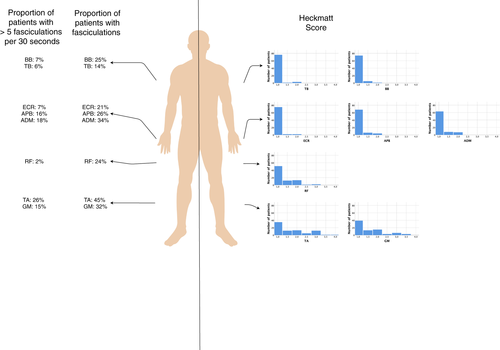
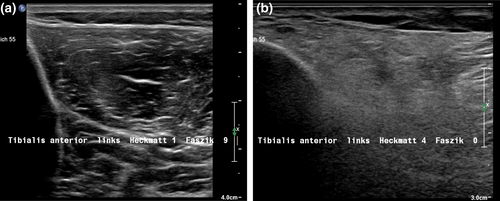
Most alterations of echointensity were detected in the distal leg muscles (median [range] Heckmatt score for distal leg muscles 1.5 [1–4]). Figure 2 (right part) shows the histograms of Heckmatt scores for each muscle. Only eight of 1269 examined muscles had a Heckmatt score of 4 (TA and GM). Figure 3 shows examples of two patients with Heckmatt scores 1 and 4 for the TA.
There were no differences between typical and atypical CIDP patients regarding number of fasciculations or Heckmatt score (Table S1).
Heckmatt scores for arm and leg muscles correlates with disability and muscle strength
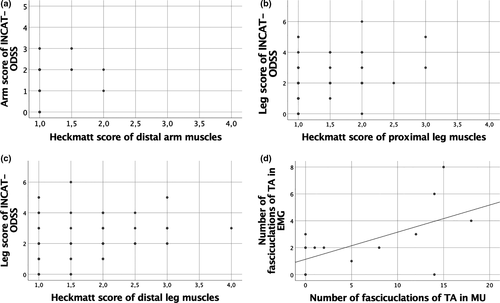
Arm score of the INCAT-ODSS correlated to Heckmatt score for the distal arm muscles (r = 0.23, p = 0.046) and leg score of the INCAT-ODSS correlated to Heckmatt scores for the proximal (r = 0.34, p = 0.002) and distal leg muscles (r = 0.33, p = 0.004; Figure 4a–c).
The MRC sum score, as well as the individual MRC arm and leg muscle scores, correlated to Heckmatt scores for the corresponding muscle groups (r = −0.25, p = 0.02 for MRC sum score and proximal arm muscle Heckmatt scores; r = −0.21, p = nonsignificant for MRC sum score and distal arm muscle Heckmatt scores; r = −0.35, p = 0.002 for MRC sum score and proximal leg muscle Heckmatt scores; r = −0.35, p = 0.002 for MRC sum score and distal leg muscle Heckmatt scores). Regarding MRC score for each individual muscle and Heckmatt scores for the same muscle, we found correlations mainly for the distal muscles of the arms and legs (Table S2).
Intra- and interrater reliability of echointensity analysis using the Heckmatt scale
Cronbach's alpha, reflecting intrarater reliability, was 0.95 for Examiner 1 (A.F.), 0.95 for Examiner 2 (S.F.) and 0.97 for Examiner 3 (Z.S.), resulting in an excellent intrarater reliability for analyzing echointensity using the Heckmatt scale.
The intraclass correlation coefficient, reflecting interrater reliability, was 0.82, which was considered to indicate good interrater reliability for analyzing echointensity using the Heckmatt scale.
Distribution of fasciculations
Fasciculations were most frequent in the distal leg muscles (mean 3.0 ± 4.5 per 30 s) and least frequent in the proximal leg muscles (mean 0.7 ± 1.9 per 30 s). In the arm muscles, fasciculations also occurred more often distally than proximally. In most cases, fasciculations occurred simultaneously in the arm and leg muscles and were rarely isolated in the proximal muscles (Table 2). Overall, fasciculations were found in 81% of all patients. The frequency of fasciculations for each muscle group is shown in Figure 2 (left panel).
| All patients | |
|---|---|
| n = 77 | |
| Arm muscles | |
| Only proximal | 5 |
| Only distal | 24 |
| Proximal and distal | 24 |
| None | 24 |
| Leg muscles | |
| Only proximal | 3 |
| Only distal | 28 |
| Proximal and distal | 21 |
| None | 25 |
| n/a | 3 |
| n = 75 | |
|---|---|
| Only arm muscles | 10 |
| Only leg muscles | 9 |
| Arm and leg muscles | 42 |
| None | 14 |
| n/a | 5 |
Number of fasciculations found on MUS correlate to those found on EMG
The number of fasciculations of the TA measured by MUS correlated to the number of fasciculations of the TA measured in EMG (r = 0.6, p = 0.03; Figure 4d).
DISCUSSION
In the present study, we found that increased muscle echointensity, reflecting fibrosis and fatty infiltration due to secondary axonal damage, correlated to muscular strength and disability in a large cohort of typical and atypical CIDP patients. Correlation of increased echointensity with INCAT-ODSS and MRC sum score was found for the distal arm as well as distal and proximal leg muscles. Echointensity therefore is a marker of disease severity caused by secondary axonal damage, confirming the findings of Hokkoku et al. [5] The correlation coefficients of approximately 0.3 correspond to a medium effect. These may not be strong enough to use MUS as a single biomarker for disease severity, but make MUS useful as a tool in addition to clinical examination, nerve conduction studies and EMG. MUS could help, in particular, to distinguish patients with severe axonal damage and worse prognosis from patients with less axonal damage and symptoms attributable to demyelination, i.e., conduction block, with better prognosis. Good intra- and interrater reliability for the analysis of echointensity using the Heckmatt scale was also described in amyotrophic lateral sclerosis [11], similar to our results.
In the present study, fasciculations occurred in many CIDP patients with considerable frequency. Other authors have described fasciculations detected by MUS in motor neuron disease, but the results of most of these studies cannot be compared directly with our results because there was either no quantification of fasciculations/time or because different methods of quantification were used. We found one study from 1990 (Walker et al. [12]) in which fasciculations were given per second, so that, calculated for 30 s, between 36 and 105 fasciculations were found in the TA of two patients with amyotrophic lateral sclerosis. Compared with these two patients with amyotrophic lateral sclerosis, we found significantly fewer fasciculations in patients with CIDP.
The origin and significance of fasciculations in CIDP are still uncertain as they also occur in radiculopathies and entrapment neuropathies, and even occur in healthy individuals. The actual site of origin may be distal in the axon or in the denervated muscle resulting from hypersensitivity and hyperexcitability [13, 14]. In healthy individuals, fasciculations probably occur with much lower frequency than that found for the CIDP patients in the present study. If associated with peripheral nerve disease, fasciculations could be considered as pathologic spontaneous activity, reflecting active or persisting axonal damage. Nevertheless, the thesis remains hypothetical and should be addressed in further studies.
We believe that MUS is even better suited than EMG for quantification of fasciculations because a much larger area of the muscle can be depicted in only one ultrasound section, observing the muscle for 30 s, than in EMG, for which each needle position usually detects potential changes up to a distance of 1–2 mm from the needle tip. This is in line with reports on MUS in the diagnosis of amyotrophic lateral sclerosis [15].
New methods to assess CIDP patients are needed, as objective disease monitoring is difficult. Nerve conduction studies are essential to diagnose CIDP [1], but doubts have been raised about whether they are useful in predicting clinical status or treatment response [16]. EMG detects pathological spontaneous activity and signs of chronic neuropathic damage in CIDP, but correlation with disease course is still under debate [17, 18]. Pathological spontaneous activity can persist several months after axonal damage regardless of ongoing damage [10]. Another everyday disadvantage of EMG, compared to MUS, is its invasiveness, resulting in patients not accepting regular detailed EMG examinations during the course of the disease. New biomarkers, such as nerve ultrasonography [19] or confocal corneal microscopy[20], are promising tools in the assessment of CIDP, but do not consider the muscular system. Correlation to clinical disability and muscular strength enables the use of MUS as a possible prognostic marker, but longitudinal follow-up of disease course, as well as evaluation of the dynamics of MUS, still need to be performed.
A limitation of the present study is that number of patients may have been too small to show differences between typical and atypical CIDP patients. Also, the lack of differences in MUS between typical and atypical CIDP patients in this study could have been a study bias, as atypical CIDP patients were less severely impaired than typical CIDP patients in our study.
In conclusion, MUS is useful to detect secondary axonal damage reflected in alterations of echointensity in CIDP patients. Alterations of echointensity occur in both typical and atypical CIDP patients, and are pronounced in the distal leg muscles. Echointensity as a marker for secondary axonal damage reflects disease severity as it correlates to muscle strength and disability. Fasciculations observed on MUS occur frequently, especially in distal muscles, in CIDP and could be a marker of active axonal damage. Future studies need to address if muscular echointensity reflecting axonal damage can be used as a prognostic marker in CIDP, and if fasciculations indeed reflect active axonal damage.
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL.
WOA Institution: Ruhr-Universitat Bochum
Blended DEAL: ProjektDEAL
Conflict of interest
Anna Lena Fisse has received research funding from Georgius Agricola Stiftung Ruhr and Ruhr-University Bochum (FoRUM-program) and honoraria and travel grants from Novartis AG, Sanofi and Eisai GmbH, none of which are related to this work, and owns shares in Fresenius SE & Co., Gilead Sciences, Medtronic PLC and Novartis AG. Jeremias Motte has received travel grants from Biogen idec, Novartis AG, Teva and Eisai GmbH, and his research is funded by the Klaus Tschira Foundation and Ruhr-University, Bochum (FoRUM-program), none of which are related to this work. Thomas Grüter has received travel reimbursement from Sanofi Genzyme and Biogen Idec, none of which are related to this manuscript. Ralf Gold has received consultation fees and speaker honoraria from Bayer, Schering, Biogen idec, Merck Serono, Novartis, Sanofi-Aventis and TEVA. He also acknowledges grant support from Bayer Schering, Biogen idec, Merck Serono, Sanofi-Aventis and TEVA, none of which are related to this work. Kalliopi Pitarokoili has received travel grants and speakers' honoraria from Novartis, Biogen idec, Teva, Bayer, Celgene, CSL Behring and Grifols, none of which are related to this work. Sean Fiegert, Zornitsa Stoykova, Jil Brünger and Diamantis Athanasopoulos have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




