Usability of the head impulse test in routine clinical practice in the emergency department to differentiate vestibular neuritis from stroke
Funding information
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; grant MA5332/3-1 to B.M.).
See editorial by C. Froment Tilikete on page 1437
Abstract
Background and purpose
The bedside head impulse test (bHIT) is used to differentiate vestibular neuritis (VN) from posterior circulation stroke (PCS) in patients presenting with acute vestibular syndrome (AVS). If assessed by neuro-otological experts, diagnostic accuracy is high. We report on its diagnostic accuracy when applied by nonexperts during routine clinical practice in the emergency department (ED), its impact on patient management, and the potential diagnostic yield of the video-oculography–supported head impulse test (vHIT).
Methods
Medical chart review of 38 AVS patients presenting to our university medical center's ED, assessed by neurology residents. We collected bHIT results (abnormal/peripheral or normal/central) and whether patients were admitted to the stroke unit or general neurological ward. Final diagnosis (VN, n = 24; PCS, n = 14) was determined by clinical course, magnetic resonance imaging, and vHIT.
Results
The bHIT's accuracy was only 58%. Its sensitivity for VN was high (88%), but due to many false-abnormal bHITs in PCS (36%), the specificity was low (64%). The vHIT yielded excellent specificity (100%) and moderate sensitivity (67%). The decision on the patient's further care was almost arbitrary and independent from the bHIT: 58% of VN and 57% of PCS patients were admitted to the stroke unit.
Conclusions
The bHIT, applied by nonexperts during routine practice in the ED, has low accuracy, is too often mistaken as abnormal/peripheral, and is not consistently used for patients' in-hospital triage. As false-abnormal bHITs can lead to misdiagnosis/mistreatment of stroke patients, we recommend that bHIT applied by nonexperts should be reassessed by a neuro-otological expert or preferably quantitative vHIT in the ED.
Abbreviations
-
- AICA
-
- anterior inferior cerebellar artery
-
- AVS
-
- acute vestibular syndrome
-
- bHIT
-
- bedside head impulse test
-
- CI
-
- confidence interval
-
- DWI
-
- diffusion-weighted imaging
-
- ECG
-
- electrocardiography
-
- ED
-
- emergency department
-
- HINTS
-
- head impulse, nystagmus, and test-of-skew
-
- HIT
-
- head impulse test
-
- MRI
-
- magnetic resonance imaging
-
- PCS
-
- posterior circulation stroke
-
- vHIT
-
- video-oculography–based head impulse test
-
- VN
-
- vestibular neuritis
-
- VOR
-
- vestibular ocular reflex
INTRODUCTION
Patients presenting to the emergency department (ED) with an acute vestibular syndrome (AVS), defined by sudden onset of vertigo or dizziness, nausea/vomiting, gait instability, and nystagmus, most often suffer from a peripheral unilateral vestibulopathy (e.g., vestibular neuritis [VN]) [1]. However, about 25% of AVS cases are caused by a posterior circulation stroke (PCS) [2-6]. The diagnosis of PCS in AVS can be challenging [7], especially because early magnetic resonance imaging (MRI) can miss about 20% to 50% of posterior fossa infarctions [2, 8]. In the first 24 to 48 h, the combined test of three eye-movement signs at the bedside (head impulse, nystagmus, and test-of-skew [HINTS[) even outperforms the MRI in differentiating peripheral from central causes of AVS [1]. Among these tests, the bedside head impulse test (bHIT) is the single best predictor for a stroke in AVS [2]. A bilaterally intact vestibular ocular reflex (VOR) without corrective saccades is found in 80% to 100% of AVS patients with PCS, if they are assessed by neuro-otological experts [9-11]. Vice versa, an abnormal bHIT result (i.e., reduced ipsilesional VOR with subsequent corrective saccade) usually indicates peripheral unilateral vestibulopathy and is found in 83% to 100% of acute VN patients [9-12]. Posterior circulation stroke only rarely causes a VOR deficit leading to abnormal bHIT, particularly if the anterior inferior cerebellar artery (AICA) is affected [4, 13, 14]. On the other hand, a mild peripheral vestibulopathy can be missed by the bHIT, because clinical detection depends on the severity of the VOR gain reduction and presence of overt large-amplitude corrective saccades [13, 15].
If the bHIT is used in an emergency setting to distinguish VN from PCS in AVS patients, a general problem of clinical bedside tests arises; the reliability of the test depends on the individual skills of the examiner. The bHIT is technically demanding to perform, and its interpretation varies significantly with the expertise of the assessor, as previously reported for vestibular patients in a nonemergency setting [16-18]. Neuro-otological experts were shown to have lower sensitivity but higher specificity than nonexperts when stating an abnormal (peripheral) bHIT, mainly because they tend to rate borderline results as normal to avoid missing central pathology [16] This is in line with the high predictive value of a normal bHIT for PCS, if AVS patients are assessed by neuro-otological experts under controlled study conditions [1, 9-12].
However, AVS patients present to the ED under real-life conditions (i.e., 24/7), during routine clinical practice, encountering different doctors at varying levels of clinical experience. In many healthcare systems, ED physicians decide on the management of AVS patients, and even if neurology or otolaryngology residents are present in the ED, they are usually not highly trained neuro-otological experts. Taking this into account, many questions arise. First, does the bHIT, when performed and interpreted by nonexperts during routine workup in the ED, still holds its diagnostic accuracy for differentiating VN from PCS in AVS patients? Second, do ED doctors rely on their bHIT result and use it for time-critical decisions on the patient's further management? To specify, will AVS patients who reveal a normal bHIT in the ED correctly be suspected of PCS and consequently admitted to a stroke unit, and will those with an abnormal bHIT instead be classified as VN and sent to a usual-care ward? Whether an AVS patient is admitted (triaged) to the stroke unit or a general neurological ward, is medically (monitoring, thrombolytic, or antithrombotic therapy) and economically (costs, resources) relevant. Third, could an immediate video-oculography–based head impulse test (vHIT) improve diagnostic accuracy in the ED and thereby change the patient management? Some argue that a vHIT, which is able to quantify the VOR gain and uncover covert corrective saccades [19], could increase the sensitivity and specificity of the bHIT [17], whereas others doubt an additional diagnostic value [11].
In this retrospective study, we searched our in-house register [6] of 610 dizzy patients presenting to our university medical center's ED for those who revealed AVS. We extracted the bHIT results obtained by neurology residents, analyzed the bHIT's accuracy (in reference to the corresponding vHIT), its sensitivity, and specificity (in relation to the final diagnosis of VN or PCS), and the impact on patients' in-hospital triage (i.e., whether they were admitted to the stroke unit or usual-care ward. We expected nonexperts in the ED to be less accurate and to misinterpret the bHIT too often as abnormal, going along with a reduced specificity for PCS due to false-abnormal peripheral bHITs in PCS patients. On the other hand, ED doctors trained to avoid missing a stroke might have a tendency to classify AVS patients as PCS and to ignore/distrust their own bHIT result, thereby causing unnecessary admissions of VN patients to the stroke unit. Given that, we expected the vHIT to have higher diagnostic accuracy than the bHIT and the potential to improve the in-hospital triage of AVS patients (electrocardiography for the eyes [17]).
METHODS
Study design and setting
Data were derived from an in-house register, originally compiled by reviewing medical charts of adult patients who presented with dizziness, vertigo, or imbalance to the ED at the University Medical Center in Lübeck, Germany between 1 January 2016 and 31 December 2018, and receiving neurological workup [6]. The ED at this tertiary-care university hospital encounters about 42,000 patients per year. It is staffed 24/7 with resident and attending internal medicine physicians as well as a neurology resident.
The study was approved by the ethics committee of the University of Lübeck (18-146A) and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Due to the retrospective design and use of anonymized data, individual written informed consent was not needed.
Study population
Within the register, we searched for patients who met the following criteria defining an AVS: (i) new and persistent vertigo or dizziness, (ii) instability of gait, and (iii) spontaneous nystagmus. We excluded patients (i) with onset of symptoms >72 h before admission to the ED, (ii) whose symptoms remitted in less than 24 h after admission, (iii) who did not undergo magnetic resonance brain imaging or video-based head impulse testing to confirm final diagnosis (to avoid misdiagnosis), (iv) with diagnosis of an inflammatory disease of the central nervous system, because focus was on PCS as the most common central cause of AVS, and (v) patients with an unclear AVS who did not meet the diagnostic criteria of VN or PCS (see Diagnostic Classification section).
Bedside head impulse test and other clinical measures
The bHIT result was extracted from the documentation of the resident's neurological examination in the ED. It was operationalized as either abnormal (peripheral) or normal (central). We also extracted information on the other two HINTS signs, namely, whether the nystagmus' fast phase alternated with gaze (bilateral gaze-evoked nystagmus) and whether a skew deviation (vertical misalignment of bulbi) was observed. Finally, we collected information on any focal neurological abnormality such as dysarthria, Horner's syndrome, unilateral facial weakness, limb ataxia, or sensory impairments. Notably, major deficits such as aphasia/anarthria or hemiparesis were exclusion criteria during the compilation of the original register and were therefore not present in the current study cohort.
Video-oculography device, performance, and analysis of vHIT
The vHIT was recorded using the EyeSeeCam HIT System (Autronics, Hamburg, Germany), a head-mounted video-oculography device [20]. The patient was seated on a chair and fixated on a laser target at a distance of 100 cm. After calibration, a medical–technical assistant standing behind the patient delivered repetitive passive and rapid head rotations (peak velocity: 200–250°/s, amplitude: 10–15°) in the plane of the horizontal semicircular canals. HITs were unpredictable for direction and onset. The device recorded head and eye velocity traces and immediately calculated and plotted the VOR gain ratio for each head impulse test (HIT) trial.
Although grossly invalid HIT trials were already rejected by the device's software during recording, all vHITs were reassessed offline by three neuro-otological experts (B.M., P.T., C.H.). They classified each vHIT as either interpretable or uninterpretable due to severe disruptive artifacts. Only interpretable vHITs were included in the further analyses.
From the device's software we exported the patient's mean VOR gain across all trials for each side (ipsilesional, contralesional) at a specific time interval (60 ms after HIT onset) [20, 21]. We calculated the left–right asymmetry of the VOR gain using a previously suggested formula [9, 17]: (higher gain–lower gain)/higher gain * 100.
Finally, one algorithm-based vHIT result (abnormal or normal) was obtained for each patient. The algorithm judged the VOR as abnormal if the ipsilesional VOR gain was equal to or below 0.7 and as normal if both VOR gain values were above this threshold. This simple cutoff criterion of a pathological VOR gain was based on previous calculations of mean VOR gains in healthy subjects [19, 22], it was shown to correctly distinguish 80% to 90% of PCS from VN patients and has good overlap with vHIT judgements by neuro-otological experts [9, 17].
Imaging parameters
All patients included in the final analysis underwent magnetic resonance brain imaging, including diffusion-weighted imaging (DWI) sequences. The results of the studies, either a DWI lesion in the cerebellum or brainstem (medulla oblongata, pons, mesencephalon) or no DWI lesion, were abstracted from the official neuroradiological reports and double-checked by a clinical neurologist and stroke expert (B.M.).
Diagnostic classification
Diagnosis of VN required the following criteria: (i) presence of spontaneous nystagmus, (ii) abnormal clinical bHIT and/or vHIT, (iii) normal MRI-DWI, and (iv) no skew deviation or clear focal neurological abnormality. Diagnosis of PCS required an acute ischemic lesion in the brainstem or cerebellum as confirmed by MRI-DWI.
Statistical analysis
Statistical analyses were performed using SPSS 22.0.0.2 (IBM, Armonk, NY, USA). Descriptive statistics were calculated for all variables of interest, and data are presented as counts and percentages. Differences between the groups (VN, PCS) were statistically compared using t tests for quantitative variables or Pearson χ2 test for categorical variables. Comparisons of sensitivity/specificity between the two HIT methods were performed using McNemar test for paired samples, applying Yates continuity correction [23]. The significance level was set at p < 0.05.
RESULTS
Selection and clinical characteristics (including bHIT) of VN and PCS patients
Of 610 patients, 193 met the inclusion criteria defining AVS (Figure 1). After exclusion of 155 patients due to predefined criteria (see Methods section), 38 patients went into the final analysis, 24 of whom had VN and 14 PCS.
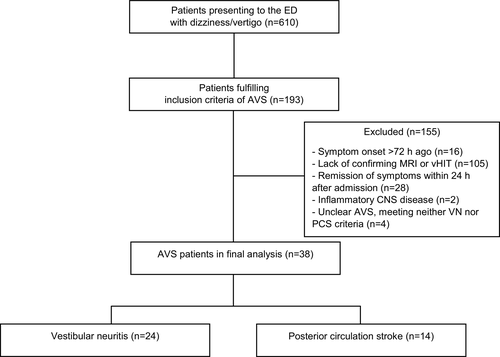
The individual clinical characteristics for each patient are provided in Table S1. The between-group comparisons (Table 1) revealed that VN patients were slightly older than PCS patients (d = 10 ± 4 years, p = 0.034). PCS patients more often exhibited a normal bHIT, positive HINTS, and focal neurological abnormalities (Table 1). The percentage of patients admitted to the stroke unit due to suspected stroke did not differ between VN (58%) and PCS (57%). The majority (11 of 14) of VN patients, who were misdiagnosed as PCS and admitted to the stroke unit, showed an abnormal (peripheral) bHIT result in the ED. Only two of them exhibited mild focal abnormalities (facial hypesthesia, discrete ataxia on pointing). Five of the six PCS patients, who were initially misdiagnosed as VN and not admitted to the stroke unit, exhibited a normal bHIT in the ED.
| Characteristics | VN | PCS | p value |
|---|---|---|---|
| Demographics | |||
| No. of patients | 24 | 14 | – |
| Age, years, mean ± SEM | 73 ± 2 | 63 ± 4 | 0.034* |
| Sex, male | 14 (56) | 11 (79) | n.s. |
| Findings on clinical examination in ED | |||
| bHIT abnormal, peripheral | 21 (88) | 5 (36) | 0.001* |
| bHIT normal, central | 3 (12) | 9 (64) | 0.001* |
| HINTS positive | 3 (12) | 11 (79) | <0.001* |
| Focal neurological abnormality | 2 (8)a | 5 (36) | 0.036* |
| Clinical management | |||
| Admitted to stroke unit due to suspected PCS | 14 (58) | 8 (57) | n.s. |
| Despite abnormal bHIT result in ED | 11 | 4 | – |
| Not admitted to stroke unit due to suspected VN | 10 (42) | 6 (43) | n.s. |
| Despite normal bHIT result in ED | 0 | 5 | – |
Note
- Data are n (%) unless otherwise indicated.
- Abbreviations: bHIT, bedside head impulse test; ED, emergency department; HINTS, head impulse, nystagmus, and test-of-skew; n.s., not significant; PCS, posterior circulation stroke; vHIT, video-oculography–based head impulse test; VN, vestibular neuritis.
- a Both patients revealed mild focal abnormalities on examination in the ED that could not be confirmed on reassessment.
- * Significant at p < 0.05.
Mean VOR gains and accuracy of clinical bHIT in relation to the vHIT
The vHIT was usually performed within 24 h after admission (median = 1 day, 95% confidence interval [CI]: 0–3 days). The vHIT was interpretable in all of the included subjects (i.e., 24 VN and 14 PCS patients). Mean VOR gain values differed significantly between VN and PCS patients (Table 2). Both ipsilesional and contralesional mean VOR gain values were lower in VN than in PCS, but the difference was particularly prominent for the ipsilesional VOR gain (d = 0.43 ± 0.09; vs. d = 0.21 ± 0.06 for contralesional VOR gain). The gain asymmetry was greater in VN than in PCS (d = 24 ± 7%, p = 0.001).
| vHIT results | VN, n = 24 | PCS, n = 14 | p value |
|---|---|---|---|
| VOR gain | |||
| Ipsilesional, mean ± SEM (95% CI) | 0.56 ± 0.06 (0.43–0.68) | 1.01 ± 0.05 (0.91–1.12) | <0.001 |
| Contralesional, mean ± SEM (95% CI) | 0.83 ± 0.04 (0.76–0.91) | 1.05 ± 0.05 (0.96–1.16) | 0.003 |
| Asymmetry, %, mean ± SEM (95% CI) | 38 ± 5 (27–48) | 14 ± 3 (9–19) | 0.001 |
| vHIT judgement by algorithm, VOR gain 0.7 as cutoff | |||
| Normal | 8 (33) | 14 (100) | <0.001 |
| Abnormal | 16 (67) | 0 (0) | <0.001 |
- Abbreviations: CI, confidence interval; PCS, posterior circulation stroke; vHIT, video-oculography–based head impulse test; VN, vestibular neuritis; VOR, vestibular ocular reflex.
Regarding the accuracy of the bHIT, we compared the bHIT result from the examination in the ED to the corresponding vHIT result, independent of the final clinical diagnosis. Taking the vHIT as reference is adequate because vHIT-derived VOR results are highly congruent to VOR measurements with scleral search coils defining the technical gold standard [19].
The bHIT and vHIT were congruent in 22 of 38 patients (58%), and both revealed an equally abnormal (n = 13) or normal (n = 9) result. In 16 patients (42%), the bHIT differed from the vHIT, with n = 3 (8%) having a normal bHIT but abnormal vHIT and n = 13 (34%) having an abnormal bHIT but normal vHIT.
A within-group analysis revealed that all the PCS patients with an abnormal bHIT (n = 5) eventually had a normal vHIT.
Sensitivity and specificity of bHIT versus vHIT for distinguishing VN and PCS
We related the individual result from (i) the bHIT assessment in the ED and (ii) the algorithm-based vHIT judgement to the patient's final diagnosis (VN or PCS). Each test's sensitivity was determined by calculating the percentage of VN patients with an abnormal/peripheral HIT result among all VN patients. The specificity was determined by analyzing the percentage of PCS patients with a normal HIT result among all PCS patients.
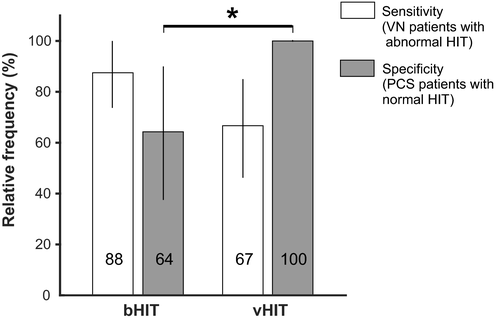
Sensitivity was 88% (n = 21 of 24; 95% CI: 73%–100%) for the bHIT and 67% (n = 16 of 24; 95% CI: 46%–85%) for the vHIT (Figure 2). Specificity was 64% (n = 9 of 14; 95% CI: 39%–88%) for the bHIT and 100% (n = 14 of 14; 95% CI: 100%–100%) for the vHIT. The sensitivity did not differ between bHIT and vHIT (p = 0.227). However, the vHIT had higher specificity than the bHIT (p = 0.044).
DISCUSSION
Accuracy of nonexperts' bHIT in the ED
The accuracy of the bHIT, when performed by nonexperts during routine clinical practice in the ED, is by far not as high as when it is assessed by neuro-otological experts under controlled conditions [9-11]. Only 58% of the bHIT results could be confirmed in the subsequent quantitative vHIT. This finding has important implications, especially with regard to the relatively high number (36%) of false-positive bHITs in PCS patients. Assigning an abnormal bHIT to a patient with normal vHIT represents a false-positive result that can lead to a dangerous false-benign misdiagnosis (VN in a patient with PCS). Moreover, the bHIT is the most important compound of the HINTS triad, which usually detects 96% to 100% of strokes in AVS patients, given that they are assessed by neuro-otological experts [1, 24]. If the bHIT is erroneously rated as abnormal, the HINTS may falsely be counted as negative, and their sensitivity for stroke decreases. This is underlined by the lower percentage (79%) of HINTS-positive PCS patients in our study.
That nonexperts have the tendency to rate the bHIT as abnormal, especially if VOR gains are at borderline values that experts would still assess as normal, is in line with previous findings in nonacute vestibular outpatients [16]. Similar to the latter study, nonexperts revealed high sensitivity (88%) for VN but at the cost of low specificity (64%), reflected by many false-abnormal bHITs in PCS. Although this may constitute only a minor problem in an elective outpatient visit, it represents a major fault in the emergency setting where a stroke must not be missed, as early treatment and monitoring is mandatory [25, 26].
The examiner's level of experience may be one reason for reduced bHIT accuracy [16]. Furthermore, a strong spontaneous nystagmus in the acute stage of an AVS may hamper correct bHIT assessment [19]. Nonetheless, 36% of PCS patients in our study were falsely assigned an abnormal bHIT in the ED, which provided the grounds for misdiagnosis. Surprisingly, nearly all the PCS patients, who were initially misdiagnosed as VN and not admitted to the stroke unit, revealed a normal bHIT in the ED, and thereby positive HINTS, which usually should have been recognized as a red flag for central pathology. This leads us to the question whether ED doctors actually rely on their own bHIT result and how this influences the further patient management in the hospital.
Reliance on the bHIT for clinical decision making
Our data indicate that doctors in the ED did not rely on their own bHIT result. In 14 VN patients, who were initially misdiagnosed as PCS and admitted to the stroke unit, 11 had an abnormal (peripheral) bHIT result in the ED. Only two of these VN patients had mild focal symptoms that could potentially be regarded as central signs indicating PCS. Hence, the majority of VN patients admitted to the stroke unit clinically presented with an isolated peripheral AVS that did not justify monitoring on a stroke unit. As mentioned above, five of six PCS patients, who were falsely suspected of VN and admitted to the general neurological ward, revealed a normal (central) bHIT result in the ED. Thus, residents in the ED did not take their own bHIT result into account when deciding on the patient's in-hospital triage. This may be due to distrust in their own clinical skills, lack of knowledge about peripheral/central signs in AVS, or a systematic bias in the ED to suspect stroke in AVS. The latter may also be related to time pressure in the ED, economic reimbursement considerations, and the fear of making a misdiagnosis (i.e., missing a stroke) with harmful consequences. However, that 58% of AVS patients were admitted to the stroke unit is in clear contrast to the 37% of patients having PCS and to the fact that the majority of AVS patients usually have a benign unilateral peripheral vestibulopathy [2, 4, 5, 9, 11].
Interestingly, if residents in the ED would have completely relied on their own—though sometimes inaccurate—bHIT result, their diagnostic assignment and patient management would have been better than their actual performance. If applying the bHIT as a single classifier, only 36% instead of 43% of PCS patients would have been misdiagnosed, and only 12% versus 58% of VN patients would have been unnecessarily monitored in the stroke unit. We admit that such an approach would misclassify PCS patients with a truly abnormal bHIT, that is, AICA strokes that collaterally damage the inner ear due to occlusion of the labyrinthine artery causing a mixed central-peripheral pathology [4, 13, 14]. However, these cases are generally rare, and none of the 14 PCS patients in our study exhibited a severe ipsilesional VOR deficit that would have led to diagnostic misclassification.
Diagnostic value of vHIT devices
Could a vHIT device, allowing quantitative VOR gain assessment, improve diagnosis and management of AVS patients in the ED? This is currently under investigation in two prospective clinical trials (NCT02483429 by Newman-Toker and collaborators; U1111-1172-8719 [27]). Data of our retrospective study suggest that an immediate vHIT could support the diagnostic distinction between VN and PCS [9, 17] and also improve patient management (e.g., in-hospital triage of AVS patients). Particularly, the vHIT's specificity was excellent (100%) and significantly higher than the bHIT's. For the ED setting, the specificity is certainly the more relevant parameter because it describes the percentage of PCS patients correctly identified by the test (normal HIT). Even not taking the remaining HINTS, central oculomotor disorders, and focal abnormalities into account, our simple algorithm-based vHIT correctly identified 100% of PCS patients and 67% of VN patients. In another study that used a different video-oculography device but comparable algorithm, the vHIT correctly classified 88% of PCS and 92% of VN patients [9].
Hence, if a vHIT would be immediately available in the ED, such as ECG [17], to support the clinician's decision, 88% to 100% of PCS patients could be correctly identified and adequately treated on the stroke unit, whereas only 8% to 33% of VN patients would be falsely classified as PCS and unnecessarily admitted to the stroke unit. This points to an additional diagnostic value of the vHIT, compared to the bHIT, that is usually assessed by doctors with limited neuro-otological expertise in the ED. Hence, implementation of a vHIT in the ED is likely to reduce the number of missed strokes and the number of VN patients who are unnecessarily admitted to stroke units. Moreover, the vHIT could help to teach and reassure nonexpert residents in the ED to improve their bHIT's accuracy and learn to rely on their clinical skills. Only in expert centers, the clinical bHIT may be as similarly reliable and precise as the vHIT in differentiating PCS from VN [9, 10, 17], and in those situations a vHIT may not have additional diagnostic yield [11].
CONCLUSION
Our findings indicate that under routine conditions in the ED, the bHIT in AVS patients has low accuracy, is too often interpreted as abnormal, and only of limited impact on further patient management (e.g., stroke unit admission). We recommend that an abnormal bHIT in the ED should be carefully reassessed by a neuro-otological expert, or preferably by vHIT, before making the diagnosis of a unilateral peripheral vestibulopathy and potentially missing a stroke. In addition to an improvement in diagnosis and the patient’s in-hospital triage, this could also help to increase ED the physician’s skills and trust in the clinical bHIT. Even if neuro-otological expertise is limited, we encourage ED clinicians to perform the bHIT and use it for diagnosis and clinical decision making in AVS patients, as we could show that taking a less accurate bHIT into account is better than completely ignoring it.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Björn Machner: conceptualization (lead), data curation (lead), formal analysis (lead), funding acquisition (lead), investigation (lead), methodology (lead), project administration (lead), resources (equal), software (equal), visualization (equal), writing original draft (lead). Kira Erber: data curation (equal), formal analysis (equal), visualization (equal), writing review editing (supporting). Jin Hee Choi: data curation (equal), formal analysis (supporting), project administration (supporting), writing review editing (supporting). Peter Trillenberg: conceptualization (supporting), formal analysis (equal), methodology (supporting), writing review editing (equal). Andreas Sprenger: data curation (equal), formal analysis (equal), software (lead), visualization (equal), writing review editing (supporting). Christoph Helmchen: conceptualization (equal), methodology (equal), resources (equal), supervision (lead), writing review editing (lead).
ETHICAL APPROVAL
The study was approved by the ethics committee of the University of Lübeck (18-146A) and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




