The economic benefit of timely, adequate, and adherence to Parkinson's disease treatment: the Value of Treatment Project 2
Abstract
Background
Parkinson's disease (PD) is a chronic progressive neurological disorder with a high psychosocial and economic burden. As part of the European Brain Council (EBC)-led Value of Treatment project, this study aimed to capture the economic benefit of timely, adequate, and adherence to PD treatment.
Methods
The EBC Value of Treatment Initiative combined different stakeholders to identify unmet needs in the patients’ journey according to Rotterdam methodology. The economic evaluation focused on three major topics identified as major gaps: start of treatment; best treatment for advanced disease; and adherence to treatment. Two separate healthcare systems (Germany and the UK) were chosen. Cost-effectiveness was determined by using decision-analytical modelling approaches. Effectiveness was expressed as quality-adjusted life-years (QALYs) gained and incremental cost-effectiveness ratio (ICER).
Results
Treatment intervention in PD was found to be cost-effective regardless of the initial health state of the patient receiving the treatment. Cost savings were between -€1000 and −€5400 with 0.10 QALY gain and -€1800 and -€7600 with 0.10 QALY gain for Germany and the UK, respectively. Treatment remains cost-effective within the National Institute for Health and Care Excellence thresholds. Availability of adequate treatment to more patients was also found to be cost-effective, with an ICER of €15,000–€32,600 across country settings. Achieving the target adherence to treatment would generate cost-savings of €239,000–€576,000 (Germany) and €917,000–€2,980.000 (UK) for every 1,000 patients treated adequately.
Conclusions
The analyses confirmed that timely, adequate, and adherence to PD treatment will not only improve care of the patients but is also cost-effective across healthcare systems. Further studies with a distinct identification of gaps in care are necessary to develop better and affordable care.
Introduction
Parkinson's disease (PD) is a chronic progressive disorder of the central nervous system, with approximately 1.42 million (0.2%) people affected in Europe [1]. It is the second most common neurodegenerative disorder after Alzheimer’s disease. As a result of the ageing European population, the number of patients is expected to double within the next 20 years [2]. Although PD is a disease more common in older age groups, one should keep in mind that approximately 10% of patients are affected at an age below 50 years. PD is characterized by the triad of symptoms bradykinesia, tremor and rigidity. In addition, a considerable number of non-motor symptoms may also occur during the course of the disease, such as gastrointestinal and autonomic disturbances, as well as behavioural and psychological symptoms (e.g., depression, cognitive impairment). Usually, the cause of the disease is not known (therefore idiopathic), but a smaller proportion of patients (<10%) have genetic disorders. PD symptoms are triggered by a decrease in the levels of the messenger dopamine, which allows messages to be sent to the parts of the brain that coordinate movement, due to the death of dopamine-producing nerve cells in the substantia nigra. With the loss of dopamine-producing nerve cells, these parts of the brain are unable to function normally, causing the symptoms of PD to appear. Typically, if first symptoms occur, a loss of more than 70% of the neuronal cells in the substantia nigra has already become obvious.
No PD is like the other and so each patient has to fight his/her own battle. The disease starts many years before the patient is aware of it. Currently there is no treatment available to slow down or reverse the disease. The goal of the treatment is to reduce symptoms with as few side effects as possible. PD does not directly cause people to die and for the majority of people it does not significantly affect their life expectancy, although some of the more advanced symptoms can lead to increased disability and poor health, which can make someone more vulnerable to infection. Thus, patients sometimes live 20 years or even longer with the disease and need to arrange their living conditions and social life accordingly. Despite little impact on life expectancy, PD patients experience progressive disability and reduced quality of life at all stages of the disease and at all ages. Several studies indicate that quality of life is affected not only by the motor symptoms of PD, but also by the non-motor symptoms such as depression and cognitive state [3]. The cost of illness escalates as PD progresses, placing an economic burden on the healthcare system, society and patients themselves. According to the European Brain Council (EBC) data for 2010, annual spend in Europe on PD was €13.9bn, consisting of €7bn in healthcare costs, €5.5bn in direct non-medical costs and €1.4bn in indirect costs [1].
In the present study, we describe some of the key issues and unmet needs along the patient’s journey, from the challenges associated with the initial diagnosis until the diverse complications of the late stages of the disease. We identified three key treatment gaps and describe potential solutions and best practices to give recommendations on how to improve care in the future. In an economic evaluation, we also assessed the impact of closing these gaps in reducing the burden of the disease on healthcare providers and society in two different European Union healthcare systems.
Methods
This study was conducted in the framework of the ‘Value of Treatment for Brain Disorders’ research project, coordinated by the EBC [4]. The PD team of the Value of Treatment project with patient representatives, clinical experts, health economic experts and industry partners worked together from May 2016 until June 2017 to evaluate diagnosis and treatment gaps in PD care in Europe. They identified best practices and solutions for better PD care models in Europe and drafted recommendations on how to implement these solutions.
The PD working group initially undertook a care pathway analysis to identify unmet needs and key issues throughout the course of the disease which prevent PD patients from receiving adequate and timely treatment. The main challenges and patient needs along the care process were described and analysed based on information from literature (review) and case studies, as well as from consultations with both patients and healthcare providers.
Secondly, an economic evaluation was performed to estimate the economic benefit of timely, adequate, and adherence to PD treatment. The aim of the economic case study analyses proposed by the Value of Treatment project was to make more and better economic evidence on the value of treatment in brain disorders available to policy decision-makers. The analyses were built on previously published research in the field, particularly where it generated evidence on effectiveness, and used methods successfully employed in published studies to explore the economic case for closing treatment gaps in brain disorders (more details on the Value of Treatment project methodology in [4]).
The economic evaluation focused on three topics that were identified as major gaps in the care pathway analysis: (i) lack of early/timely treatment (caused by delayed or inadequate diagnosis); (ii) lack of adequate treatment, and (iii) lack of adherence to treatment. The type of evidence available varied across the three topics considered and therefore influenced the type of analyses conducted. Two separate healthcare systems, in Germany and the United Kingdom (UK), were chosen as examples of European nations with different healthcare systems, considering the delivery of services, financing and coverage (details on the differences between healthcare systems are summarized in Appendix 1 in Data S1). They were also taken as case studies reporting on relevant evidence in the literature that could be used for the purposes of our analyses.
Lack of early/timely treatment
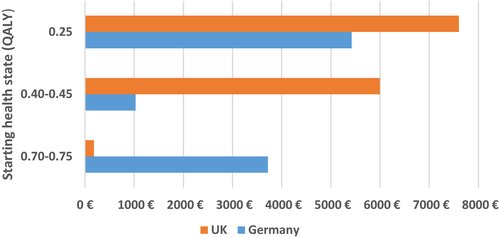
The first economic analysis looked at the short term cost-effectiveness gains attached to treatment start at different stages in the patient journey (graded according to Hoehn and Yahr (H&Y) stages [5]) compared with no treatment. Published economic and QALY data for different H&Y stages [5] were used to create a matrix that enabled annual direct costs to be attached [6-8] (Fig. 1). With a decision analytical modelling approach, we evaluated the impact of a hypothetical treatment (with fixed gain in effectiveness compared with no treatment, as per published data on early PD interventions [9, 10] when given to patients in different health states [5]. Annual direct cost estimates were reported from a healthcare provider perspective [6] (inflated to 2017 figures from the European Central Bank Eurosystem [11], in Euros). Effectiveness was expressed in terms of quality-adjusted life-year (QALY) gains. Cost-effectiveness was reported in terms of incremental cost-effectiveness ratio (ICER). Sensitivity analyses were applied to test the robustness of the model according to variation in the QALY improvements (0.05, 0.10 and 0.15 QALYs) and intervention costs (€0 up to €6 daily). The analyses included data sourced from both German [6] and UK settings [7].
Lack of access to adequate treatment for advanced Parkinson's disease
A second set of analyses evaluated the cost-effectiveness of best treatment in advanced PD (deep brain stimulation [DBS] and best medical treatment [BMT]) compared with current care. The analysis looked at direct costs (2017 figures from [11], in Euros) and QALYs comparing a current scenario where only a small proportion of eligible patients receive best treatment (2% on DBS + BMT vs. 88% on BMT vs. 10% no treatment), with a target scenario where a larger number of patients receive best treatment (15% on DBS + BMT vs. 85% on BMT only). Input from the Expert Working Group was crucial to agree the decision analytical mode structure as well as the current (suboptimal) and target model of care (Fig. 2). Published economic evidence representing clinical progression and capturing treatment effect (QALY) and costs using Markov modelling techniques were used to provide long-term (5-year) cost and QALY evidence for two different healthcare settings (Germany [12] and the UK [13], discount rates 3% and 3.5% per annum, respectively). Appendix 2 in Data S1 provides the patient-level cost and effectiveness data estimates considered for the different treatment options (BMT, DBS + BMT, no treatment). Details of the Markov models are presented elsewhere [12-14].

Lack of adherence to drug treatment
A third set of analyses looked at the economic impact of adherence to treatment (e.g., looking at the change in average patient healthcare costs according to level of adherence); and of a shift towards increased adherence to treatment in the country-specific PD patient population. Using a decision analytical model (Fig. S3 in Data S1), we calculated the economic savings (2017 figures from [11], Euros) when moving from current (suboptimal) to a target care scenario, with improved adherence rates. Outcomes for the economic evaluation were healthcare costs (medication costs, accident and emergency department visits, hospitalizations, general practitioner [GP] visits, day care and care home stay). The perspective adopted was for the public health insurance (Germany) and National Health Service (NHS; UK). A timeframe of 18 months was considered for analysis to cover the same period considered by relevant published literature. Sensitivity analyses looked at grouping patients according to different definitions of adherence as follows:
- Duration of therapy: assesses the duration, or persistence that a patient is treated with atypical Parkinsonism disorders. Duration of therapy was measured as the number of days between the first and last filled prescription of all PD drugs and the days’ supply of the last fill, the date of death, or the end of 19 months or whichever came first.
- Medication possession ratio: assesses how regularly patients take anti-PD drugs while in their possession. Calculated as the total days’ supply for all drug classes (numerator) divided by the aggregate duration of therapy of all drug classes (denominator).
Use-of-resources data were extracted from previous publications ([15-17] Appendix S3 and S1 in Data S1]. Unit costs for Germany and UK were sourced elsewhere ([18-23]; Appendix S3 and S4 Data S1). A public health insurance perspective for Germany and an NHS perspective for the UK were adopted. With the support of the experts we adapted findings and updated model variables using fresh evidence to reflect what could be expected in Europe today, at today’s prices.
Finally, potential solutions, best practices and policy recommendations (on how to improve the situation of the patients and caregivers and how to optimize the use of available resources) emerged from the care pathway analysis and economic evaluation, and were extracted from consensus reports of the European Parkinson’s Disease Association.
Results
Care pathway analysis/patient journey narrative
The author group undertook a structured discussion on currently felt treatment gaps during in-person meetings. This resulted in the following main issues. The economic analyses below were focused on these treatment gaps.
Delayed or inadequate diagnosis and misdiagnosis
Barriers to optimal treatment are numerous. Nearly a third of all patients who notice first symptoms wait 12 months or more before seeking medical help [24]. Furthermore, long waiting times to see a PD expert also contribute to delay in diagnosis. Although, the symptoms of PD are well known, the issue of missed or mis-diagnosis is relevant as well [24] for several reasons, including delay in improvement for the patient. There is evidence showing that nearly half of diagnoses (47%) are incorrect when performed in the primary care setting [24]. This high percentage might be explained by the extremely diverse range of non-motor symptoms and the fact that many symptoms are common to other diseases too. The absence of well-established biomarkers also increases the risk of misdiagnosis. As the deterioration in quality of life is already significant in the early phase of the disease, the diagnosis should be given as early as possible.
Lack of access to adequate treatment
The treatment of each patient needs to be adopted individually and tailored carefully to patient needs and disease stage. In the beginning, the medication helps to control the symptoms (this initial phase is the so-called ‘honeymoon’ phase), but these positive effects wane from year to year. No disease-modifying therapies are currently available. The impact of the disease increases over time and, in the advanced stage, PD may lead to a considerable loss of quality of life, disability and care dependency. Recommended therapies in more advanced disease stages, although only for selected patients, include DBS and pump therapies. Access to these therapies, however, is quite limited in some European countries.
Treatment of non-motor symptoms, such as depression, pain and other symptoms, should be focused on PD care as well, as they have a major impact on the patient's quality of life [25]. Patients' perceptions of symptoms often differ from the clinician's view, which may have an impact on their effective management of PD. Most patients depend on the help of their partners, families and/or the support of healthcare professionals (PD is often called a ‘family's disease’) and the burden to them is extremely high compared to other non-neurological chronic disorders [26]. Patients in Central and Eastern Europe especially often feel left alone with their problems, from the time of diagnosis to the later stages of the disease, when carers seem to be ignored or excluded from the decision-making process.
Lack of adherence to drug treatment
Patients with PD, in general, seem to have poor adherence to prescribed therapies, which is not only critical for their well-being, but also costly for the health system. Reasons for this non-adherence might be the fear of secondary effects, existing comorbidities and the complexity of dosing schedules, especially in patients with cognitive deficits [24].
Economic evaluation
The purpose of the economic analysis was to measure the economic impact of closing the current treatment gaps in PD, with particular attention given to providing timely and optimal care to PD patients. In particular, we have focused on three major topics.
Lack of early/timely treatment
Our model suggests that, at 1 year, the hypothetical PD treatment intervention is cost-effective regardless of the initial health state of the patient receiving the treatment (Germany cost savings between −€1,000 and −€5,400 with 0.10 QALY gain per patient; UK cost saving of −€1,800 and −€7,600 with 0.10 QALY gain per patient; Fig. 1). When the treatment enables the patient to improve to a less severe H&Y stage (e.g., transitions from H&Y stage 2 to 1, from stage 3 to 2 or from stages 4/5 to 3), it was found to be not only a more effective but also less costly option (compared to no treatment; Fig. S2). The cost savings increased with the severity of the disease (e.g., the transition from H&Y stage 4/5 to 3) were more cost saving than from H&Y stage 3 to 2 (e.g., −€5,400 vs. −€1,030 as the economic impact of 0.10 QALY gain in Germany; −€7,600 vs. −€6,000 as the economic impact of 0.10 QALY gain in UK). If we extrapolate the study findings to a long period (5 years or more) we can anticipate that timely/early intervention practices would enable the reduction of disease symptoms and related societal and healthcare costs across healthcare systems. Sensitivity analyses (Appendix 5 in Data S1) showed that the treatment remains cost–effective within the National Institute for Health and Care Excellence (NICE) thresholds (or cost-saving when shifting between H&Y stages) even in the worst scenario (with the costliest intervention option). When doubling or tripling the treatment effectiveness from 0.05 to 0.10 or 0.05 to 0.15, the window of opportunity to move to a less severe H&Y stage increased proportionally. The findings were consistent across healthcare systems. If we extrapolate the results to model the economic impact of early/timely treatment to a longer period (5 years or more) we can anticipate that such practices would enable a decrease in the related societal and healthcare costs across healthcare systems.
Lack of access to adequate treatment for advanced Parkinson's disease
Results showed that making the adequate treatment available to more patients is cost-effective (ICER €15,000 to €32,600 across country settings), where an increase in direct costs is accompanied by a gain in QALYs (compared with current care; Table 1; Fig. S2; Table S1). Sensitivity analyses are reported elsewhere (Dams et al. 2013 [12], Eggington et al. 2014 [13], McIntosh et al. 2016 [14]).
| Country (source of data) | Scenarios | Cost per 1000 people (5 years; Euros, 2017) | QALY gain per 1000 people (5 years) | ICER |
|---|---|---|---|---|
| Germany (Dams et al., 2013) [12] | Target | € 38 041 643 | 3134 | €14 836 |
| Baseline | € 31 540 872 | 2696 | ||
| UK (Eggington, et al., 2014) [13] | Target | € 64 369 795 | 1360 | €32 681 |
| Baseline | € 54 944 966 | 1072 | ||
| UK (McIntosh et al., 2016) [14] | Target | € 168 183 714 | 7145 | €28 127 |
| Baseline | € 138 680 392 | 6096 |
- ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
- Cost-effectiveness of best treatment (target scenario) vs. current suboptimal care (baseline scenario). This table reports data on the cost-effectiveness of access to adequate treatment for advanced Parkinson's disease
Lack of adherence to drug treatment
Results showed that, over a time frame of 1.5 years, low levels of adherence would correspond to an increase in annual patient costs (an increase of 20%–40% in Germany and 80%–300% in the UK, depending on the definition of adherence used; Figs. S3 and S4; Fig. 2). Intensified use of hospital and residential/nursing care home services were the main drivers of such increases. Meeting the target adherence to treatment rates (defined by the experts) would generate a cost saving of €239.000–€576,000 (Germany) and €917,000–€2,980,000 (UK) for every 1,000 patients treated adequately. Sensitivity analyses according to different levels of adherence are reported in Appendix 5 in Data S1. The major voice if saving were hospitalization, day care at home and residential/nursing home.
Discussion
The EBC provided an exact calculation of the costs attributable to the care of brain disorders and especially PD in the different European healthcare settings in previous publications [1, 27]; a total of €13,9bn is spent on the care of patients with PD in the European Union [3]. The present study concerns the major cost drivers based on the specific needs of the patients identified in the patients' journey. We have focused on three major topics: lack of early/timely treatment (caused by delay or inadequate diagnosis); lack of adequate treatment; and lack of adherence to treatment. The analyses confirmed that a timely, adequate and adherent approach to PD treatment is paramount to reducing the risk of disease progression; limiting the effects of PD on quality of life; and tackling the economic impact on service providers across healthcare systems.
It is important to note that the data presented in this study are based on the limited economic evidence available to describe the impact of the three treatment gaps of interest in Europe (lack of early/timely treatment: only effectiveness data were available as per published data of early PD interventions [8, 9]; lack of access to adequate treatment for advanced PD: economic data from previous cost-effectiveness analyses in Germany [11] and UK [12]; and lack of adherence to drug treatment: use-of-resources data were extracted from previous publications in the US setting [13, 15, 16]). Expert opinion was crucial to adapt the published data to current European Union clinical pathways and fill possible gaps in the analyses. The time frames considered for the three analyses were short term (1 year) up to medium term (up to 5 years, depending on the specific topic and evidence retrieved). The data showcased the impact of closing the treatment gaps on the healthcare providers in two different healthcare systems; more evidence would be needed to analyse the long-term consequences for healthcare providers and society across country systems.
Evidence from the qualitative analysis of the PD patient journey emerging from the Value of Treatment project confirmed that barriers to optimal treatment across Europe are numerous and span from diagnosis to treatment of the disease and its follow-up. Although the majority of patients with PD are in the older age groups, to close such gaps these patients should be actively involved in treatment decisions and receive sufficient attention to their quality-of-life concerns and specific needs. This will help identify adequate treatment of the individual symptoms and reduce the potential side effects of PD medication. Better information and empowerment of patients will lead to increased treatment adherence, especially if the carers are also involved. New techniques might support the patient to identify the best individual treatment.
Empower patients and involve families/caregivers
Good coordination and communication among the various healthcare providers is another important aspect, which leads to high patient and carer satisfaction. Studies from several European countries reveal considerable differences between the existing healthcare systems and identify that the creation of multidisciplinary care systems is still a long way off. However, the Netherlands and Israel have already established integrated, multidisciplinary care models which focus on the patient’s needs and could serve as examples for other countries [28, 29].
Promote a multidisciplinary approach involving all concerned parties
Patients with PD in the advanced stages of the disease may benefit from a team effort including neurologists, GPs, occupational therapists and physiotherapists, who may assist whenever a problem occurs. Communication and information transfer generally need to be improved; more efficiency is not only desirable for the patient–doctor relationship, but also for cooperation between the different European countries. It has already been demonstrated that this approach will even lead to significant savings of healthcare costs, which could, in turn, be invested in better education and training of providers. Currently, two PD networks are in place in Europe: the Dutch ParkinsonNet [30] and the Lombard regional network [31].
Individualized treatment as well as access to new and advanced therapies is vital
The public needs to be much better informed about PD, its typical symptoms and the particular needs of patients with PD. Patients should not need to worry about stigmatization; the disease itself is more than enough to cope with. Increasing public awareness of PD and the needs of PD patients (including among employers) will help not only the individual patient but also society as a whole to identify solutions for the increasing impact that PD has on health and economic systems in Europe [32-34].
Raise disease awareness and promote research
Parkinson's disease has many different facets, which require the joint effort of all stakeholders. Decision- and policy-makers need to realize that they have to act now to adequately face the 'tide' of the upcoming high occurrence of brain disorders so as not to be drowned. More funding for research is needed at different levels, including basic science, disease-oriented research, and healthcare research. Funding allocation to brain disorders is not adequate, and is considerably lower compared to that for other, non-neurological, disorders, such as cancer [25, 35]. The US Institute of Medicine panel, as well as European governmental institutions, proposed the concept that the amount of disease-specific research funding should be systematically and consistently allocated depending on the prevalence, impact of the disease on the population and the economy of the respective society [36]. These aims have not yet been reached.
In conclusion, decision- and policy-makers are asked to act soon in order to face the financial and societal burden resulting from an increasing number of patients with PD in Europe. Our analyses confirmed that timely and adequate treatment, and adherence to this, are pivotal to improve care of the patients and secure cost-effective care delivery across healthcare systems. A good balance is needed between cost-effectiveness of PD diagnosis and treatment and the well-being of the individual with the disease. Because of the high complexity of the disease, better knowledge and well-coordinated care models are needed as are already available in some countries in Europe. The support of politicians is requested to broadly implement available solutions and known best practices in all European member states.
Acknowledgements
This study was part of the EBC-led Value of Treatment Project. The work was supported by an unrestricted grant from Grünenthal, Pfizer and Medtronic to the EBC. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Open access funding enabled and organized by Projekt DEAL.
Disclosure of conflicts of interest
R.D. reports personal fees from AbbVie, personal fees and non-financial support from Axon Neuroscience, non-financial support from the Akademie für medizinische Fortbildung, personal fees from Agentur Süß, personal fees and non-financial support from Bayer, grants from Baxter, personal fees from Bial, personal fees and non-financial support from Biogen, personal fees and non-financial support from Bayer Vital, personal fees and non-financial support from BB-Biotech, grants and non-financial support from the Deutsche Gesellschaft für Neurologie, non-financial support from the Deutscher Hausärzteverband, personal fees from the Deutsches Zentrum für neurodegenerative Erkrankungen, grants and non-financial support from the Deutsche Parkinson Vereinigung, non-financial support from the Deutsche Gesellschaft Parkinson, personal fees and non-financial support from Desitin, non-financial support from the EBC, grants from EU-Horizon, personal fees and non-financial support from Eisai, personal fees from Elsevier, grants from Ernst und Margot Faber Stiftung, personal fees and non-financial support from Gemeinsamer Bundesausschuss, personal fees and non-financial support from GE Healthcare, grants from GE Healthcare General Electric, personal fees and non-financial support from the International Parkinson and Movement Disorder Society, personal fees and non-financial support from the Institut für hausärztliche Fortbildung im Deutschen Hausärzteverband, personal fees and non-financial support from IAK+, personal fees and non-financial support from Johanniter, from Kohlhammer, grants and personal fees from Lilly, grants from the Michael J. Fox Foundation, grants, personal fees and non-financial support from Novartis, personal fees from Ono Pharma, personal fees and non-financial support from Pfitzer, personal fees and non-financial support from Paul-Martini-Stiftung, non-financial support from the Programm Demenz Prävention, personal fees and non-financial support from Roche, personal fees and non-financial support from Schwabe, personal fees from Springer Verlag, grants from Stichting International Parkinson Fonds, personal fees from Thieme Verlag, personal fees and non-financial support from UCB Pharma, outside the submitted work. In addition, R.D. has patents issued for: Alpha-Synuclein Epitopbereiche (Bindungsstellen) natürlich vorkommender humaner Alpha-Synuclein Autoantikörper zur Diagnostik und Therapie von Morbus Alzheimer und anderen neurodegenerativen Erkrankungen; Diagnosis of Alzheimer’s Disease and other neurodementia diseases of AD-type and AD-therapeutic application; Diagnosis, Prohylaxis and Therapy of Alzheimer’s Disease and other neurodementing disorders; ELISA-Test zur Bestimmung von Amyloid Beta Auto-Antikörpern in humanen Serum-/Plasma-/CSF-Proben; Herstellung von oligomeren (trimeren) beta-Amyloid Molekülen mittels spezifischer Mutationen; Künstliche Mini-Amyloide zur Diagnose und Behandlung der Alzheimer Demenz; Naturally occurring autoantibodies against alpha-synuclein that inhibit the aggregation and cytotoxicity of alpha-synuclein with Dr. Rentschler Holding GmbH & Co KG; and Verfahren, insbesondere Enzyme-linked Immunosorbent Assay (ELISA) zum in vitro Nachweis von Amyloid beta Autoantikörpern, Mikrotiterplatte und Testkit. M.T. has nothing to disclose. G.D. reports personal fees from Boston Scientific, Cavion, Functional Neuromodulation, Thieme publishers, grants from Medtronic, outside the submitted work. He receives funding for his research from the German Research Council (SFB 1261, B5). G.P. is an employee of Gruenenthal. W.O. reports personal fees from Roche, UCB, Bial, Abbvie, Desitin, Synovion, Novartis GmbH Germany, outside the submitted work, received grants from the German Research Council, the Michael J Fox Foundation, the ParkinsonFonds Deutschland, the Charitable Hertie Foundation, and holds shares in Biogen, Medigene, Merck and Roche. J.A.-B. has nothing to disclose.
Open Research
Data availability statement
The data that support the findings of this study are available in the supplementary material of this article. The data that support the findings of this study are available from the corresponding author upon reasonable request.




