Transient ischemic attack without self-awareness of symptoms witnessed by bystanders: analysis of the PROMISE-TIA registry
Abstract
Background and purpose
A transient ischemic attack (TIA) can occur without self-awareness of symptoms. We aimed to investigate characteristics of patients with a tissue-based diagnosis of TIA but having no self-awareness of their symptoms and whose symptoms were witnessed by bystanders.
Methods
We used data from the multicenter registry of 1414 patients with a clinical diagnosis of TIA. For patients without evidence of ischemic lesions on imaging, clinical characteristics were compared between patients with and without self-awareness of their TIA symptoms.
Results
Among 896 patients (559 men, median age of 70 years), 59 (6.6%) were unaware of their TIA symptoms, but had those symptoms witnessed by bystanders. Patients without self-awareness of symptoms were older and more frequently female, and more likely to have previous history of stroke, premorbid disability, and atrial fibrillation, but less likely to have dyslipidemia than those with self-awareness. Patients without self-awareness of symptoms arrive at hospitals earlier than those with self-awareness (P < 0.001). ABCD2 score was higher in patients without self-awareness of symptoms than those with self-awareness (median 5 vs. 4, P = 0.002). Having no self-awareness of symptoms was a significant predictor of ischemic stroke within 1 year after adjustment for sex, ABCD2 score, and onset to arrival time (hazard ratio = 2.44, 95% confidential interval: 1.10–4.83), but was not significant after further adjustment for arterial stenosis or occlusion.
Conclusions
Patients with a TIA but having no self-awareness of their symptoms might have higher risk of subsequent ischemic stroke rather than those with self-awareness, suggesting urgent management is needed even if patients have no self-awareness of symptoms.
Introduction
Transient ischemic attack (TIA) is a medical emergency because of the elevated risk of subsequent ischemic stroke [1], which was highest within the first 24 h [2, 3]. Urgent evaluation and treatment of patients with a TIA in specialized clinics can greatly reduce the risk of stroke [4, 5]. However, because of its short-lasting nature [6], a TIA can be overlooked if patients are unaware of their symptoms, unless these symptoms are witnessed by bystanders. This situation means that some patients with a TIA are brought to stroke centers without self-awareness of their symptoms, because the symptoms were witnessed by family members or surrounding people. However, the frequency and characteristics of patients with a TIA but having no self-awareness of their symptoms are not well established. In addition to poor insights into the disease, the absence of ischemic lesions in a recent tissue-based diagnosis of TIA [7] can interfere with treatment decisions. Therefore, clinical evidence for a TIA without self-awareness of symptoms would contribute to the management of a TIA in clinical practice. Given this background, we aimed to elucidate the frequency and characteristics of patients with a tissue-based diagnosis of TIA but no self-awareness of their symptoms that were witnessed by bystanders, using data from the Prospective Multicenter Registry to Identify Subsequent Cardiovascular Events After Transient Ischemic Attack (PROMISE-TIA) study.
Materials and methods
The PROMISE-TIA study was a multicenter, prospective, observational study, and its methods are described elsewhere [8]. Briefly, patients with a clinical diagnosis of TIA who arrived within 7 days of onset were consecutively enrolled between June 2011 and December 2013 from 57 teaching hospitals certified by the Japan Stroke Society and followed up for 1 year. A clinical diagnosis of TIA was made if focal neurological symptoms attributable to a vascular etiology lasted for <24 h, irrespective of the presence of ischemic insults observed on imaging examinations [9]. The attending physicians made the diagnosis of TIA and management decisions. If patients were diagnosed with diseases other than a vascular etiology after registration, the diagnosis was changed to TIA mimics. Acute ischemic lesions were evaluated by computed tomography or diffusion-weighted imaging (DWI), and in this analysis, patients having at least one ischemic lesion were excluded as a diagnosis of minor stroke. Each local ethics committee approved the study. Written informed consent was obtained from all patients prior to enrollment.
The following clinical information was systematically collected: age; sex; vascular risk factors such as hypertension, diabetes mellitus, and dyslipidemia; and previous history of stroke or myocardial infarction. Premorbid functional status was estimated by the modified Rankin Scale (mRS), with premorbid disability defined as mRS ≥ 3. All patients and/or bystanders were interviewed using preconceived questionnaires regarding neurological symptoms during the index TIA by neurologists or stroke physicians at the participating institutions. Several yes/no questions on neurological symptoms, including hemiparesis, sensory disorder, speech difficulty, visual disturbance, and other nonfocal symptoms were included in the questionnaires, together with questions on who noticed the symptoms of the index TIA. In this study, the patients were divided into two groups: those with no self-awareness of their TIA symptoms, potentially including cases who neglected or did not pay attention to their TIA symptoms, and those with self-awareness of their symptoms regardless of the symptoms being witnessed by bystanders. The duration of symptoms was divided into two categories: ≤60 and >60 min. Time from onset to presentation was divided into five categories: <3, 3 to 6, 6 to 12, 12 to 24, and ≥24 h. Blood pressure was measured on arrival, and the ABCD2 score was calculated to estimate risk of ischemic stroke. Atrial fibrillation (AF) was diagnosed based on electrocardiographic findings on admission, 24-h Holter monitoring and/or electrocardiographic monitoring findings after admission, and medical history based on the medical charts. Extracranial and intracranial arteries were evaluated by carotid duplex ultrasonography, transcranial color flow imaging, magnetic resonance angiography, computed tomography angiography, and/or conventional angiography. Vascular lesions were defined as stenosis ≥50% or occlusion detected by any modality. Distribution of lesions was categorized into three territories: the right or left anterior circulation (internal carotid artery [ICA], middle cerebral artery [MCA], and/or anterior cerebral artery [ACA]), and posterior circulation (posterior cerebral artery [PCA], basilar artery [BA], and/or vertebral artery [VA]). Patients were assessed in the outpatient clinic or by telephone interview at 3 months and 1 year after TIA onset, and follow-up data including occurrence of ischemic stroke were obtained.
All statistical analyses were performed with JMP version 9.0 software. Data were expressed as median and interquartile range for continuous variables and count and percentage for categorical variables. Clinical characteristics were compared between patients with and without self-awareness of their TIA symptoms using Mann-Whitney U test for continuous variables, and the χ2 test or Fisher's exact test for categorical variables. Cumulative risks of ischemic stroke in patients with and without self-awareness of symptoms were estimated with Kaplan-Meier models. Hazard ratios (HRs) for ischemic stroke within 1 year of TIA onset were calculated with Cox regression analyses adjusted for age, sex, ABCD2 score, onset to arrival time, and vascular lesions assessed at baseline. Values of P < 0.05 was considered statistically significant.
Results
Among the 1414 consecutively enrolled patients, 77 were excluded because they had a final diagnosis of TIA mimics (n = 42), incomplete data on symptoms of the index TIA (n = 10), lack of information on who noticed the neurological symptoms (n = 20), or lack of imaging data (n = 5). Ischemic lesions were examined using DWI (n = 1300) in the remaining 1337 patients, and 441 patients were excluded with a final diagnosis of minor stroke. Among patients with ischemic lesions, 51 (11.6%) were unaware of their symptoms. Finally, 896 patients (559 men, median age of 70 years) with a diagnosis of tissue-based TIA were included in the analysis. Among them, 59 (6.6%) were unaware of their symptoms, but had their symptoms witnessed by bystanders. Out of remaining 837 patients with self-awareness, bystanders also witnessed symptoms in 234 patients (28.0%).
The characteristics of the patients with and without self-awareness of their TIA symptoms are listed in Table 1. Patients with no self-awareness of their TIA symptoms had higher frequencies of female sex (57.6% vs. 36.2%, P = 0.001), older age (median 81 years vs. 70 years, P < 0.001), previous history of stroke (40.7% vs. 19.8%, P < 0.001), premorbid disability (23.7% vs. 1.7%, P < 0.001), and AF (32.2% vs. 10.5%, P < 0.001), but lower frequencies of dyslipidemia (62.7% vs. 76.3%, P = 0.019) than those with self-awareness of their symptoms. Regarding neurological symptoms of the index TIA, patients with no self-awareness of their symptoms were more likely to have speech difficulty (69.5% vs. 47.0%, P < 0.001) and less likely to have sensory disorder (8.5% vs. 35.1%, P < 0.001) than those with self-awareness of their symptoms. All sensory disorders in patients with no self-awareness of their symptoms were accompanied by hemiparesis.
| Variable | Total, n = 896 | Self-awareness of TIA symptoms | P value | |
|---|---|---|---|---|
| No, n = 59 | Yes, n = 837 | |||
| Sex, female | 337 (37.6) | 34 (57.6) | 303 (36.2) | 0.001 |
| Age, years | 70 (62–78) | 81 (75–88) | 70 (62–77) | <0.001 |
| Premorbid disability | 28 (3.1) | 14 (23.7) | 14 (1.7) | <0.001 |
| Risk factors | ||||
| Hypertension | 599 (66.9) | 43 (72.9) | 556 (66.4) | 0.309 |
| Dyslipidemia | 676 (75.4) | 37 (62.7) | 639 (76.3) | 0.019 |
| Diabetes mellitus | 214 (23.9) | 20 (33.9) | 194 (23.2) | 0.062 |
| Atrial fibrillation | 107 (11.9) | 19 (32.2) | 88 (10.5) | <0.001 |
| Previous history of stroke | 190 (21.2) | 24 (40.7) | 166 (19.8) | <0.001 |
| Previous history of MI | 26 (2.9) | 2 (3.4) | 24 (2.9) | 0.687 |
| Clinical findings | ||||
| Hemiparesis | 641 (71.5) | 45 (76.3) | 596 (71.2) | 0.405 |
| Side of hemiparesis, right, n = 641 | 337/641 (52.6) | 20/45 (44.4) | 317/596 (53.2) | 0.257 |
| Sensory disorder | 299 (33.4) | 5 (8.5) | 294 (35.1) | <0.001 |
| Speech difficulty | 434 (48.4) | 41 (69.5) | 393 (47.0) | <0.001 |
| Visual disturbance | 79 (8.8) | 3 (5.1) | 76 (9.1) | 0.473 |
| Nonspecific symptoms | 166 (18.5) | 13 (22.0) | 153 (18.3) | 0.473 |
| Duration of symptoms > 60 min | 361 (40.3) | 25 (42.4) | 336 (40.1) | 0.736 |
| Onset to arrival time | ||||
| <3 h | 491 (54.8) | 49 (83.1) | 442 (52.8) | <0.001 |
| 3–6 h | 205 (22.9) | 8 (13.6) | 197 (23.5) | |
| 6–12 h | 83 (9.3) | 1 (1.7) | 82 (9.8) | |
| 12–24 h | 72 (8.0) | 1 (1.7) | 71 (8.5) | |
| ≥24 h | 45 (5.0) | 0 (0) | 45 (5.4) | |
| Blood pressure, mmHg | ||||
| Systolic | 153 (137–172) | 145 (132–177) | 153 (137–172) | 0.297 |
| Diastolic | 83 (73–95) | 76 (66–88) | 84 (74–95) | <0.001 |
| ABCD2 score | 4 (4–5) | 5 (4–6) | 4 (3.5–5) | 0.002 |
| ≥4 | 680 (75.9) | 52 (88.1) | 628 (75.0) | 0.026 |
| Examination findings | ||||
| Glycemia, mg/dl | 112 (100–136) | 122 (101–160) | 111 (100–135) | 0.032 |
| HbA1c, % | 5.7 (5.4–6.2) | 5.7 (5.3–6.6) | 5.7 (5.4–6.2) | 0.877 |
| LDL-C, mg/dl | 114 (94–137) | 99(83–115) | 115 (95–138) | <0.001 |
| HDL-C, mg/dl | 51 (41–61) | 49 (39–67) | 51 (41–61) | 0.775 |
| TG, mg/dl | 115 (82–168) | 90 (65–148) | 119 (83–171) | <0.001 |
| Vascular lesions, n = 880 | 328/880 (37.3) | 25/54 (46.3) | 303/826 (36.7) | 0.157 |
| Distribution of the lesions, n = 328 | ||||
| Anterior circulation, right | 84/328 (25.6) | 4/25 (16.0) | 80/303 (26.4) | 0.291 |
| Anterior circulation, left | 87/328 (26.5) | 7/25 (28.0) | 80/303 (26.4) | |
| Posterior circulation | 49/328 (14.9) | 2/25 (8.0) | 47/303 (15.5) | |
| ≥2 vascular territories | 108/328 (32.9) | 12/25 (48.0) | 96/303 (31.7) | |
- Data are presented as n (%) or median (interquartile range).
- Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; mRS, modified Rankin Scale; TG, triglyceride; TIA, transient ischemic attack.
Patients with no self-awareness of their symptoms arrived earlier (P < 0.001) and had higher ABCD2 score (5 [4–6] vs. 4 [3.5–5], P = 0.002) than those with self-awareness of their symptoms. Vascular lesions were examined in 880 patients (98.2%), and 328 (37.3%) had vascular lesions including ICA (n = 86), BA (n = 42), VA (n = 60), MCA (n = 137), ACA (n = 38), and/or PCA (n = 48). The laterality of hemiparesis and distribution of vascular lesions were not significantly different between patients with and without self-awareness of their TIA symptoms.
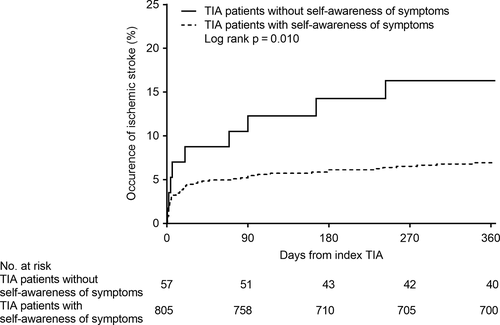
During the 1-year follow-up period after TIA onset, ischemic stroke occurred in nine patients without self-awareness of their TIA symptoms and 56 patients with self-awareness. Death from any cause occurred in 13 patients: three patients without self-awareness and 10 patients with self-awareness. None of the patients died from cardiovascular disease. Kaplan-Meier curves for the cumulative incidence rate of occurence of ischemic stroke are shown in Fig. 1. A higher risk of ischemic stroke during follow-up was observed in patients without self-awareness than in those with self-awareness (16.3% vs. 6.9%, log-rank; P = 0.010). Having no self-awareness remained as an independent risk factor for ischemic stroke after adjusting for sex, ABCD2 score, and onset to arrival time (adjusted HR = 2.44 [95% confidence interval: 1.10–4.83]), but was not significant after further adjustment for vascular lesions (Table 2).
| Variable | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| Having no self-awareness, unadjusted | 2.44 (1.13–4.69) | 0.026 |
| Model 1 | 2.58 (1.15–5.20) | 0.024 |
| Model 2 | 2.44 (1.10–4.83) | 0.029 |
| Model 3 | 1.69 (0.64–3.73) | 0.267 |
- Model 1: adjusted for age, sex. Model 2: adjusted for sex, ABCD2 score and onset to arrival time. Model 3: adjusted model 2 for vascular lesions.
Discussion
This study clarified the frequency and characteristics of patients with a tissue-based diagnosis of TIA without self-awareness of their symptoms. Among the patients with a TIA who arrived at stroke centers, 6.6% were unaware of their symptoms, but had those symptoms witnessed by bystanders. This suggests that some TIA events can be overlooked due to the absence of both self-awareness of symptoms and witnessing by bystanders. In previous studies, the annual standardized incidence rate of TIA ranged from 28.6 to 98.7 per 100,000 in Western countries [10, 11], and the annual incidence rate was 0.56 per 1000 Hisayama residents from Japan [12]. Further to previous studies showing that patients with a TIA do not always seek medical attention [13, 14], the present findings indicate that the incidence of TIA is actually much higher than previously reported, considering that TIA events can be overlooked.
The present study identified several factors, including higher age, previous history of stroke, and premorbid disability, that were associated with patients having no self-awareness of their TIA symptoms and these symptoms being witnessed by bystanders. There are two potential reasons for this finding; first, older patients and patients with a high mRS score had more chances for caregivers to be present and to witness the symptoms than younger patients and patients with a low mRS score. Second, these patients were more likely to have physical and psychological frailty [15], dementia [16], and disorders of the locomotive system including deteriorated motor functions and musculoskeletal pathologies [17, 18]. Although a previous history of stroke has been associated with stroke symptom recognition [19, 20], the opposite result was seen in this study. The poststroke cognitive decline might interfere with self-noticing of TIA symptoms by patients themselves.
Among the neurological symptoms, speech difficulty was a more frequent symptom in patients without self-awareness of their symptoms than those with self-awareness, which was consistent with a previous study, in which difficulty in speaking was the most frequent symptom observed by bystanders [21]. The time from TIA onset to hospital arrival was significantly shorter in patients without self-awareness of their symptoms than in those with self-awareness of their symptoms. This may be because of the difference in frequency of symptom witnessing by bystanders between the two groups. Bystanders were less likely to wait and see whether symptoms improved spontaneously than patients themselves when faced with a stroke [22]. Bystanders' awareness of symptoms and immediate response are important for seeking medical attention in patients with stroke and TIA [23-25]. The present findings support the importance of bystanders' witnesses of transient symptoms, which is the only clue for seeking medical attention for patients without self-awareness of symptoms but with a high risk of subsequent ischemic stroke.
Patients without self-awareness of symptoms had higher ABCD2 score and high incidence of ischemic stroke than those with self-awareness. Our result can be the basis for managing patients without self-awareness of symptoms and poor insights into the cerebrovascular disease. Moreover, the significance in association between having no self-awareness and risk of ischemic stroke disappeared after adjustment with vascular lesions. Because large artery lesions are predictive for subsequent stroke after a TIA [26, 27], present findings indicate urgent vascular evaluations and management are required for patients with a TIA who are unaware of their symptoms, but have these symptoms witnessed by bystanders.
Our study has several limitations. First, the number of patients with no self-awareness of their TIA symptoms was small and may have led to some statistical errors. Moreover, the characteristics of these patients may simply reflect the noticeability of their symptoms by bystanders rather than the patients having no self-awareness of their symptoms. Second, some clinical information, including details of premorbid disabilities, the cognitive status, initial perceptions of symptoms in the index TIA, detailed profiles of bystanders, and routes of arrival, was not available. This information could negate previous findings such as perception of an emergency situation and correct diagnosis of stroke by bystanders leading to seeking medical attention [28, 29], and referral from another medical facility being associated with a delay in seeking assessment after a TIA [30]. Moreover, patients with cognitive impairment may be more likely to be unaware of a TIA or to forget it. Third, emergency doctors might have made some treatment decisions in the initial emergency response before neurological consultation, which could have affected the clinical outcomes. Finally, the association between self-awareness of symptoms and anosognosia due to right hemispheric lesions has not been clear. Further studies are needed to elucidate whether the intensive antithrombotic therapy can prevent ischemic stroke in patients without self-awareness of symptoms.
In conclusion, 6.6% of TIA patients arriving at hospitals had no self-awareness of their symptoms. Patients with a TIA and no self-awareness of their symptoms might have higher risk of subsequent ischemic stroke rather than those with self-awareness, suggesting urgent management is needed even if patients have no self-awareness of symptoms.
Acknowledgments
The authors acknowledge the contributions of Dr. Shigeharu Takagi, Department of Neurology, Tokai University Hospital (now deceased) to this work. The authors thank Alison Sherwin, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript. This study was supported in part by Grants-in-Aid (H21-Junkanki-Ippan-017 and H24-Junkanki-Ippan-011) from the Ministry of Health, Labour and Welfare of Japan. This study was not funded by any private companies.
Disclosure of conflicts of interest
Dr. Sato reports employment by the Japanese subsidiary of Bayer Healthcare Pharmaceuticals, Bayer Yakuhin Ltd., from January 2018, but all of his contributions to the present study were made during his employment at the National Cerebral and Cardiovascular Center. Dr. Okada received lecture honoraria from Daiichi-Sankyo, Bayer Yakuhin Ltd., and Pfizer Co. outside of the submitted work. Dr. Ogasawara reports consigned research funds from Nihon Medi-Physics Co. Ltd. Dr. Toyoda received lecture honoraria from Daiichi-Sankyo, Bayer Yakuhin Ltd., Nippon Boehringer Ingelheim, and Bristol-Myers Squibb Co. outside of the submitted work. Dr. Minematsu reports compensation for seminar presentations by Bayer Yakuhin Ltd., Otsuka Pharmaceutical Co. Ltd., Boehringer Ingelheim Japan Inc., AstraZeneca KK, Pfizer Japan Inc., Mitsubishi Tanabe Pharma Corporation, Stryker Japan KK, Kowa Company Ltd., Nihon Medi-Physics Co. Ltd., Bristol-Myers Squibb Co., Sawai Pharmaceutical Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Daiichi-Sankyo Co. Ltd., Astellas Pharma Inc., and Nippon Chemiphar Co. Ltd., and for advisory boards at CSL Behring KK, Medico's Hirata Inc., and Bayer Yakuhin Ltd. The other authors report no conflicts of interest.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




