Monogenic cerebral small-vessel diseases: diagnosis and therapy. Consensus recommendations of the European Academy of Neurology
Abstract
Background and purpose
Guidelines on monogenic cerebral small-vessel disease (cSVD) diagnosis and management are lacking. Endorsed by the Stroke and Neurogenetics Panels of the European Academy of Neurology, a group of experts has provided recommendations on selected monogenic cSVDs, i.e. cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL), autosomal dominant High Temperature Requirement A Serine Peptidase 1 (HTRA1), cathepsin-A-related arteriopathy with strokes and leukoencephalopathy (CARASAL), pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL), Fabry disease, mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS) and type IV collagen (COL4)A1/2.
Methods
We followed the Delphi methodology to provide recommendations on several unanswered questions related to monogenic cSVD, including genetic testing, clinical and neuroradiological diagnosis, and management.
Results
We have proposed ‘red-flag’ features suggestive of a monogenic disease. General principles applying to the management of all cSVDs and specific recommendations for the individual forms of monogenic cSVD were agreed by consensus.
Conclusions
The results provide a framework for clinicians involved in the diagnosis and management of monogenic cSVD. Further multicentre observational and treatment studies are still needed to increase the level of evidence supporting our recommendations.
Video Interview
Watch Marco Pasi interviewing Prof. Hugh S. Markus.
Introduction
Although the most common hereditary cerebral small-vessel disease (cSVD), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), was first described over two decades ago, in recent years additional monogenic cSVD genes have been identified. Apart from Fabry disease (FD), there are no available disease-modifying therapies. Therefore, cSVD treatment focuses on symptomatic management, using approaches for which there is often no clear evidence base. As a consequence, there are inconsistencies in treatment and preventive care regimens. Diagnosis can also provide challenges, particularly with the increasing use of next-generation sequencing techniques and the need to determine whether or not variants are disease causing.
This article reports the results generated by a European Academy of Neurology Delphi consensus panel on important clinical questions related to management of monogenic cSVD.
Materials and methods
The Delphi method provides a systematic approach for collecting opinions from experts (the ‘Delphi panel’) and has been widely applied to obtain consensus or recommendations on well-defined topics. Although described as ‘panel’, experts provide their opinions freely, individually and anonymously.
Phase I: pre-meeting
Endorsed by the Neurogenetics and Stroke Panels of the European Academy of Neurology, two facilitators (M.M. and H.S.M.) invited experts from established centres of excellence in cSVD within Europe. All experts are opinion leaders in the fields of neurogenetics, neuroimaging and cSVD. The experts decided to focus on the following selected monogenic cSVDs and working groups were created: CADASIL (led by S.L.O., with A.Be., A.Fe., H.C. and H.S.M.), cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) (led by A.Be., with A.Fe., H.S.M. and E.T.-L.), cathepsin-A-related arteriopathy with strokes and leukoencephalopathy (CARASAL) (J.F.), pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL) (led by H.C., with A.Be. and E.T.-L.), FD (led by A.Bu., with J.F. and M.A.), mitochondrial encephalopathy, lactic acidosis and stroke-like episodes (MELAS) (led by M.M., with J.F.), type IV collagen (COL4)A1/2 (led by H.C., with A.Be. and E.T.-L.) and retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations (RVCL-S) (D.H.).
Each working group leader executed a comprehensive search strategy to November 2018 in the MEDLINE database. Only English language studies were included. Each leader screened citations, reviewed full-text papers for inclusion, appraised study quality and abstracted the data. Consensus was achieved for inclusion of papers by discussion within working groups (Fig. 1). Finally, each group provided a list of queries to be voted on, as well as the references used to address the specific disease (File S1), which was distributed to all experts.
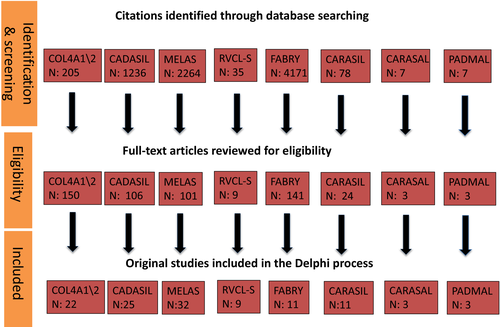
The two facilitators created a survey (File S2) to gauge the level of consensus among experts; responses and votes were collected anonymously through the Google forms platform (https://docs.google.com/forms/u/0/) and analysed prior to the face-to-face meeting.
Participants voted using a five-point Likert scale 1 to indicate their level of agreement on each statement (1, absolutely disagree; 2, disagree; 3, no judgement; 4, more than agree; 5, absolutely agree). A ‘strong consensus’ was defined if >70% of scores were ≥4 and the mean score was ≥4. If only one of these two parameters was met, the consensus was considered a ‘good consensus’. If both parameters were not met, the statement was considered to lack consensus agreement.
Phase II: Delphi panel
The 14 experts met in a face-to-face meeting in Vienna from 28 February to 1 March 2019. A delegate from the Lily Foundation participated in the meeting as patient advocate, without voting.
M.M. introduced the workshop aims. H.S.M. gave a brief overview on proposed red flags suggesting a diagnosis of monogenic cSVD. M.M. presented results from Phase I. Statements from Phase I without consensus were selected for discussion. The working group leaders presented individual diseases, discussing results of the first round of the survey and presenting the available data supporting the queries. After discussion, when required, statements from the first round were modified or deleted and participants voted again on the revised statements. All participants were involved in all Delphi phases.
Statements were divided into three main areas: (i) identification of monogenic cSVD; (ii) clinical and features and diagnosis; and (iii) management.
Results
Table 1 presents the results of all statements.
| Query | Mean score | Percentage of people voting 4 or 5 |
|---|---|---|
| General table: Thinking about the red flags of monogenic cSVD, which are the ones that a general neurologist should keep in mind to suspect a monogenic cSVD | ||
| Family history | 5 | 100 |
| Young age at onset | 4.92 | 100 |
| Characteristic neuroimaging features | 4.54 | 84.6 |
| Consanguinity | 4.92 | 100 |
| Extracerebral/systemic features | 4.54 | 100 |
| Clinical phenotype characteristic of specific monogenic disease | 4.61 | 92.8 |
| Negative clinical workup for other causes of cSVD | 4.15 | 69.2 |
| Even in patients with traditional vascular risk factors we should consider monogenic stroke | 4.54 | 84.6 |
| Standard clinical care including general indications, contraindications, clinical monitoring and side effects for all drugs must always be kept in mind with or without genetic defect causing cSVD | 4.46 | 84.6 |
| General principles for all monogenic cSVDs | ||
| Patients with monogenic cSVD should be followed up by specialized centres and/or an expert neurologist | 4.77 | 100 |
| Genetic counselling should include discussion of family planning, including the option of pre-natal diagnostics and pre-implantation genetic diagnosis | 4.86 | 100 |
| Family members of the proband should be offered at least one consultation with a clinical geneticist or equivalent for genetic counselling | 4.84 | 100 |
| At (genetic) diagnosis, patients with cSVD should be offered psychological support if needed | 4.77 | 100 |
| Because vascular risk factors have been shown to increase the severity of CADASIL and may well do the same for other rarer monogenic cause of cSVD, after genetic diagnosis the vascular risk factor profile of patients with monogenic cSVD should be carefully evaluated and lifestyle changes such as stopping smoking, healthy diet and physical exercise should be advised | 4.84 | 100 |
| Patients with cSVD should have a yearly evaluation of their vascular risk factor profile | 4.92 | 100 |
| At genetic diagnosis of cSVD, a cerebral MRI should be considered, irrespective of signs or symptoms | 4.15 | 84.6 |
| Follow-up cerebral MRI is not indicated unless new signs or symptoms appear | 4.85 | 100 |
| CADASIL | ||
| CADASIL is caused by NOTCH3 mutations leading to an uneven number of cysteine residues (usually 5 or 7) in one of the NOTCH3 protein’s EGFr domains | 4.85 | 100 |
| NOTCH3 mutations leading to a cysteine alteration in one of the NOTCH3 EGFr domains 1–6 may predispose to an earlier onset of stroke than mutations located in one of the EGFr domains 7–34 | 4.46 | 92.8 |
| CADASIL can only be definitively confirmed by genetic testing, revealing a NOTCH3 mutation altering the number of cysteines in one of the 34 EGFr domains of the NOTCH3 protein | 4.92 | 100 |
| CADASIL can be confirmed by skin biopsy, with electron microscopy showing GOM | 4.38 | 92.8 |
| CADASIL can be established by skin biopsy, but genetic testing should be the first diagnostic line investigation | 4.61 | 92.8 |
| In the case of a NOTCH3 variant of unknown significance, CADASIL can be confirmed using a skin biopsy for electron microscopy and/or NOTCH3 immunostaining | 4.54 | 92.8 |
| WMHs located in the anterior temporal lobes are present in 90% of cases but can be absent | 4.38 | 92.8 |
| The diagnosis of CADASIL should be considered in any patient with unexplained symmetrical periventricular WMHs and a positive family history of migraine with aura, stroke, mood disorders or dementia | 4.92 | 100 |
| CADASIL cannot be ruled out in the absence of a medical or family history of migraine with aura | 4.38 | 84.6 |
| CADASIL cannot be ruled out in the presence of common cerebrovascular risk factors and extensive WMHs | 4.30 | 84.6 |
| CADASIL can present with mild signs or symptoms, even in elderly individuals (>70 years) with a negative medical history of stroke and a negative family history | 4.61 | 100 |
| Most strokes in CADASIL are ischaemic, but intracerebral haemorrhage can rarely occur, especially in patients treated with anticoagulants | 4.69 | 100 |
| CADASIL should be considered in the differential diagnosis of multiple sclerosis | 4.77 | 100 |
| Patients with CADASIL should be monitored for subjective or objective cognitive impairment and when present should be offered neuropsychological assessment and support | 4.77 | 100 |
| Many clinicians prescribe an antiplatelet agent in a patient with CADASIL who has suffered a clinical ischaemic stroke, although there is no trial evidence supporting their use in CADASIL as opposed to stroke more generally | 4.61 | 100 |
| There is no evidence to support the use of antiplatelet agents in patients with CADASIL who have not suffered a clinical ischaemic stroke | 4.85 | 100 |
| Patients with CADASIL should not receive thrombolysis for acute ischaemic stroke unless it is due to a large-artery occlusion secondary to a concurrent cause (this is very uncommon) | 4.69 | 100 |
| Statin therapy is not recommended for a patient with CADASIL who has normal cholesterol even after stroke, but is not contraindicated if there is another reason for its use | 4.77 | 100 |
| There is no evidence contraindicating the use of triptans in CADASIL | 4.54 | 100 |
| There is no evidence to recommend the use of acetazolamide; trials of its use are required | 4.61 | 100 |
| Patients with CADASIL can be treated with psychiatric drugs according to guidelines for other cerebrovascular disorders | 4.61 | 100 |
| Anticoagulants are not recommended for the prophylaxis of stroke in CADASIL due to the risk of intracerebral haemorrhage, but are not contraindicated if there is another indication for it (e.g. atrial fibrillation, pulmonary embolus) | 4.77 | 100 |
| There is no evidence to contraindicate the use of oral contraceptives in CADASIL | 4.23 | 92.8 |
| Anaesthetic procedures maintaining haemodynamic stability and avoiding hypotension are recommended for patients with CADASIL undergoing surgery | 4.77 | 100 |
| Pregnancy and labour are generally uncomplicated in CADASIL and do not warrant specific precautions or medical interventions | 4.31 | 84.6 |
| Pregnancy and labour are associated with an increased risk of transient neurological events especially during the puerperium, mostly consistent with migraine aura, of which the patient and obstetrician should be aware | 4.69 | 92.8 |
| CARASIL | ||
| CARASIL is caused by biallelic mutations of HTRA1 gene | 5 | 100 |
| Homozygous stop codon mutations, homozygous missense or frameshift and compound heterozygous mutations of HTRA1 gene have been identified as causative in CARASIL | 5 | 100 |
| HTRA1 gene heterozygous mutation can cause autosomal dominant cSVD, distinct from CARASIL | 4.92 | 100 |
| HTRA1 mutations behave as autosomal recessive mutations with heterozygous carriers clinically unaffected but also as autosomal dominant mutations, with heterozygous carriers being clinically affected | 4.23 | 84.6 |
| Skin biopsy examination does not show GOM typical of CADASIL | 5 | 100 |
| Premature diffuse scalp alopecia is seen in the majority of patients | 4.85 | 100 |
| The diagnosis CARASIL should be considered in any patient with early-onset premature scalp alopecia, severe spondylosis and unexplained early lacunar strokes and/or symmetrical WMHs | 4.92 | 100 |
| White matter changes in the anterior temporal lobe and external capsule may occur in CARASIL | 4.77 | 92.8 |
| HTRA1 heterozygous mutations may lead to a late-onset syndrome characterized by gait disturbances, mood depression, cognitive impairment, stroke, migraine as well as WMHs on MRI | 4.77 | 100 |
| CARASIL should be considered in the differential diagnosis of CADASIL, CARASAL and early-onset cSVD | 4.7 | 92.8 |
| No data to recommend antiplatelet therapy in CARASIL | 4.92 | 100 |
| HTRA1-autosomal dominant disease | ||
| Heterozygous HTRA1 mutations cause an autosomal dominant cSVD | 4.71 | 92.8 |
| Clinical features may include lacunar strokes, cognitive impairment and encephalopathy | 4.36 | 85.7 |
| COL4A1-COL4A2 | ||
| An autosomal dominant cSVD of haemorrhagic type can result from missense or null mutations of COL4A1 or COL4A2 | 5 | 100 |
| Missense mutations of COL4A1 or COL4A2 leading to replacement of a glycine of a GLY-X-Y motif in the triple helix and mutation leading to premature stop codon are pathogenic | 4.78 | 100 |
| A mutation of COL4A1 or CO4A2 should be sought in a patient with deep intracerebral haemorrhage of undetermined origin or WMHs of undetermined origin or porencephaly haemorrhage of undetermined origin when there is a family history of cerebral haemorrhage, porencephaly, retinal vessel tortuosities, haematuria, glomerular dysfunction, renal insufficiency, renal cysts, infantile hemiparesis, early cataracts, abnormalities of the anterior segment of eyes, multiple intracranial aneurysms, muscle cramps or hepatic cyst is present in at least one first- or second-degree relative | 4.78 | 100 |
| A non-invasive workup at different organ levels (including cerebral MRI, intracranial and cervical MR angiography, cardiac echography, detailed ocular and retinal vessel examination with visual acuity and ocular pressure measures, renal echography, muscular creatine kinase) is recommended in individuals with COL4A1 or COL4A2 mutation | 4.93 | 100 |
| Pre-symptomatic testing of family members is recommended because of potential benefit of preventive measures, including avoiding complications during childbirth | 4.71 | 100 |
| Antiplatelet and anticoagulant treatments are not recommended in COL4A1/2 cSVD | 4.71 | 100 |
| Intravenous thrombolysis is not recommended in a patient with a diagnosis of COLA1/2 cSVD | 4.71 | 100 |
| Sporting activities with a high risk of head trauma or excessive or prolonged exercise should be avoided in a patient with COL4A1/2 cSVD | 4.78 | 92.8 |
| A Caesarean section should be considered in women giving birth where the fetus contains a COL4A1/2 mutation | 4.78 | 100 |
| Mutations of COL4A1 at the binding site of MIR-29 gene can cause a distinct ischaemic cSVD with early onset in adults called PADMAL | 4.86 | 92.8 |
| CARASAL | ||
| CARASAL manifests clinically not only in the central nervous system but also in other organs/tissues | 4.46 | 84.6 |
| Dystonia is a rare phenotypic feature of CARASAL | 4.23 | 92.8 |
| Extensive subcortical and brainstem hyperintensity is commonly observed in CARASAL | 4.84 | 100 |
| Clinical manifestations can be absent or mild despite extensive leukoencephalopathy | 4.92 | 100 |
| CTSA is the only gene mutated known to cause CARASAL | 5 | 100 |
| There are insufficient data to recommend any specific treatment in patients with CARASAL | 4.92 | 100 |
| Both ischaemic and haemorrhagic strokes occur in patients with CARASAL | 4.61 | 100 |
| RVCL-S | ||
| Heterozygous C-terminal frameshift mutations in the TREX1 gene have been identified as causative in RVCL-S | 5 | 100 |
| Pathogenic frameshift mutations cause protein truncation in the C-terminus, which leads to subcellular mislocalization of the enzyme, but does not affect enzyme activity | 4.38 | 100 |
| Ophthalmological examination shows a distinctive and progressive retinal vasculopathy in almost all symptomatic patients | 4.69 | 100 |
| Brain involvement typically develops in the fourth to fifth decade | 4.30 | 92.8 |
| Neurological symptoms include focal deficits and progressive cognitive deficits | 4.84 | 100 |
| Focal brain disease can occur, in particular in association with mass-like lesions on brain MRI scanning | 4.77 | 100 |
| Brain MRI changes in RVCL-S typically involve punctate T2 hyperintensities that may also display nodular enhancement | 4.46 | 92.8 |
| Individuals with RVCL-S may develop larger T2 hyperintense white matter mass lesions with rim enhancement, mass effect and surrounding oedema | 4.84 | 100 |
| There is no evidence of efficacy of antiplatelet therapy in RVCL-S | 4.77 | 100 |
| There is no evidence of efficacy of immunosuppressive therapy in RVCL-S and clinical trials are needed | 4.84 | 100 |
| FD | ||
| WMHs can be seen in patients with FD and progression of WMHs occurs in significant proportion of the patients with FD | 5 | 100 |
| Ischaemic stroke is the most common brain stroke subtype in patients with FD, but haemorrhage strokes have been reported | 4.92 | 100 |
| Early diagnosis is important for correct management of patients with FD | 4.61 | 100 |
| FD can explain <1% of the cryptogenic ischaemic strokes in young adults | 4.92 | 100 |
| Neuropsychological testing in FD is recommended | 4.38 | 92.8 |
| Systemic thrombolysis is not contraindicated in FD | 4.69 | 100 |
| Thrombectomy can be applied in case of proximal occlusion of cerebral arteries in patients with FD | 4.54 | 92.8 |
| There is no evidence to recommend antithrombotic treatment for primary prevention of stroke in FD | 4.84 | 100 |
| Patients with FD should receive antithrombotic therapy after the first cerebrovascular event | 4.46 | 92.8 |
| ERT is usually recommended to reduce complications of FD although there is no good evidence that such a treatment can prevent stroke recurrence | 4.46 | 84.6 |
| There is a causal relationship between FD and ischaemic stroke, but the underlying mechanisms are unclear | 4.46 | 100 |
| MELAS | ||
| Diagnosis of an SLE requires the combination of clinical assessment, brain MRI and EEG | 4.85 | 100 |
| Fibrinolysis is not indicated to treat acute SLEs | 4.85 | 100 |
| Antiplatelet therapies are not indicated for secondary prevention of SLEs | 4.61 | 100 |
| If an SLE is suspected and focal seizures are evident, they should be managed with antiepileptic drugs | 4.92 | 100 |
| Seizures in SLEs should be treated with AEDs, with the exception of valproic acid, which is contraindicated, mainly in patients with POLG mutations | 4.92 | 100 |
| There is not enough evidence to recommend the use of intravenous venous L-arginine to treat acute SLEs | 4.46 | 84.6 |
| Acute psychiatric complications related to SLEs are common | 4.61 | 100 |
| Drugs like haloperidol, benzodiazepine, quetiapine, olanzapine may be safely used to treat psychiatric complications in MELAS | 4.46 | 92.8 |
| Because of the frequent occurrence of arrhythmia in MELAS (especially if caused by m.3243A>G), ECG monitoring during an SLE, especially after the introduction of an antipsychotic drug with QT-prolonging side effect, should be considered | 4.77 | 100 |
| Urgent genetic testing for MELAS should be considered in patients presenting with suspected SLE | 4.69 | 100 |
| As for the genetic testing, urgent search for the M.3243A>G mutation (in urine where possible) and, if M.3243A>G mutation is negative, POLG sequencing are required | 4.69 | 100 |
| Muscle biopsy should be considered after excluding M.3243A>G and POLG mutations | 4.38 | 92.8 |
| Even though there is no scientific evidence of positive impact of steroids in SLEs, their use is not contraindicated | 4.46 | 92.8 |
| Increased lactate in blood and/or CSF or detected by proton magnetic resonance spectroscopy could provide additional evidence of mitochondrial aetiology of an SLE, although neither specific nor highly sensitive | 4.30 | 84.6 |
- CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CARASAL, cathepsin-A-related arteriopathy with strokes and leukoencephalopathy; CARASIL, cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy; COL4, type IV collagen; CSF, cerebrospinal fluid; cSVD, cerebral small-vessel disease; ECG, electrocardiogram; EEG, electroencephalogram; EGFr, epidermal growth factor-like repeat; ERT, enzymatic replacement therapy; FD, Fabry disease; GOM, granular osmiophilic material; HTRA1, High Temperature Requirement A Serine Peptidase 1; MELAS, mitochondrial encephalopathy, lactic acidosis and stroke-like episodes; MR, magnetic resonance; MRI, magnetic resonance imaging; PADMAL, pontine autosomal dominant microangiopathy and leukoencephalopathy; QT, QT interval (the time from the start of the Q wave to the end of the T wave at the electrocardiogram); RVCL-S, retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations; SLE, stroke-like episode; WMH, white matter hyperintensity.
Clues for diagnosis of monogenic cerebral small-vessel disease
Experts were asked to identify ‘red-flag’ features that suggested to them that a diagnosis of monogenic disease should be considered in a patient with cSVD. Strong consensus was reached on the following: family history, young age at onset, consanguinity, suggestive extracerebral/systemic features and clinical and neuroimaging characteristics of a specific monogenic disease. There was strong consensus that, even in patients with conventional vascular risk factors, a diagnosis of monogenic cSVD should not be excluded. Negative workup for other causes of cSVD reached good consensus.
A number of specific causes of cSVD were individually considered. These, with the associated key genetic, clinical and neuroimaging features are shown in Table 2.
| Disease | CADASIL | CARASIL | AD-HTRA1 | COL4A1/2 | PADMAL | CARASAL | RVCL-S | FD | MELAS |
|---|---|---|---|---|---|---|---|---|---|
| Gene | NOTCH3 | HTRA1 | HTRA1 | COL4A1/2 | COL4A1 | CTSA | TREX1 | α-galactosidase A | Variety of mitochondrial DNA genes or POLG |
| OMIM | 125310 | 600142 | 616779 | 614519 | 618564 | 256540 | 192315 | 301500 | 540000 |
| Inheritance | AD | AR | AD | AD | AD | AD | AD | X-linked but females can also be affected | Maternally inherited or AR (POLG) |
| Clinical features | |||||||||
| Ischaemic strokes | X | X | X | X | X | X | X | ||
| Intracerebral haemorrhage | X (rarely) | X | X | X | |||||
| SLEs | X | ||||||||
| Cognitive impairment/dementia | X | X | X | X | X | X | X | X | |
| Migraine | X | X | X | X | |||||
| Encephalopathy | X | X | X | ||||||
| Seizures | X (rarely) | X | X | ||||||
| Mood disorders | X | X | X | X | X | X | |||
| Alopecia | X | X | |||||||
| Lumbar spine problems | X | X | |||||||
| Extracerebral features | X | X | X | X | X | X | |||
| Neuroimaging | |||||||||
| Lacunar infarcts | X | X | X | X | X | X | |||
| WMHs | X | X | X | X | X | X | X | ||
| Cerebral microbleeds (no specific location) | X | X | X | ||||||
| Anterior temporal pole WMH | X | X | X | ||||||
| Large contrast-enhanced white matter lesions | X | ||||||||
| Cortical and subcortical signal abnormalities often crossing vascular territories | X | ||||||||
- AD, autosomal dominant; AR, autosomal recessive; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CARASAL, cathepsin-A-related arteriopathy with strokes and leukoencephalopathy; CARASIL, cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy; COL4, type IV collagen; FD, Fabry disease; HTRA1, High Temperature Requirement A Serine Peptidase 1; AD-HTRA1, High Temperature Requirement A Serine Peptidase 1; MELAS, mitochondrial encephalopathy, lactic acidosis and stroke-like episodes; OMIM, Online Mendelian Inheritance in Man; PADMAL, pontine autosomal dominant microangiopathy and leukoencephalopathy; RVCL-S, retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations; SLE, stroke-like episode; WMH, white matter hyperintensity.
Management recommended for all monogenic cerebral small-vessel diseases
General principles for all monogenic cSVDs, discussed and elaborated during the face-to-face meeting, are listed in Table 1.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
CADASIL, caused by NOTCH3 gene variants 2, is the most prevalent monogenic cSVD, with a minimal estimated prevalence of 2–4:100 000 3, although the true prevalence is likely to be much higher 4. NOTCH3 is a single-pass receptor protein that plays an important role in vascular smooth muscle cell differentiation and maturation, and mutant NOTCH3 protein deposits are found in small arteries and capillaries throughout the body. However, despite this widespread vascular pathology, clinical manifestations are only related to the central nervous system, in the form of migraine with aura, acute encephalopathy, recurrent lacunar strokes, cognitive decline, gait and mood disorders 5, 6. Most strokes are ischaemic, but intracerebral haemorrhage can rarely occur, especially in patients treated with anticoagulants 6, 7.
Stroke onset was classically described to be between the ages of 40 and 60 years, with a severe progression to a mute and bedridden state after 10–20 years. However, individuals with milder disease are increasingly reported 4. NOTCH3 variants leading to a cysteine alteration in one of the NOTCH3 epidermal growth factor-like repeat (EGFr) domains 1–6 have been recently reported to predispose to an earlier onset of stroke than variants located in one of the EGFr domains 7–34 4. Classical vascular risk factors, such as smoking and hypertension, have been associated with earlier age of stroke onset 8.
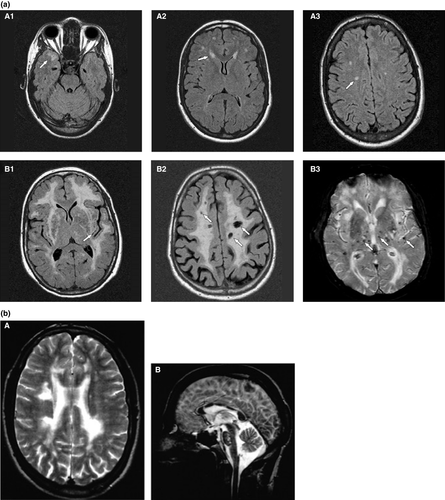
The magnetic resonance imaging (MRI) features are central to diagnosis and features include symmetrical and progressive subcortical white matter hyperintensities (WMHs), lacunes, microbleeds and enlarged perivascular spaces (Fig. 2a) 9. WMHs are widespread. Anterior temporal lobe WMHs are highly suggestive and often considered a radiological hallmark for CADASIL, but can be lacking in some cases and are also seen in CARASIL and High Temperature Requirement A Serine Peptidase 1 (HTRA1)-autosomal dominant disease.
Pathological hallmarks of the disease are small-vessel wall abnormalities, which typically include an increased and granular pattern of NOTCH3 staining using an antibody with an epitope in the extracellular domain and pathognomonic granular osmiophilic material (GOM) adjacent to mural cells using electron microscopy 10. There is usually a family history, but this may not be apparent due to mild disease course or misdiagnosis in other family members or occasionally due to a de-novo variant.
CADASIL is confirmed by genetic testing, revealing a NOTCH3 variant altering the number of cysteines in one of the 34 EGFr domains of the NOTCH3 protein encoded by exons 2–24, thereby disrupting normal disulphide bridge formation and resulting in a pro-aggregatory mutant NOTCH3 protein that sequesters extracellular matrix proteins.
There are few data on management of CADASIL and a paucity of randomized controlled trials. A multicentre trial of the cholinesterase inhibitor donepezil showed no improvement in the primary endpoint of the V-ADAS-cog score in CADASIL with cognitive impairment 11.
Consensus statement results
Genetic testing
- CADASIL diagnosis can be established by skin biopsy with electron microscopy showing GOM, but genetic testing should be the first diagnostic line of investigation.
- In the case of a NOTCH3 variant of unknown significance, diagnosis can be investigated using a skin biopsy for electron microscopy and/or immunostaining to the NOTCH3 extracellular domain.
All or almost all variants leading to CADASIL result in a loss or gain of a cysteine in EGFr repeats. Some non-cysteine-changing variants have been reported but the consensus was that the vast majority of these variants are not pathogenic. In such cases, electron microscopy revealing GOM can be a useful diagnostic tool.
Clinical and neuroimaging diagnosis
- The diagnosis of CADASIL should be considered in any patient with unexplained symmetrical periventricular WMHs and a positive family history of migraine with aura, stroke, mood disorders or dementia.
- WMHs located in the anterior temporal lobes are often present, but can be absent.
- CADASIL cannot be ruled out in the absence of a medical or family history of migraine with aura.
- CADASIL can present with mild signs or symptoms, even in elderly individuals (>70 years) with a negative medical history of stroke and negative family history.
- Although most patients have a family history, if the clinical and imaging phenotype is consistent with CADASIL the diagnosis should be considered.
- CADASIL cannot be ruled out in the presence of common cerebrovascular risk factors and extensive WMHs.
- CADASIL should be considered in the differential diagnosis of multiple sclerosis.
CADASIL with established WMH has been diagnosed as multiple sclerosis. Corpus callosal involvement, typical of multiple sclerosis but rare in sporadic small-vessel disease, can occur in CADASIL9 (Fig. 2b).
Management
- Patients with CADASIL should be monitored for cognitive impairment and, when present, neuropsychological assessment and support should be offered.
- There is no evidence to support the use of antiplatelet agents in patients with CADASIL who have not suffered clinical ischaemic stroke.
- Patients with CADASIL should not receive thrombolysis for acute small-vessel ischaemic stroke (which is almost always the case).
- Statin therapy is not recommended for a patient with CADASIL who has normal cholesterol, even after stroke, but is not contraindicated.
- There is no evidence contraindicating the use of triptans in patients with CADASIL with migraine.
- There is no evidence to recommend acetazolamide; trials of its use are required.
- Patients with CADASIL can be treated with psychiatric drugs according to guidelines for other cerebrovascular disorders.
- Anticoagulants are not recommended for stroke prophylaxis in CADASIL due to the risk of intracerebral haemorrhage, but they are not contraindicated if there is another strong indication (e.g. atrial fibrillation, pulmonary embolus).
- There is no evidence to contraindicate the use of oral contraceptives.
- Anaesthetic procedures maintaining haemodynamic stability, and avoiding hypotension, are recommended for patients with CADASIL undergoing surgery.
- Pregnancy and labour are associated with an increased risk of transient neurological events, mostly consistent with migraine aura and especially during the puerperium, of which the patient and obstetrician should be aware.
Pregnancy is not contraindicated in CADASIL, and no prophylaxis with heparin or aspirin is required. However, migraine can worsen especially around the puerperium when confusional migraine and encephalopathy can occur.
Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy
CARASIL is a rare autosomal recessive disorder caused by biallelic variants in the HTRA1 gene on chromosome 10q26 13, 14, mainly reported in Japan but also in Chinese and Caucasian cases 15, 16. HTRA1 encodes a homotrimeric serine protease that probably has a role in maintaining cerebral small-vessel integrity by regulating transforming growth factor beta signalling.
Recurrent lacunar stroke with onset from 20 to 44 years, early vascular dementia (usually by age 30–40 years), gait disturbance, depression, premature head alopecia in 90% of patients during adolescence and severe back pain with lumbar disc herniation are the main clinical features. Additional manifestations include seizures, psychiatric disturbances, pseudobulbar palsy and spondylosis deformans. The disease is rapidly progressive with stepwise deterioration of motor and cognitive function, and the average illness duration is 20–30 years, although most patients became bedridden within 10 years after onset 16. T2-weighted cerebral MRI by age 20 years usually shows diffuse and symmetrical WMHs and lacunar infarcts (Fig. 3) 17. WMHs sometimes involve the temporal lobes as in CADASIL.
In contrast to CADASIL, histological changes are not disease specific and are characterized by arteriolosclerosis and loss of vascular smooth muscle cells with subsequent hyalinization, intima fibrous thickening and internal elastic lamina disruption. GOM deposits have not been found.
No effective treatment of CARASIL is available.
Consensus statement results
Genetic testing
- Carriers of heterozygous HTRA1 variants do not manifest with CARASIL but with a different form of heritable cSVD, i.e. HTRA1 autosomal dominant disease (described below).
Clinical and neuroimaging diagnosis
- CARASIL should be suspected in cases of recurrent unexplained lacunar strokes with severe WMHs, particularly when associated with premature alopecia, premature lumbago or spondylosis.
Management
- There are not enough data to recommend antiplatelet therapy in CARASIL, even in the presence of cerebrovascular events.
HTRA1-autosomal dominant disease
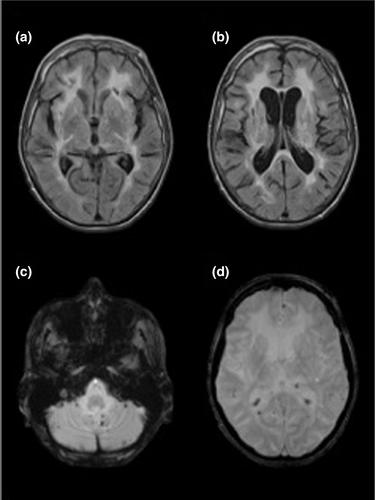
In addition to biallelic HTRA1 variants that cause CARASIL, heterozygous HTRA1 variants have been shown to cause an autosomal dominant cSVD18. These dominant negative variants lead to decreased residual activity of the HTRA1 trimer through different mechanisms 19. Clinical features differ from CARASIL by a later age of stroke onset (around 60 years) and less frequent alopecia and spondylosis deformans (Fig. 4). Cognitive impairment and encephalopathy also occur. Histological findings demonstrate diffuse and focally intensive myelin pallor in the white matter sparing U-fibres, resembling CARASIL 19.
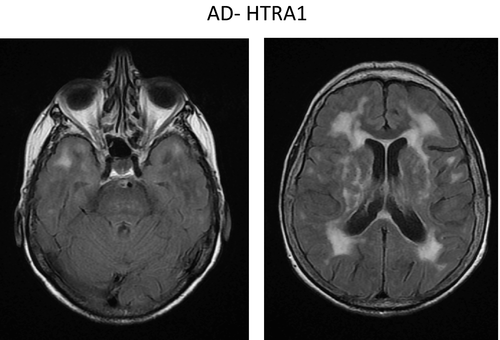
Not all rare missense HTRA1 variants are pathogenic. In order to establish pathogenicity of a HTRA1 variant, the activity of the mutated enzyme and its ability to form trimers should be evaluated, as well as whether it segregates with disease within a family.
Collagen 4A1 and collagen 4A2 microangiopathy
Type IV collagen is a component of basement membranes and expressed ubiquitously in blood vessels and organs throughout the body. Missense or null variants in COL4A1 and COL4A2 cause an autosomal dominant cSVD of the haemorrhagic type that can be associated with extracerebral clinical manifestations 20. These two genes encode the α1 and α2 chains of COL4 and share a common locus on chromosome 13 (13q34). Most pathogenic variants of COL4A1/2 lead to either substitution of a glycine with a different amino acid or haploinsufficiency.
The clinical spectrum associated with pathogenic variants of monogenic COL4A1/2 is highly variable 21, 22. Mean age at stroke onset is 30–40 years but intracerebral haemorrhage can occur in fetuses, children and adults, and has been associated with physical activity, trauma and anticoagulant therapy. Therefore, it is recommended that a carrier should avoid hard physical activity and anticoagulants. Due to risk of haemorrhage, a fetus carrying a pathogenic COL4A1/2 variant should be delivered by Caesarean section. Haemorrhagic strokes are reported twice as often as ischaemic strokes. COL4A1/2 present in the paediatric and neonatal period with porencephaly or schizencephaly. Other manifestations include: intracerebral aneurysms, proteinuria, renal insufficiency, renal and hepatic cysts, tortuosities of retinal arteries, focal retinal haemorrhages, and early cataract or abnormal development of the anterior segment of the eye 23, 24.
The clinical phenotype can vary widely between different affected family members.
Brain imaging may show intracerebral haemorrhages or cerebral microbleeds, WMHs and lacunes that are usually subcortical (Figs 5 and 6) 25.
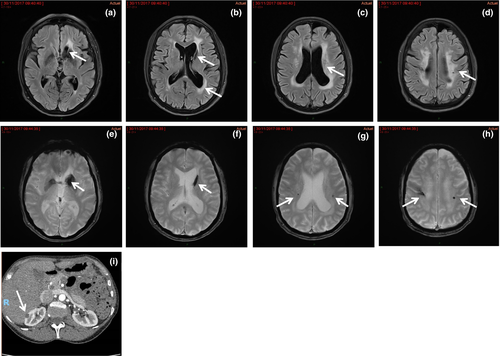
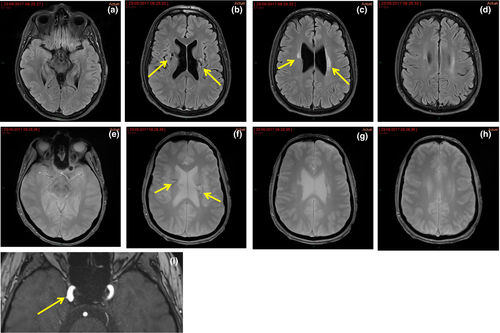
Missense variants of COL4A1 that disrupt the NC1 domain seem to be associated with antenatal cerebral haemorrhages and porencephaly, but not with kidney disease. Missense variants of COL4A1 that encode the CB3 (IV) segment of the triple helical domain (exons 24 and 25) have been associated with hereditary angiopathy, nephropathy, aneurysms and cramps (HANAC) syndrome 24.
In contrast with COL4A1/A2 microangiopathies associated with either glycine or stop codon variants, PADMAL is an autosomal dominant ischaemic cSVD caused by variants in the 3’ untranslated region of COL4A1. These variants affect a binding site of a microRNA, miR29, and lead to upregulated expression of COL4A1. PADMAL clinical features are characterized by adult early-onset pons infarcts and early death 26. No clinical manifestations have been reported so far in children.
Consensus statement results
Genetic testing
- Missense variants of COL4A1 or COL4A2 leading to replacement of a glycine of a gly-x-y motif in the triple helix and variant leading to premature stop codon are pathogenic.
- Variants of COL4A1 at the binding site of mir-29 microRNA within COL4A1 3’UTR can cause a distinct ischaemic cSVD with early onset in adults called PADMAL.
Clinical and neuroimaging diagnosis
- A variant of COL4A1 or COL4A2 should be considered in a patient with deep intracerebral haemorrhage of undetermined origin OR WMHs of undetermined origin OR a cerebral haemorrhage, porencephaly, retinal vessel tortuosities, haematuria, glomerular dysfunction, renal insufficiency, renal cysts, infantile hemiparesis, early cataracts, abnormalities of the anterior segment of eyes, multiple intracranial aneurysms, muscle cramps or hepatic cyst is present in at least one first- or second-degree relative OR early-onset pontine infarcts.
- A multi-system non-invasive workup (including cerebral MRI, intracranial and cervical magnetic resonance angiography, cardiac echography, detailed ocular and retinal vessel examination with visual acuity and ocular pressure measures, liver and renal echography) is recommended in individuals with a COL4A1/2 variant.
- Pre-symptomatic testing of family members is recommended, except in PADMAL, because of potential benefit of preventive measures, including avoiding complications during childbirth.
Management
- Antiplatelet and anticoagulant treatments are not recommended in COL4A1/2 cSVD.
- Intravenous thrombolysis is not recommended in COL4A1/2 cSVD.
- Sporting activities with a high risk of head trauma or excessive or prolonged exercise should be avoided.
- A Caesarean section should be considered in women giving birth where the foetus harbours a COL4A1/2 mutation.
Cathepsin-A-related arteriopathy with strokes and leukoencephalopathy
CARASAL is an adult, ultra-rare, hereditary cSVD reported in only 19 patients 27-29, caused by a dominant variant in the CTSA gene, located on chromosome 20q13.12 27, with the variant c.973C> T detected in 14/19 patients 27, 28. CTSA encodes the serine carboxypeptidase cathepsin-A, predominantly located in lysosomes where it stabilizes a multi-enzyme complex built up of beta-galactosidase and neuraminidase, and degrades endothelin-1 27.
CARASAL manifests clinically as ischaemic or haemorrhagic stroke, cognitive impairment, dementia, headache, migraine, vertigo, movement disorders including, rarely, dystonia, gait disturbance or depression. Some patients present with extra-central nervous system manifestations, such as dysphagia, dry mouth, dry eyes, facial pain, muscle cramps, arterial hypertension or diabetes 27-29. Cerebral imaging shows leukoencephalopathy involving the brainstem (pyramidal tracts, tegmental tracts, middle and superior cerebellar peduncles) and the subcortical white matter sparing the U-fibres 27. Grey matter, including the thalamus, basal ganglia and dentate nuclei can be also affected 28. Clinical manifestations can be absent or mild also in cases with extensive leukoencephalopathy.
There is no causal treatment available and no evidence that antithrombotic medication, anticoagulation or thrombolysis is indicated.
Consensus statement results
Management
- ▪ There are insufficient data to recommend any specific treatment in CARASAL.
Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations
RVCL-S is a genetic multi-organ small-vessel disease. The brain and retina are almost always affected, but microangiopathic disease can develop in other organs, most notably the kidneys and liver. The terms cerebroretinal vasculopathy, hereditary vascular retinopathy and hereditary endotheliopathy, retinopathy, nephropathy and stroke (HERNS) were initially used to describe affected families. However, the identification of a common genetic basis in 2007 suggested a single microvascular disorder, subsequently termed RVCL-S 30. RVCL-S is an autosomal dominant disease caused by heterozygous C-terminal frameshift variants in the TREX1 gene 30. Although the biological functions of TREX1 are increasingly well described, the exact molecular mechanism whereby RVCL-S variants cause disease is unknown. TREX1 is a negative regulator of innate immune pathways such as the type I interferon response, but prominent activation of this pathway is not typically observed in RVCL-S 31.
RVCL-S is a very rare disease and our understanding of clinical phenotype is largely based on details from an international study of 11 unrelated families with 78 variant carriers 32. The clinical picture is usually dominated by microangiopathy of the retina and brain. Disease onset is typically in middle age 32. Ophthalmological examination shows progressive retinal vasculopathy in almost all symptomatic patients. Clinical manifestations of the cerebral microangiopathy often develop in the fourth to fifth decade. Although the majority of patients develop cognitive impairment, brain disease can manifest as a large enhancing lesion, which can mimic tumours with pronounced mass effect or tumefactive inflammation 32. Focal deficits can be associated with the development of contrast-enhancing lesions on MRI. These can become large and confluent, sometimes with perilesional oedema (Fig. 7). Calcifications in the white matter detected on computed tomography are common and were identified in 52% of mutation carriers 32. Lacunar stroke is not a common manifestation and systematic screenings for TREX1 in 1000 young-onset lacunar stroke patients detected no TREX1 pathogenic variants 33.
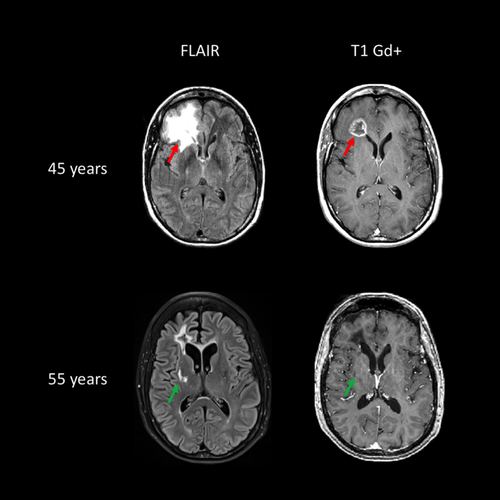
Microvascular disease affecting other organs is commonly seen and can be severe, mainly in kidney and liver, and can cause organ failure. Raynaud’s disease, gastrointestinal bleeding, bone disease and thyroid disease are also seen.
Consensus statement results
Genetic testing
- Pathogenic frameshift variants in TREX1 cause protein truncation in the C-terminus, which leads to subcellular mislocalization of the protein, but does not affect enzyme activity.
Management
- There is no established treatment of RVCL-S.
- There is no evidence of efficacy of antiplatelet therapy in RVCL-S.
- There is no evidence of efficacy of immunosuppressive therapy in RVCL-S and clinical trials are needed.
Fabry disease
Fabry disease is an inherited metabolic disease that results from a deficiency or absence of the enzyme α-galactosidase A due to a variant in the GLA gene. FD is inherited as an X-linked trait. Male patients can develop a classic severe phenotype of the disease (type 1), whereas heterozygous female patients present with phenotypes ranging from asymptomatic to severe involvement of vital organs. The classic forms exhibit early manifestations, whereas later-onset forms (type 2) can present clinical signs later in life. About 900 variants, most of them private, have been reported 34. FD is a lysosomal storage disease, due to accumulation of the sphingolipid globotriaosylceramide (GL-3 or Gb3) and its deacetylated derivative lyso-globotriaosylceramide (lyso-GL-3 or lyso-Gb3).
Many different cell types are involved in FD, including vascular cells (endothelial cells and vascular smooth muscle cells), cardiomyocytes, renal cells, enteric neurons and nerve cells 34. Therefore, heart, kidney, brain, peripheral nervous system and the gastrointestinal system are heavily involved in the disease 34, 35.
There is a causal relationship between FD and ischaemic stroke, but the underlying mechanisms are still unclear. Ischaemic stroke is the most common subtype, but intracerebral haemorrhages and cerebral venous thrombosis have been reported 36. The posterior circulation seems to be predominantly affected. The most common age of stroke onset is 20–50 years 36. WMHs seen on T2-weighted MRI are a common finding in patients with FD, present in up to 46% (Fig. 8) 37, and progression of WMHs occurs in a significant proportion of them. Other neuroradiological features are basilar artery dolichoectasia and the pulvinar sign 36.
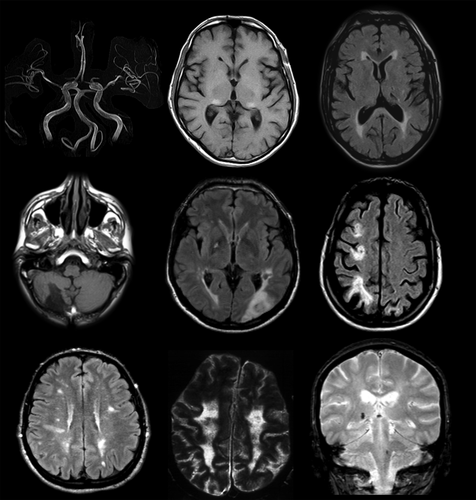
Adapted from
Burlina and Politei 2018. Reproduced with permission from Springer Nature. Authorization for this adaptation has been obtained from the publisher.Fabry disease explains less than 1% of cryptogenic ischaemic strokes in young adults. An early study 38 suggested that FD might account for 4.9% of ischaemic stroke in young male patients but this has not been confirmed in replication studies 39, 40.
Cognitive function has been less studied than other neurological complications in FD.
Besides symptomatic drugs, specific therapies are available. The latter include enzymatic replacement therapy (ERT) (agalsidase beta or alfa) and chaperone therapy for amenable variants. Recent recommendations regarding diagnosis, monitoring and treatment have been published 35.
Consensus statement results
Management
- Early diagnosis is important for the correct management of patients with FD.
- Systemic thrombolysis is not contraindicated in FD.
- Thrombectomy can be applied in case of proximal occlusion of cerebral arteries in patients with FD.
- There is no evidence to recommend antithrombotic treatment for primary prevention for stroke in FD.
- Primary stroke prevention in FD includes lifestyle modifications.
- Patients with FD should receive antithrombotic therapy after the first cerebrovascular event. Regarding stroke prevention, there are no data yet on whether antithrombotic drugs are effective in FD. Recent experts’ recommendations include lifestyle modifications to control common risk factors and antithrombotic drugs for secondary prevention 35.
- ERT is usually recommended to reduce complications of FD although there is no good evidence that ERT can prevent stroke recurrence.
Stroke recurrence in patients with FD, with and without ERT, has been reported 34. There was strong consensus that there was no clear evidence that ERT reduced stroke risk in FD. It was agreed that more data are required. Two recent systematic reviews reported a lower incidence of cerebrovascular events with agalsidase beta 41 and ERT 42, the latter suggesting an approximate halving of stroke risk. These are based on observational data and it was agreed that more data are required, ideally from randomized trials.
Mitochondrial encephalopathy, lactic acidosis and stroke-like episodes
MELAS is one of the most severe syndromes in mitochondrial disease. Although the majority of patients (80%) have the common MELAS pathogenic variant m.3243A>G in the mitochondrial DNA tRNA-leucine (MT-TL1) 43, the number of genotypes causing the MELAS phenotype continues to expand and may include nuclear genes, mainly POLG 44.
One of the clinical hallmarks of MELAS, the stroke-like episode (SLE), is characterized by headache, nausea and vomiting, encephalopathy, focal-onset seizures and focal neurological deficits. On conventional MRI, the affected areas do not correspond to vascular territories and involve the cortex and juxtacortical white matter (Fig. 9). Lesions may spread over days, weeks and even months or go to the contralateral side after the acute episode. Some lesions reverse completely with time; however, most severe lesions develop into cortical laminar necrosis, gliosis and atrophy.
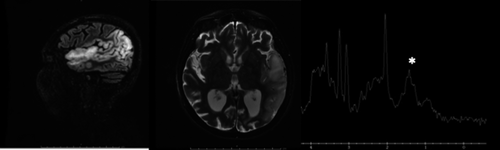
Although SLEs were originally described in individuals<40 years of age, late-onset presentation is recognized. Although SLEs cannot be strictly classified as cSVD, they have to be considered in the differential diagnosis of cSVD and therefore MELAS was included in the consensus statement.
Additional common clinical and laboratory findings in MELAS are eyelid ptosis, cardio(myo)pathy, muscle weakness, diabetes and lactic acidosis.
Compared with strokes of vascular origin, there is higher incidence of bilateral clinical symptoms such as cortical blindness or auditory agnosia.
The underlying pathophysiological mechanism of SLEs remains unclear 45. Limitations in the understanding of the disease mechanism are reflected in the controversies about the acute management of SLEs.
Consensus statement results
Genetic
- Urgent genetic testing for MELAS should be considered in patients presenting with suspected SLE. Search for the m.3243A>G variant (in urine where possible) and, if m.3243A>G variant is negative, POLG sequencing is required. Muscle biopsy should be considered after excluding m.3243A>G and POLG variants.
Clinical and neuroimaging diagnosis
- A mitochondrial SLE is a subacute, evolving brain syndrome driven by seizure activity in genetically determined mitochondrial disease, which can be present at any age with neurological symptoms typically associated with cortical/subcortical MRI changes and electroencephalogram abnormalities 45.
- The diagnosis of an SLE requires the combination of clinical assessment, cerebral MRI and electroencephalogram.
Management
- Fibrinolysis is not indicated to treat SLE.
- Antiplatelet therapies are not indicated for secondary prevention of SLEs.
- If an SLE is suspected and focal seizures are evident, they should be treated urgently with intravenous antiepileptic drugs, including levetiracetam, benzodiazepines or lacosamide.
- Valproic acid is contraindicated, mainly in patients with POLG variants.
- There is not enough evidence to support the use of intravenous L-arginine to treat SLEs.
- Even though there is no scientific evidence of positive impact of steroids in SLEs, their use is not contraindicated.
- Drugs like haloperidol, benzodiazepine, quetiapine, olanzapine may be safely used to treat psychiatric complications in MELAS.
- Because of the frequent occurrence of arrhythmia in MELAS (especially if caused by m.3243A>G), electrocardiogram monitoring during an SLE, especially after the introduction of an antipsychotic drug with QT (QT interval; the time from the start of the Q wave to the end of the T wave at the electrocardiogram)-prolonging side effect, should be considered.
Acknowledgements
The authors thank Lyndsey Butterworth from the mitochondrial disease patient advocacy group Lily Foundation for participation in the meeting. M.M. is grateful to Telethon and MITOCON association for support (GUP09004 and GSP16001). H.S.M. is supported by an NIHR Senior Investigator award and his work is supported by Cambridge University Hospitals NIHR Biomedical Research Centre.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.