A native apex predator limits an invasive mesopredator and protects native prey: Tasmanian devils protecting bandicoots from cats
Abstract
Apex predators can limit the abundance and behaviour of mesopredators, thereby reducing predation on smaller species. We know less about whether native apex predators are effective in suppressing invasive mesopredators, a major global driver of vertebrate extinctions. We use the severe disease-induced decline of an apex predator, the Tasmanian devil, as a natural experiment to test whether devils limit abundance of invasive feral cats and in turn protect smaller native prey. Cat abundance was c. 58% higher where devils had declined, which in turn negatively affected a smaller native prey species. Devils had a stronger limiting effect on cats than on a native mesopredator, suggesting apex predators may have stronger suppressive effects on evolutionarily naive species than coevolved species. Our results highlight how disease in one species can affect the broader ecosystem. We show that apex predators not only regulate native species but can also confer resistance to the impacts of invasive populations. Apex predators could therefore be a powerful but underutilised tool to prevent biodiversity loss.
Introduction
Apex predators play crucial roles in structuring ecosystems, but much of the Earth is now devoid of large predators (Estes et al. 2011; Ripple et al. 2014). Declines of these species can trigger trophic cascades, whereby herbivorous prey relax their anti-predator behaviours, increase in abundance, and overconsume vegetation (Estes et al. 1998; Ripple et al. 2001; Terborgh et al. 2001; Ripple & Beschta 2007). Apex predator declines can cause mesopredator release, defined as an increase in the density or change in behaviour of mid-ranked predators (Prugh et al. 2009), which can in turn lead to increased predation on smaller animals (Crooks & Soulé 1999; Johnson et al. 2007; Ritchie & Johnson 2009). The top-down effects of predators can be mediated by bottom-up drivers; for instance, declining lynx (Lynx lynx) densities released red foxes (Vulpes vulpes), but this effect was most pronounced in productive environments, highlighting the need to simultaneously consider bottom-up and top-down processes (Elmhagen & Rushton 2007). Predators clearly play integral roles in structuring food webs (Estes et al. 2011; Ripple et al. 2014), but we know considerably less about how apex predators affect invasive mesopredators, and how this in turn affects smaller prey species.
Invasive predator populations – those that have spread from introduced populations and maintain themselves without human assistance – are a major cause of global biodiversity loss (Simberloff et al. 2013). Invasive populations have contributed to 58% of bird, mammal and reptile extinctions (Doherty et al. 2016), and exert a heavy toll on many surviving species (Loss et al. 2013). This is particularly true in Australia, where feral cats (Felis catus) now occupy the entire continent (Legge et al. 2017), and together with invasive red foxes (Vulpes vulpes) are a major driver of most of Australia's c. 34 mammalian extinctions since 1788 (Woinarski et al. 2015; Woinarski et al. 2019). Apex predators could reduce the harm caused by invasive mesopredators if they limit their abundance through direct lethal effects or indirect behavioural effects (Ritchie & Johnson 2009). Despite a solid theoretical grounding, however, there is still debate over whether apex predators can be a useful tool to protect native biodiversity. For example, it has been repeatedly questioned whether dingoes (Canis lupus) or Tasmanian devils (Sarcophilus harrisii) limit the abundance of invasive mesopredators in Australia (Allen et al. 2013; Allen et al. 2015; Fancourt et al. 2015; Fancourt & Mooney 2016).
The Tasmanian devil (6–14 kg; hereafter ‘devil’) is the apex predator on the large island of Tasmania (c. 65 000 km2) following the extinction of the thylacine (Thylacinus cynocephalus) in the mid-20th century. Recently the devil has suffered severe population declines due to the emergence of a novel, transmissible cancer, devil facial tumour disease (DFTD). DFTD first arose in north-east Tasmania in 1996 (Hawkins et al. 2006) and now occupies 80% of the devil's range (Fig. 1a) (Lazenby et al. 2018), causing population declines of 80% on average (Lazenby et al. 2018) and up to 95% (Hollings et al. 2014). Unlike on the mainland of Australia, cats have not caused any confirmed extinctions in Tasmania. One hypothesis explaining this is that devils have so far limited the harm caused by cats. The progressive spread of DFTD across Tasmania has established a gradient of time since the arrival of DFTD (Fig. 1a), causing devil densities to range from very low in north-east Tasmania, where DFTD has been present for the longest, to high in areas of north-west Tasmania not yet affected by DFTD. Unlike almost all declines of apex predators (Ripple et al. 2014), devil population declines are not caused by humans, providing the rare opportunity to study the effects of a predator with little anthropogenic confounding.
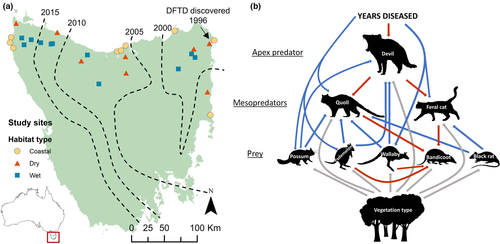
Disease-induced changes in population density, as well as other environmental perturbations, can provide useful natural experiments that advance our understanding of the processes that shape ecosystems (Lindström et al. 1994; Holdo et al. 2009). In this study, we treat the disease-induced decline of the Tasmanian devil as a large-scale natural experiment on the role of this top predator in structuring the mammal community, especially by limiting the abundance of feral cats and their impact on prey. We analysed the cascading effects of devil declines as a network of species using structural equation modelling. We predicted changes in the mammal community based on trophic cascade theory, mesopredator release hypothesis, and bottom-up drivers, which are reflected in our a priori structural equation model (described in Box 1 and visualised in Fig. 1b). Further, the presence of similar-sized native (spotted-tail quoll, Dasyurus maculatus; hereafter ‘quoll’) and invasive (feral cat; hereafter ‘cat’) mesopredators in this community enabled us to test whether an apex predator has a stronger limiting effect on an invasive mesopredator than on a coevolved mesopredator, similar to the stronger effects of predators on species of prey that have not coevolved with them (Salo et al. 2007).
Box 1. The mammal community and a priori predictions for the cascading effects of devil population declines.
We predicted shifts in the mammal community based on the mesopredator release hypothesis and trophic cascade theory (defined in the Introduction), while also assessing the bottom-up influence of different vegetation types, which reflect environmental productivity (resulting from the effect of elevation on rainfall and temperature). We predicted that DFTD would cause substantial declines in devil activity (Lazenby et al. 2018), resulting in mesopredator release of one or both of the native spotted-tailed quoll (1.8–6 kg) and the invasive feral cat (2–5 kg). Because invasive predators have caused a disproportionately high rate of small mammal extinctions in Australia (Burbidge & McKenzie 1989), we hypothesised that release of cats would in turn cause the decline of smaller native mammals in their preferred prey size-range (i.e. rabbit-sized or smaller; Doherty et al. 2015). The southern brown bandicoot (Isoodon obesulus, c. 1 kg) is a good example of these species and has suffered population declines on the Australian mainland (Burbidge 2016), where it is classified as endangered (Brown & Main 2010). We did not include an a priori relationship between cats and quolls because the directionality of this relationship is unclear; however, the test of SEM fit assesses whether a relationship should be present among unconnected variables (see Methods). We hypothesised that increasing time since local DFTD outbreak, and therefore increasing time since the onset of devil population declines, would result in more pronounced release of both mesopredators and the primary prey of devils.
The vegetation types (dry eucalypt, wet eucalypt/rainforest or coastal) differ in structure and availability of resources, which we predicted would affect the abundance of prey species, and that higher prey abundance would lead to higher predator abundance. Devils and quolls have high dietary overlap, both feeding mainly on Bennett's wallaby (Macropus rufogriseus, hereafter ‘wallaby’) and Tasmanian pademelon (Thylogale billardierii, hereafter ‘pademelon’)(Andersen et al. 2017). Conversely, cats prey mostly on smaller animals of rabbit size or less (Doherty et al. 2015). We therefore predicted that devils and quolls would respond positively to wallaby and pademelon abundance, and that cats would respond positively to the abundance of smaller mammals. Concerningly, of the small mammals detected in this study, only 5% were native species, while the invasive black rat (Rattus rattus) comprised 81% of detections. Because of limited sample size, we restricted our a priori SEM to include only black rats, and hypothesised that cats would respond positively to black rats.
Materials and methods
Study area and camera trapping
DFTD first emerged in north-east Tasmania in c. 1996 and has since spread to c. 80% of the devil's range (Lazenby et al. 2018), establishing a gradient of disease-induced population decline. We selected 28 independent study sites spanning this gradient, from the long-diseased north-east of Tasmania where DFTD was present for c. 20 years to the disease-free north-west of Tasmania (Fig. 1a). Each study site comprised a 2-km transect of 14 remote cameras (explained below) and was on average 15 km from the nearest study site. We selected study sites to sample three of Tasmania's major vegetation communities: wet eucalypt/rainforest, dry eucalypt forest and coastal vegetation (TASVEG 3.0 GIS layer). We ensured comparability of sites of the same vegetation type by ensuring similar average rainfall (dry: 750–1500 mm, wet: 1100–2000 mm; coastal: 650–1200; Bureau of Meteorology GIS layer) and elevation (dry < 500 m, wet < 800 m, coastal < 100 m). Each vegetation type was approximately equally represented across the gradient of population decline to ensure that vegetation type was not confounded with time since DFTD arrival (Fig. 1a). All sites were in reserves, which are the areas of Tasmania where human influence is least (i.e. no hunting or recent logging).
We deployed 14 remote cameras (Reconyx PC-800 infrared) at each study site for at least 39 days, giving a total of 392 remote cameras and at least 15 288 camera nights (between March and August 2017). Cameras were spaced 100–200 m apart and deployed > 30 m into the forest alongside a 2-km section of a low-use, unsealed road. We focussed on surveying many sites using a moderate number of cameras at a relatively fine spatial scale rather than surveying fewer sites in detail. This enabled us to survey many sites with disease-induced differences in devil abundance and have replication across the gradient of the natural experiment. Cameras were fastened to a tree c. 75 cm above the ground and were positioned facing animal trails or small clearings. To increase detections, we suspended a general-purpose olfactory and visual lure from an overhanging branch 2–3 m in front of the camera. The lure consisted of a perforated PVC cannister containing dried beef liver, tuna oil, peanut butter, rolled oats and sardines, with a CD suspended below.
Statistical analyses
Our analysis took a two-stage approach. We first derived a measure of each species’ abundance that accounts for imperfect detection, and then fed this information into a piecewise structural equation model to investigate the community-wide cascading effects of declining devil abundance.
Abundance of cats
We estimated the abundance of cats at each independent study site using a mark-resight model (McClintock et al. 2009). Mark-resight models estimate abundance when some but not all individuals are uniquely identifiable (McClintock et al. 2009) and have been used elsewhere in Australia to estimate the abundance of feral cats (McGregor et al. 2015). To estimate the contribution of unmarked individuals to the overall population, the model assumes that marked and unmarked individuals have identical sighting probabilities (McClintock et al. 2009; McClintock 2018).
Most cats with tabby or classic patterns could be confidently identified as individuals. We created unique encounter histories for each identifiable cat at each site, consisting of the number of times an individual was encountered during a 39-day camera survey. For example, if an individual was detected five times during the survey, its capture history was ‘05’. Cats with no unique markings were labelled as ‘unmarked’. Cats with markings that could not be identified to the individual level in a particular detection event were labelled as ‘marked unidentified’; this usually occurred because of a poor or partial photo. Detections of ‘unmarked’ and ‘marked unidentified’ cats were included as counts for each study site (McClintock 2018). We used a zero-truncated Poisson log-normal mark-resight model, which derives an estimate of abundance by first estimating three parameters: the intercept for the mean resighting rate (α), the number of unmarked individuals in the population during the sampling occasion (U), and individual heterogeneity (σ) (McClintock et al. 2009; McClintock 2018).
When estimating the abundance of low-density, elusive carnivores, like feral cats in Tasmania, Gerber et al. (2014) recommend that information about the detection process be shared across study sites. For instance, information about the resighting rate of cats can be shared across sites to inform the abundance of cats at sites with very small populations and consequently few detections. Information theoretic model selection can then be used to test whether sharing information is supported by the data (White 2005; Gerber et al. 2014). We constructed 11 biologically plausible models (Table S1), some of which shared information across sites. We modelled α in response to a combination of three variables: (1) a binary variable for whether DFTD was present at a study site (which could affect devil abundance, and in turn cat behaviour), (2) the number of devil detections at a site, (3) years since DFTD outbreak, and (4) vegetation type. We modelled σ as (1) a constant intercept for all sites, (2) fixed to zero, and (3) individually for each site. We modelled the intercept for U as (1) constant across all sites, or (2) individually for each site. We excluded models that did not converge, and selected the best models using information-theoretic model selection (Burnham & Anderson 2002). Eight models were within 7ΔAICc (Burnham et al. 2011) (see Table S1 for model selection table). We therefore performed model averaging (Burnham & Anderson 2002) by first deriving estimates of cat abundance from each model, and then multiplying each estimate by that model's AICc weight. This produced a model-averaged estimate of cat abundance at each site. Because sites were not geographically bounded and cats are not thought to be territorial in Tasmania, the estimated abundance relates to the ‘super population’ of cats available for detection on the camera array (McClintock 2018), and therefore relates to an area larger than the 2-km transect. The mark-resight analysis was performed using the ‘RMark’ package (Laake 2013) in R (R Core Team 2019).
Abundance of other species
Because the remaining species in the hypothesised food-web (Fig. 1b) were difficult or impossible to identify to the individual level, we derived detectability-corrected measures of abundance, either using the N-mixture model (Royle 2004) or the Royle–Nichols model (Royle & Nichols 2003). These models are extensions of occupancy modelling (MacKenzie et al. 2002) that in addition to modelling detection probability also model abundance. Both models rely on temporally and spatially replicated detection histories, which are counts in the case of the N-mixture model (Royle 2004) and presence-absences for the Royle–Nichols model (Royle & Nichols 2003). Because the N-mixture model uses count data rather than presence–absence data, we used it to estimate the abundance of species where counts of detections often exceeded one (i.e. devils, wallabies, possums and pademelons). Because the Royle–Nichols model uses presence-absence data, we used it to estimate abundance for the species where counts of detections rarely exceeded one (i.e. quolls, bandicoots and black rats). For these species, we truncated the small number of counts larger than one to one.
To create the detection history, we partitioned each 39-day survey into five periods for each camera (four 10-day periods and one 9-day period). We recorded the number of independent detections of a species in each period at each camera. We defined a detection as independent if at least 30 min separated the next detection of that species at that study site, as is common in similar studies (e.g. Brook et al. 2012).
For the species analysed using the N-mixture model, we first tested whether the detection histories best conformed to the Poisson or zero-inflated Poisson distributions. To do this, we created an intercept-only N-mixture model for both distributions and then proceeded with the distribution with the lowest AICc value (e.g. Ficetola et al. 2018). For each species, the winning distribution was the zero-inflated Poisson distribution. We did not consider the negative binomial distribution because it can produce biologically unrealistic results (Joseph et al. 2009; Dennis et al. 2015). The Royle–Nichols model does not require this step.
We then created nine biologically plausible models. The most complex model consisted of detection probability modelled in response to ‘lure age’ and ‘date’ (both also with quadratic terms to allow for nonlinear effects), and abundance modelled in response to ‘study site’. ‘Lure age’ increased from 1 in the first period to 5 in the fifth period. ‘Date’ was set at 1 for the beginning of the first survey and increased for every day of the study. We modelled detection probability in response to ‘date’ because cameras were moved between study sites over the course of approximately six months, which could cause cameras to detect behaviours that differ among seasons and potentially affect detection probability. We did not expect that date would substantially affect abundance because the survey was conducted in autumn and winter, which is after the time (most commonly spring) when juveniles enter the population for most species.
We created all simpler combinations of the most complex model and selected the best-performing models using AICc (Burnham & Anderson 2002). We assessed whether high-ranking models contained uninformative parameters, which are often present when comparing nested models, simply because the inclusion of an uninformative parameter receives a penalty of 2 AIC points (Anderson 2007; Leroux 2019). Uninformative parameters can be identified when their addition to a simpler nested model causes little improvement in the log-likelihood and when confidence intervals for the parameter estimate span zero (Anderson 2007; Leroux 2019). In such cases, we omitted the model (Leroux 2019). We predicted abundance and standard errors for each of the 28 study sites, either from the best model when there was a clear winning model, or a model-averaged prediction when competing models were within 7ΔAICc (Burnham et al. 2011). We fitted the models using the ‘pcount’ (N-mixture) and ‘occuRN’ (Royle–Nichols) functions within the ‘unmarked’ package (Fiske & Chandler 2011) in R.
The motivation for the analysis was to compare trends in the abundance of species at sites with differing abundance of devils, not to estimate the absolute densities of species. We did not attempt to estimate the area from which animals were available for detection. In such situations when the sample area is unknown, Royle (2004) states that the derived estimates should still serve as a useful measure of abundance that accounts for detection probability, which should be sufficient for evaluating geographic differences in abundance. We therefore treat the estimates from the N-mixture and Royle–Nichols models as detectability-corrected indices of abundance that enable us to compare trends in abundance between sites.
Structural equation modelling
To model the community-wide effects of devil population declines, we used the detectability-corrected measures of abundance, detailed in the previous two sections, as variables in a piecewise structural equation model (SEM). In contrast to classical SEM, which calculates parameter estimates globally, piecewise SEM uses individual regressions to calculate local estimates for each pathway in a hypothesised causal network (Grace et al. 2012; Lefcheck 2016). Because each response variable is modelled individually, piecewise SEM can accommodate a wide range of distributions and model types and is therefore useful for ecological datasets, which often violate the assumptions of classical SEM (Grace et al. 2012; Lefcheck 2016).
We developed an a priori SEM (Fig. 1b) based on previous research involving these species and a combination of trophic cascade theory, mesopredator release hypothesis, and possible bottom-up drivers. See Box 1 for a detailed justification for the a priori SEM. To construct the SEM, we fitted an individual regression for each species either using a generalised linear model (GLM) or ordinary least squares regression (see Table S2). Mixed models were not necessary because we modelled a single abundance estimate for each independent study site, which meant that the structure of the data was not nested.
For bandicoots, we initially modelled abundance with a GLM, but this performed poorly because bandicoots showed a negative triangular relationship with the abundance of cats and wallabies. In such situations, standard regression methods that estimate changes in the mean are not appropriate because of heterogeneous variance. Instead, quantile regression can be used to model the edges of a triangular scatter and the limiting effect of one variable on another (Cade et al. 1999; Cade & Noon 2003; Johnson & VanDerWal 2009). In a SEM context, Grace et al. (2012) recommend using local approaches that best meet the need of a pathway. Because we aimed to investigate whether cats or wallabies impose an upper limit on bandicoot abundance, we therefore used quantile regression to model bandicoot abundance at the 0.99th quantile with bootstrapped standard errors (Feng et al. 2011) using the ‘quantreg’ package (Koenker 2018) in R (R Core Team 2019). The use of quantile regression implies that some important factors that affect the ecological process have not been measured (Cade & Noon 2003).
To produce a parsimonious SEM, we used backward stepwise model reduction by sequentially removing non-significant paths (α = 0.05) until only significant predictors remained (for the same approach, see Gordon et al. (2017)). We calculated standardised path coefficients using the relevant range method (Grace & Bollen 2005) and R2 for each species (‘rsq’ package). We did not calculate standardised coefficients and R2 for quantile regression because it does not have a comparable interpretation. We assessed overall fit of the SEM using Shipley's test of d-separation (Shipley 2000, 2009), which tests whether all unconnected variables are conditionally independent, and considered the final SEM consistent with the data if Fisher's C had P> 0.05. This test revealed positive wallaby–pademelon and wallaby–possum associations; we did not have a theoretical expectation about these relationships, so we specified them as partial correlations (i.e. accounting for the effect of covariates), which assumes the association is driven by an unmeasured underlying process (Lefcheck 2016).
Results
Estimates of abundance
We first derived detectability-corrected estimates of each species' abundance, which we then fed into a structural equation model. We present the estimates of abundance in Table S3. Table S1 shows the model selection table for estimating cat abundance, and Table S4 shows the model selection table for estimating abundance indices for all other species.
Structural equation modelling reveals cascading effects of devil declines
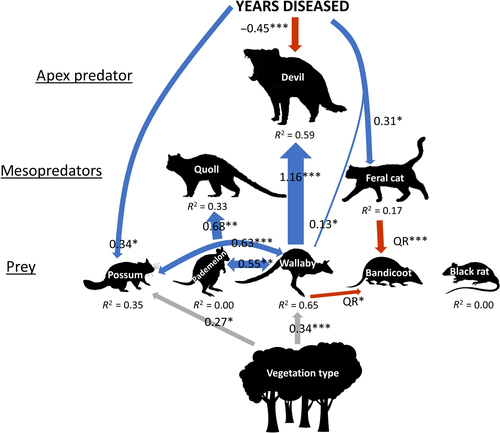
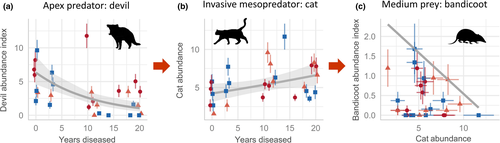
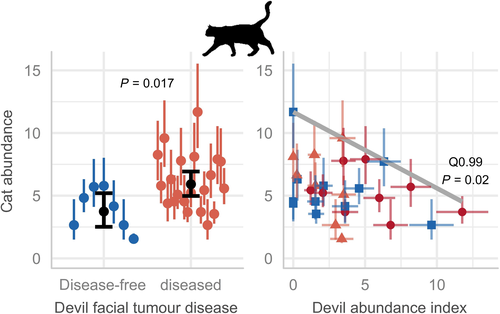
Devil facial tumour disease caused an average decline in devil abundance of 83% at long-diseased sites (as estimated by the GLM; Fig. 3a), which seemingly triggered a reorganisation of the food web. The SEM (Fig. 2) revealed that cat abundance increased with increasing time since disease arrival to a site (Fig. 3b), which in turn had a limiting effect on the abundance of bandicoots (Fig. 3c). Cat abundance was on average 58% higher at sites with DFTD than sites without DFTD (Fig. 4a). The relationship between devil and cat abundance was triangular in shape; where devil abundance was high, cat abundance was consistently low, and where devils were rare, cats were sometimes highly abundant but were not always so (Fig. 4b). Similarly, the relationship between cat and bandicoot abundance was negative and triangular in shape: bandicoots were most abundant at sites with lower cat abundance (Fig. 3c). The abundance of two major prey species of devils (but not of cats), the Bennett's wallaby and brushtail possum (Jones & Barmuta 1998; Andersen et al. 2017; Ingram 2018), increased with time since DFTD arrival (Fig. 2; Figure S1), suggesting these species have been released from top-down control. See Table 1 for results of the final regressions.
| Coefficient (SE) | P-value | |
|---|---|---|
| Tasmanian devil; GLM | ||
| (Intercept) | 1.451 (0.174) | < 0.0001*** |
| yearsDFTD | −0.09 (0.02) | 0.0001*** |
| Wallaby | 0.089 (0.02) | 0.0002*** |
| Spotted-tailed quoll; OLS | ||
| (Intercept) | 0.51 (0.478) | 0.295 |
| Pademelon | 0.169 (0.047) | 0.0012** |
| Feral cat; OLS | ||
| (Intercept) | 2.056 (0.139) | <0.0001*** |
| yearsDFTD | 0.026 (0.008) | 0.0301* |
| Bennett's wallaby; GLM | ||
| (Intercept) | 1.708 (0.307) | <0.0001*** |
| HabitatDry | −1.197 (0.358) | 0.0027** |
| HabitatWet | −2.322 (0.534) | 0.0002*** |
| YearsDFTD | 0.051 (0.021) | 0.02* |
| Tasmanian pademelon; OLS | ||
| No significant paths | ||
| Brushtail possum; OLS | ||
| (Intercept) | 1.526 (0.372) | 0.0004*** |
| yearsDFTD | 0.058 (0.023) | 0.0169* |
| HabitatDry | −0.626 (0.444) | 0.171 |
| HabitatWet | −0.941 (0.408) | 0.03 |
| Southern brown bandicoot, 0.99th quantile | ||
| (Intercept) | 2.299 (0.137) | < 0.0001*** |
| Cats | −0.124 (0.033) | 0.001** |
| Wallaby | −0.055 (0.02) | 0.013* |
| Black rat; OLS | ||
| No significant paths | ||
- ‘OLS’ refers to ordinary least squares regression and ‘GLM’ refers to a generalised linear model.
- *P < 0.05, **P < 0.01, ***P < 0.001.
The simultaneous role of top-down and bottom-up drivers in shaping ecosystems (Sinclair et al. 2003; Elmhagen & Rushton 2007; Elmhagen et al. 2010) was evident in the final SEM by the presence of top-down and bottom-up pathways. In contrast to the feral cat, the native mesopredator – the spotted-tailed quoll – showed no change in abundance in response to devil declines. Instead, quolls were positively associated with the abundance of their primary prey, pademelon (Fig. 2; Figure S1; Jones & Barmuta 1998; Andersen et al. 2017). Similarly, devils were strongly positively associated with wallaby abundance (Fig. 2; Figure S1), and the GLM showed that wallaby abundance was highest in coastal vegetation, where the structure is most open and forage most accessible (abundance was 10.2-fold higher than in wet forest/rainforest, and 3.3-fold higher than in dry eucalypt forest, as estimated by the GLM; Figure S1). This offers a mechanistic explanation for the apparent preference of devils for coastal vegetation (Hollings et al. 2016), suggesting they prefer coastal vegetation because of the higher abundance of wallabies, their largest common prey (Andersen et al. 2017). The final SEM fitted the data well (Fishers C = 23.02, p = 0.81), suggesting there were no missing paths between unconnected variables.
Discussion
The severe disease-induced decline of the Tasmanian devil, an apex predator, seemingly caused a reorganisation of the food web, including the release of feral cats and a concomitant decline of native bandicoots. Our findings highlight that apex predators not only have important regulatory effects on native prey species – in this case, possums and wallabies – but they also confer resistance to the impacts of invasive populations, which are a major global extinction threat (Doherty et al. 2016).
By estimating the abundance of cats at many sites across the full range of devil densities and disease outbreak times, we provide evidence that devils limit the abundance of feral cats, helping to clarify a previous debate. In a remote-camera study, Fancourt et al. (2015) claimed that devils do not limit cats. That study, however, used an inappropriate design by only surveying sites where DFTD had been present for > 5 years, therefore including no sites with high devil densities. Our finding adds to those from two other studies, one using longitudinal spotlight surveys and one using hair traps (Hollings et al. 2014; Hollings et al. 2016), that both show an increase in cat detections following devil declines. We show that although devils never eliminate cats, they do limit their abundance, and this seemingly facilitates the persistence of bandicoots.
Other research shows that devil declines have resulted in the behavioural release of quolls. For instance, where devils are rare, quolls consume more carrion (Cunningham et al. 2018) and increase their activity during the period of the day preferred by devils (Cunningham et al. 2019c). Despite this behavioural release, no study has found evidence for increased abundance of quolls following devil declines (Hollings et al. 2014; Troy 2014; Hollings et al. 2016), and our study further supports those findings.
The divergent responses of the two mesopredators – the invasive cat and the native quoll – raises the question of whether native apex predators could, in general, have a stronger suppressive influence on invasive mesopredators than on coevolved mesopredators. This could arise because evolutionary naivete may leave an invasive mesopredator without beneficial behaviours or morphologies (Sih et al. 2010), similar to the way that invasive predators have stronger effects on evolutionarily naïve prey than on coevolved prey (Salo et al. 2007). Although this hypothesis applied to effects of apex predators on mesopredators is speculative and requires testing in other systems, there is some support for it from other studies. For instance, Crooks & Soulé (1999) showed that the presence of a native apex predator, the coyote (Canis latrans), had a stronger negative effect on introduced mesopredators (feral cat and Virginia opossum Didelphis virginiana) than on native mesopredators (grey fox Urocyon cinereoargenteus, racoon Procyon lotor, and striped skunk Mephitis mephitis). Similarly, the Iberian lynx (Lynx pardinus) had a stronger negative effect on introduced mesopredators (Egyptian mongoose Herpestes ichneumon and common genets Genetta genetta) than on native mesopredators (red foxes and European badgers Meles meles) (Palomares et al. 1996). In theory, the weaker effect of apex predators on coevolved mesopredators could arise because eco-morphological divergence over evolutionary time-scales gives rise to niche partitioning (Jones 2003), leading to behaviours that reduce encounter rates and facilitate coexistence (Schoener 1974; Linnell & Strand 2000). Others have shown that apex predators can confer resistance to the effects of invasive populations (Ritchie & Johnson 2009; Wallach et al. 2010; Letnic et al. 2011; Ritchie et al. 2012; Gordon et al. 2015; Derham et al. 2018). We extend this to suggest that native apex predators could potentially have even stronger effects on introduced than coevolved mesopredators.
The greater abundance of possums in long-diseased areas agrees with other research that shows declining devil abundance has released possums from top-down control. For example, possums relaxed their risk-sensitive foraging behaviours following devil population declines (Hollings et al. 2015), and reinstated these behaviours following the introduction of devils to the previously devil-free Maria Island (Cunningham et al. 2019a). Wallabies also responded to the introduction of devils to Maria Island by increasing activity at periods of the day when devils are inactive (Cunningham et al. 2019c). Because possums are typically arboreal but often forage on the ground, the trends we show here could reflect changes in abundance or increased ground-based activity by possums in response to a relaxed landscape of fear (Hollings et al. 2015; Cunningham et al. 2019a).
Disease outbreaks and other environmental perturbations provide valuable natural experiments that can improve our understanding of how ecosystems function (Holdo et al. 2009). This is particularly so when perturbations are independent of human effects, like DFTD, because these cases reduce anthropogenic confounding. This is significant because many other studies of the effects of large-carnivore declines have been conducted on cases where carnivores have declined because of human effects (Ripple et al. 2014), which are also likely to affect many other species. These anthropogenic effects could mask or confound the relationships between carnivore decline and changes in other species, and so far, has been one of the major challenges in disentangling mesopredator release from land-use change (Prugh et al. 2009). Disease-induced natural experiments have helped shape our understanding of broad-scale processes that would otherwise be unfeasible or unethical to manipulate. For instance, the eradication of rinderpest in the Serengeti caused an eruption of wildebeest, which in turn suppressed fire and facilitated tree regeneration (Holdo et al. 2009). Similarly, a mange outbreak in Scandinavian red foxes (Vulpes vulpes) led to severe population declines, revealing predation by foxes as a crucial process regulating the abundance of several prey species (Lindström et al. 1994). Of course, natural experiments are not true manipulative experiments. Most notably for our study, the results need to be interpreted in the context of the east-west correlative design; at a regional scale, the west of Tasmania tends to be wetter, but importantly our site selection controlled for rainfall, vegetation type, and elevation.
A growing body of research highlights the importance of apex predators in protecting small prey species (Crooks & Soulé 1999; Prugh et al. 2009), yet this potential is rarely harnessed to reduce the harm caused by invasive predators (Derham et al. 2018). In Australia, the global hotspot of small-mammal extinctions (Woinarski et al. 2015), there is compelling evidence that dingoes sometimes benefit small mammals by suppressing mesopredators and by promoting vegetation cover through the suppression of large herbivores (Johnson et al. 2007; Johnson & VanDerWal 2009; Letnic et al. 2009; Wallach et al. 2010; Brook et al. 2012; Colman et al. 2014). Despite these benefits, dingoes are lethally controlled across much of the continent. The Australian Government plans to kill 2 million feral cats by 2020 in a “war on cats”, but this has a weak scientific basis because this target is not linked to conservation outcomes (Doherty et al. 2019), is difficult to achieve at broad scales and does not attempt to harness the potential for apex predators to indirectly protect smaller wildlife (i.e. by relaxing lethal control of apex predators; Cunningham et al. 2019b). In areas of the Australian mainland where restoring dingo populations remains socially unacceptable, it is worth exploring whether devils could fill the void, given they were present on the mainland until approximately 3200 years ago (White et al. 2018) and the synergistic causes of their extinction (climate, dingoes and human intensification) are sufficiently understood (Brown 2006; Brüniche-Olsen et al. 2014; Prowse et al. 2014; Brüniche–Olsen et al. 2018). This could begin with a carefully controlled experimental reintroduction of devils to a bounded landscape to assess whether they can perform key top-down functions in ecosystems on mainland Australia, as modelling suggests (Hunter et al. 2015).
Following the extinction of the larger thylacine (c. 20–30 kg), the Tasmanian devil has ascended to the role of Tasmania's apex predator. Our findings provide rare evidence of a trophic cascade caused by changes in the abundance of a marsupial predator, and we suggest the trophic effects of the thylacine, at approximately twice the mass of the devil, may have been even stronger. The conservation implications of our findings therefore need to be interpreted in the context of shifting baselines. The term ‘apex predator’ is context-specific, referring to species at the top of food webs with no significant predators themselves (Prugh et al. 2009; Ritchie & Johnson 2009). In ecological communities where the largest apex predators have been extirpated, top-down control of invasive predators may still be effective if the remaining predators are sufficiently large (the general rule is at least twice as large; Donadio & Buskirk 2006; Ritchie & Johnson 2009). Humans typically have less conflict with medium-sized native carnivores than large carnivores. This suggests our findings have management implications for areas where large carnivores will never be tolerated and where harm is caused by invasive mesopredators. We speculatively suggest that the effects of larger native predators may be stronger on evolutionarily naive mesopredators than on coevolved mesopredators, and we encourage more work in other systems to test if this is a general phenomenon. Overall, our results should reinforce the importance of apex predators in promoting the inherent strengths that enable resilient ecosystems (Wallach et al. 2010), and inspire a more self-sustaining, ecosystem-based approach to managing the harm caused by invasive predators.
Acknowledgements
We thank the staff at Parks and Wildlife for assisting with accommodation and many volunteers for assisting with field work. We thank four anonymous reviewers and the editor for their very constructive feedback. The project was funded by the Australian Research Council (ARC DP110103069) and a Holsworth Wildlife Research Endowment. CC was supported by a top-up scholarship from the Save the Tasmanian Devil Appeal, MJ by an ARC Future Fellowship (FT100100031) and CJ by an ARC Professorial Fellowship (DP110103069).
Ethics statements
This study was conducted in accordance with the University of Tasmania Animal Ethics Committee Permit A15274 and a scientific permit (TFA 16161) from the Department of Primary Industries, Parks, Wildlife and Environment.
Author contributions
All authors designed the study. CC conducted the field work, statistical analysis and led the writing. All authors contributed to writing.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the Figshare Repository: https://doi.org/10.6084/m9.figshare.11661696.




