Biodiversity in cities needs space: a meta-analysis of factors determining intra-urban biodiversity variation
Abstract
Understanding varying levels of biodiversity within cities is pivotal to protect it in the face of global urbanisation. In the early stages of urban ecology studies on intra-urban biodiversity focused on the urban–rural gradient, representing a broad generalisation of features of the urban landscape. Increasingly, studies classify the urban landscape in more detail, quantifying separately the effects of individual urban features on biodiversity levels. However, while separate factors influencing biodiversity variation among cities worldwide have recently been analysed, a global analysis on the factors influencing biodiversity levels within cities is still lacking. We here present the first meta-analysis on intra-urban biodiversity variation across a large variety of taxonomic groups of 75 cities worldwide. Our results show that patch area and corridors have the strongest positive effects on biodiversity, complemented by vegetation structure. Local, biotic and management habitat variables were significantly more important than landscape, abiotic or design variables. Large sites greater than 50 ha are necessary to prevent a rapid loss of area-sensitive species. This indicates that, despite positive impacts of biodiversity-friendly management, increasing the area of habitat patches and creating a network of corridors is the most important strategy to maintain high levels of urban biodiversity.
Introduction
Urbanisation is tantamount to a near-permanent alteration of land use, which eliminates the locally dominant natural ecosystem (Güneralp & Seto 2013). It is often regarded as one of the largest societal transformation processes (Kareiva et al. 2007) and a major threat to global biodiversity (Grimm et al. 2008). Consequently, urbanisation research is predominantly directed towards the negative impact of urbanisation on ecosystems, biodiversity hotspots or protected areas (Ricketts & Imhoff 2003; Seto et al. 2011; Güneralp & Seto 2013). This stigmatisation of the urban landscape can distract from the high levels of biodiversity that may flourish inside cities (Aronson et al. 2014) and that interact with the unique features of this environment in the same dynamic way as it is observed in natural ecosystems. It also distracts from positive effects that urban biodiversity has on ecosystem services (Bolund 1999), including human well-being (Fuller et al. 2007).
A recent comparison of biodiversity levels between more than a hundred cities worldwide (i.e. gamma diversity) showed that bird and plant species densities vary substantially among cities and were explained best by a city's urban land cover, age of urban area as well as an intact urban vegetation cover (Aronson et al. 2014a). Besides varying levels of biodiversity among cities, biodiversity levels also vary within cities (Sushinsky et al. 2013). Profound differences in species richness or species diversity are detectable among intra-urban localities (i.e. alpha diversity), confirmed by a great number of studies looking into the distribution of numerous taxonomic groups within cities globally (Dickman 1987; Tilghman 1987; Hobbs 1988; Natuhara & Imai 1999; Cornelis & Hermy 2004; Pacheco & Vasconcelos 2007; Bickford et al. 2010; Sattler et al. 2010; Bates et al. 2011; Fontana et al. 2011; Kappes et al. 2012; Lizee et al. 2012; Goertzen & Suhling 2013).
Analyses of intra-urban biodiversity levels often focus on changes in species richness, measures of species diversity and species’ abundance along urban–rural gradients (McKinney 2008; Niemela & Kotze 2009; Martinson & Raupp 2013). This gradient is delineated from the border of a city towards a city's centre and is often used as the exclusive explanatory variable determining biodiversity of urban areas (Niemela 1999; Kotze et al. 2011; McDonnell 2011). This approach equates the position along the urban–rural gradient with the degree of urbanisation and has the advantages of being intuitive and easily measured. Despite its practicability, it greatly generalises the urban landscape and makes an interpretation of results ambiguous with regard to decisive underlying specific features of the urban landscape, which are not quantified (McDonnell & Hahs 2008). The ramifications of this approach become especially apparent when considering the heterogeneity of urban areas: in a European comparison, the percentage of green space was found to vary from 2 to 46% among cities (Fuller & Gaston 2009); heterogeneity that is hardly captured by a simple gradient approach. Although gradients or categories may explain some variation in biodiversity levels (Dunn & Heneghan 2011), a transformation of the urban landscape into these gradients or categories, e.g. of urbanisation or socioeconomic parameters, masks the underlying driving factors of the biophysical urban landscape. In consequence, alpha diversity can only be linked crudely, if at all, to habitat features of cities. In their review of urban–rural gradient analyses McDonnell & Hahs (2008) pointed out that the relationship between biodiversity and the urban–rural gradient follows a wide range of predictive curves, depending largely on the taxa under investigation: species richness of animals usually declines from rural to more urban areas (McKinney 2008; Faeth et al. 2011), whereas plant species richness often increases towards the city centre (McKinney 2008). To answer the question of what determines intra-urban variation in biodiversity we need to precisely quantify the individual factors that affect alpha diversity.
The literature investigating biodiversity relationships at a finer scale is growing. Previously utilised approximations, e.g. the urban–rural gradient, are replaced by individually quantified habitat features and often distinguish precisely between different aspects of urban features, such as patch area, patch area-perimeter ratio, temperature, the application of pesticides, the degree of fragmentation or various vegetation variables, to mention just a few (Chace & Walsh 2006; Hamer & McDonnell 2008; Shwartz et al. 2013). The range of variables quantified varies widely and thus needed simplification to retain a high sample size in the study at hand. Population ecology often distinguishes local habitat features from those of the surrounding landscape (‘matrix’) (Ricketts 2001). While local factors determine habitat suitability (in terms of species survival), landscape factors define the permeability of the surrounding landscape for species’ dispersal. We applied this local-landscape dichotomy to compare the importance of habitat suitability vs. habitat permeability. The effects of the matrix can be highly variable and gave rise to numerous hypotheses, emphasising either negative effects of the matrix (e.g. ‘fragmentation hypothesis’, Debinski & Holt 2000) or different degrees of positive effects (e.g. ‘habitat compensation hypothesis’, Norton et al. 2000; ‘landscape supplementation hypothesis’, Dunning et al. 1992). Another way to categorise the factors is to distinguish them based upon their traits. Traditionally, ecological factors can be divided into biotic and abiotic factors (e.g. Hooper et al. 2005). While abiotic factors are usually considered crucial for plant diversity, vegetation is believed to be a major factor influencing the fauna, providing habitat and food. This biotic–abiotic dichotomy is a second major classification in biodiversity research (Benton 2009). However, some factors (e.g. area, disturbance) cannot be clearly assigned to either of these categories (Jackson et al. 2011). These remaining factors either define the geographic properties of a site (‘design’) or its anthropogenic influences (‘management’) (Bazelet & Samways 2011). This design-management dichotomy is a critical question in conservation biology and of crucial importance for landscape planning. However, it never has been tested in an urban context.
We here present the first comprehensive meta-analysis, spanning 75 cities on all inhabited continents, on the importance of individual habitat variables for determining levels of intra-urban biodiversity (Fig. 1). We specifically focus on factors which have often been put forward as key determinants of intra-urban biodiversity, such as patch area, fragmentation and vegetation (Faeth & Kane 1978; Drinnan 2005; Cushman 2006). A systematic and global quantification of the effects of these variables on biodiversity across taxonomic groups within cities is lacking. Species–area relationships (Arrhenius 1921; MacArthur & Wilson 1963, 1967) generally have a large impact on biodiversity, and, accordingly, we hypothesise that area is an important factor explaining intra-urban biodiversity. We also hypothesise that vegetation plays an important role for intra-urban biodiversity, and test whether various taxonomic groups react differentially to these habitat characteristics due to taxon-specific habitat requirements.
Methods
Study selection
We performed a literature review in ISI Web of Knowledge for the period 1900 – 16th August 2013 matching the following search term: TS = (urban* OR town* OR city OR cities) AND TS = (biodiversity OR species richness OR diversity) AND TS = (species OR taxa OR taxon). This yielded a total of 3956 publications. In addition, we also surveyed reviews and meta-analyses on the subject for relevant publications that had remained undetected previously. Only studies published in peer-reviewed journals were included in the meta-analysis, relying on the peer-review process as a first step of quality control. We then applied the following criteria that had to be met by a publication to be included in our meta-analysis: (1) use of primary data; (2) three or more study sites, species and habitat variables used in analysis; (3) the statistical procedure employed different levels of significance to individual variables; (4) more than 50% of study sites had to be located in urban areas themselves (and not in the rural surroundings); (5) the response variable had to be a measure of biodiversity (e.g. species richness or an index of diversity); (6) the taxonomic group under investigation had to live terrestrially at least during one life stage; (7) the study had to be published in English.
The first screening of publications, based on title and field of publication, left 1116 candidate publications within the scope of ecology. Of these, 318 publications were selected on the basis of information delivered in abstracts and checked against the above mentioned seven criteria. However, 229 of them had to be excluded for the following reasons: habitat variables were not analysed separately, instead proxies or categories, such as the rural–urban gradient, were used (n = 71); less than three study sites, species or habitat variables were analysed (n = 56); more than 50% non- or only near-urban study sites were included (n = 30); insufficient statistical information (n = 22; see below for details); no primary data source, i.e. data were gathered from other surveys (n = 16); other reasons, e.g. only species level analysis, publication in another language, focus on life-history traits or otherwise different scope of the paper (n = 34). Many studies had to be excluded on the basis of more than one criterion; numbers given here indicate the first criterion of exclusion that had been identified. A total of 87 publications met our criteria. In addition to the many different habitat variables quantified, most of these publication also analysed separately the effects on different measures of biodiversity, often also for different taxonomic groups, and sometimes even in different cities. Therefore, in total, the meta-analysis is based on 2021 separate combinations of factors, biodiversity measures, taxa and cities.
A major challenge in urban ecology is clearly delineating the extent of urban landscapes. Regional differences in urban planning and construction make it difficult to find criteria that adequately describe urban landscapes at a global scale (Adams & Lindsey 2011) and common criteria are missing (United Nations 2012). Frequently, human population size or density as well as the relative cover of sealed surfaces are adopted as proxies. Nonetheless, country-specific definitions of what is ‘urban’ were employed for the compilation of a global dataset for the 2011 Revision of the World Urbanization Prospects (United Nations 2012). Due to this heterogeneity of approaches, we relied on the categorisation of study sites as urban or non-urban by the primary authors of publications when determining their suitability for our meta-analysis.
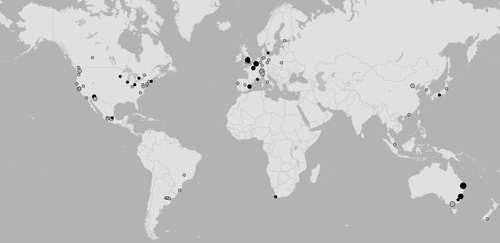
Similar to a previous review on studies of urban wildlife research (Magle et al. 2012) the cities included in our meta-analysis were not equally spread globally, with 51 lying in Europe and North America (68% of all studied cities; see Fig. 1). A similar bias was evident with respect to the taxa covered: 938 combinations of factors, biodiversity measures, taxa and cities were available for birds (149 of the detailed data set; 789 of the generalised data set), representing almost half of all included analyses (see Supporting information).
Data extraction
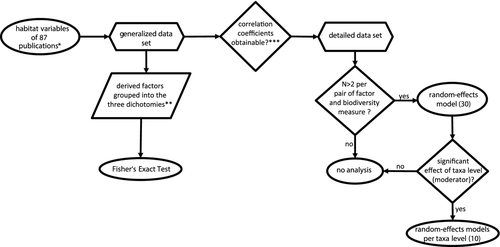
The studies varied substantially in the types of data reported. We therefore created two data sets: (1) a detailed data set with a reduced number of case studies allowing to quantify the effect of each individual factor in random-effects models, and (2) a generalised data set, including a maximum number of case studies, but with simplified explanatory categories to test the influence of the three dichotomies: ‘local’ vs. ‘landscape’, ‘abiotic’ vs. ‘biotic’ and ‘design’ vs. ‘management’ (Fig. 2).
Depending on the definitions given by the respective authors, we transformed the response variables used in the 87 papers to a reduced set of biodiversity measures: species richness and species diversity. Raw species richness, i.e. derived from surveys, was treated equally as estimated species richness from species accumulation curves. Species density was treated as species richness (n = 1); Shannon-index (n = 4) and Evenness (n = 1) were grouped together as diversity while functional richness (n = 1) and urban diversity index (n = 1) were excluded from further analyses. Following Felton et al. (2010) and due to a lack of consistent taxonomic grouping of the data (e.g. amphibians and reptiles are analysed either separately or jointly as ‘herpetofauna’) the taxonomic categories we employed do not necessarily translate to ecological distinctiveness, evolutionary relatedness or functional properties, rather they followed operational necessities.
The numerous different independent variables used to describe the urban landscape were grouped in 69 factors (see Table in Supporting information, listing all original variables used in the source publications and their assigned new factor name). If a variable used in a publication could not be assigned to any of these factors in a meaningful way, it was omitted from further analyses (n = 178).
For the detailed data set, we noted the effect of each factor on the respective response variables (the measures of biodiversity) using rho and sample size (number of study sites). We changed the sign of a correlation coefficient if studies calculating effects for the same factor, e.g. habitat connectivity, used opposing measures, e.g. proximity or distance to suitable habitat patches. If, finally, more than three entries existed for a given factor (Figs. 3-5), it was used in our meta-analysis and we computed a summary effect using a random-effects model. This provided the detailed data set which was used to quantify the effect of each single factor on measures of biodiversity. In cases where publications fulfilled all requirements to be included in a meta-analysis, but did not provide correlations of variables employed, we asked the respective authors for the missing statistical information for integration into our database (11% responded positively).
For the generalised data set, and if we could not obtain correlation coefficients from the respective authors, we noted a factor's significant or non-significant effect on the measure of biodiversity. If a study only published a final simplified multivariate model, e.g. following AIC (Akaike information criterion) or DIC (Deviance information criterion) model selection procedures, variables were accepted as positively or negatively significant if they were part of the final model. If they were not included in the model they were considered non-significant. Effects were likewise registered, if variables were found significant in simple regression models. In cases where effects were not indicated separately for single habitat variables, but were expressed as explanatory multivariate axes (e.g. PCA, principle component analysis, or CCA, canonical correspondence analysis), an effect was accepted if significance levels of correlations of habitat variables with the explanatory axes with greatest explanatory power were supplied. If several models were provided as being equally suitable to explain the variation in a response variable, only those habitat variables were taken as significantly positive/negative that occurred in at least 50% of the models presented. If studies did not deliver any of this information, they were excluded from analyses for statistical reasons (n = 22, see also paragraph above).
Finally, this yielded a detailed data set of correlations for the quantification of effects of individual factors and a separate generalised data set for comparisons between local and landscape level factors, biotic and abiotic factors as well as between design and management factors.
In addition, threshold values of patch area were noted, indicating sizes of study sites below which species richness declined significantly.
Statistical analyses
The detailed data set, based on correlation analyses, contained 331 combinations of factors, biodiversity measures, taxa and cities from 24 publications. We calculated random-effect models for 27 factors and species richness and for three factors and species diversity, all with three or more entries per factor. Generally, random-effect models also attribute the distribution of effect sizes to real differences among studies and do not assume sampling error as the only source of differences in effect sizes between studies (Borenstein et al. 2009). This better accommodates that our data originates from all biogeographical regions and from divergent experimental setups. Furthermore, estimates of the variance of summary effects are more accurate in random-effects models, and facilitate more robust inferences of effect sizes (Hedges & Vevea 1998), which is important for predictions for cities not covered by the database.
Following Stein et al. (2014), we omitted results potentially affected by covariates, such as multiple regressions or partial correlations and only included raw correlation coefficients on relationships between a measure of biodiversity and an explanatory category, thus providing a better comparability across studies.
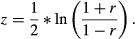
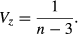
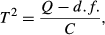
Using the above formulas, we calculated estimates of effect size for the relationship between the two measures of biodiversity and each factor. To determine levels of heterogeneity of effects between studies we calculated the following measures: Q, T² and I² (Borenstein et al. 2009). These measures of heterogeneity are complementary and we employed them collectively to adequately describe the variation in the data set. Q, as the weighted sum of squares within a data set, can be tested against the expected deviation, assuming a common effect size of all studies, for significant heterogeneity. As the measure of Q is standardised and independent of the metric of effect size, we use T² to estimate the amount of variation in the same metric as the effect size, giving an indication of the range of effect sizes that can be assumed for future studies. I² is a proportional measure describing the amount of variation in a data set that can be attributed to real differences in effect sizes between studies. Meta-analyses are often subjected to publication bias resulting in missing studies and a potentially biased result of effect size. Publication bias can be identified using funnel plots (see Supporting information) (Borenstein et al. 2009), which show the effect size of studies and their standard error. In the absence of publication bias, they should be symmetrically distributed around the mean effect size. The studies at the top of the funnel plot are usually those with large sample sizes (~ small standard errors), while the studies with smaller sample sizes (~ large standard errors) are located at the bottom. Since standard errors can be expected to be randomly distributed, independent of effect size, gaps in the funnel plot are indicative of missing publications. We tested for publication bias using a regression test for asymmetry (Borenstein et al. 2009). Significantly asymmetric results were then augmented using the Trim and Fill method (Sutton et al. 2000). This method calculates the number of missing studies, due to publication bias, estimates their effect sizes as well as standard errors and adds them to the data set of the meta-analysis. This aims to retrieve a summary effect size that is closer to the true effect, which could not be calculated directly because of publication bias. Although asymmetry was significant in some cases, augmentation of data using the Trim and Fill method did not change the significance of results, except for edge-effects (see Table 1 and Trim and Fill plots in Supporting information).
| Factors | n | Taxa levels | P (Q-d.f.) | Tau² | I² | Missing studies |
|---|---|---|---|---|---|---|
| Species richness | ||||||
| Area | 22 | 6 | <0.001*** | 0.19 | 86.65 | 0 |
| Habitat richness | 5 | 3 | 0.016* | 0.07 | 69.01 | 0 |
| Age | 7 | 4 | 0.001** | 0.09 | 71.81 | 2 |
| Edge-effects | 10 | 6 | <0.001*** | 0.35 | 90.14 | 3 |
| Managed | 12 | 5 | 0.021* | 0.05 | 50.37 | 0 |
| Disturbance | 7 | 4 | <0.001*** | 0.20 | 81.64 | 0 |
| Pesticide | 4 | 4 | 0.530 | 0.00 | 0.00 | 0 |
| Herb density | 3 | 3 | 0.173 | 0.03 | 43.95 | 0 |
| Herb cover | 11 | 3 | <0.001*** | 0.15 | 82.83 | 0 |
| Herb structure | 11 | 4 | 0.284 | 0.00 | 0.02 | 0 |
| Shrub structure | 6 | 4 | 0.875 | 0.00 | 0.00 | 0 |
| Shrub cover | 6 | 3 | 0.252 | 0.01 | 25.03 | 1 |
| Tree structure | 11 | 5 | 0.111 | 0.02 | 34.68 | 2 |
| Tree cover | 17 | 4 | 0.002** | 0.05 | 62.39 | 0 |
| Tree density | 4 | 1 | 0.047* | 0.03 | 64.58 | 0 |
| Vegetation structure | 10 | 4 | 0.067 | 0.04 | 41.21 | 0 |
| Vegetation richness | 4 | 3 | 0.087 | 0.06 | 53.57 | 1 |
| Microclimate | 4 | 3 | 0.005** | 0.19 | 76.87 | 0 |
| Bare soil cover | 4 | 2 | <0.001*** | 0.25 | 93.06 | 0 |
| Water cover | 9 | 4 | 0.315 | 0.00 | 5.34 | 1 |
| Water body structure | 4 | 4 | 0.773 | 0.00 | 0.00 | 0 |
| Corridor | 5 | 5 | 0.437 | 0.00 | 0.00 | 0 |
| Connectivity | 10 | 5 | 0.006** | 0.06 | 64.01 | 0 |
| Distance to water body | 7 | 5 | 0.007** | 0.09 | 63.61 | 0 |
| % green area | 21 | 7 | <0.001*** | 0.08 | 77.88 | 0 |
| % agriculture | 4 | 3 | 0.003** | 0.05 | 78.40 | 1 |
| % sealed | 17 | 6 | <0.001*** | 0.16 | 85.25 | 0 |
| Species diversity | ||||||
| % green area | 3 | 2 | 0.179 | 0.00 | 0.01 | 0 |
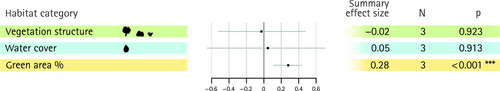
| Vegetation structure | 3 | 3 | 0.024* | 0.17 | 73.34 | 0 |
| Water cover | 3 | 2 | <0.001*** | 0.48 | 91.75 | 0 |
- Signif. codes: < 0.0001 **** < 0.001 *** < 0.01 ** < 0.05 *.
Factors that we could perform a meta-analyses for were further analysed to test for an effect of the different taxa included in the analysis. Using taxa levels as a moderator in a mixed-effects model (Borenstein et al. 2009), we tested for a significant effect for single taxonomic groups.
The generalised data set, compiled for comparisons of landscape vs. local variables, biotic vs. abiotic and design vs. management was based on 87 publications and a total of 1690 entries. The previously introduced factors were grouped into either landscape or local, either abiotic or biotic and either design or management variables (see Table in Supporting information). The ratio of significant vs. non-significant effects on the response variables (i.e. biodiversity measures) was then analysed using Fisher's exact test (see Fig. 2).
To get an indication of the size of habitat patches necessary to sustain levels of biodiversity, we analysed spatial thresholds that authors identified to prevent species loss. Due to the heterogeneity of approaches for this procedure, we grouped results in two categories: high- or low-level thresholds. High-level thresholds contain values found necessary for the protection of a significant number of area-sensitive species (e.g. forest interior species), while the low-level thresholds contain values necessary to sustain urban-adapter species numbers before they decrease exponentially. The results for these two groups were tested for significant differences using a Wilcoxon rank-sum test.
All statistical analyses were performed with R version 3.0.2 (R Core Team 2013) using the packages rmeta (Lumley 2012) and metafor (Viechtbauer 2010).
Results
Detailed data set
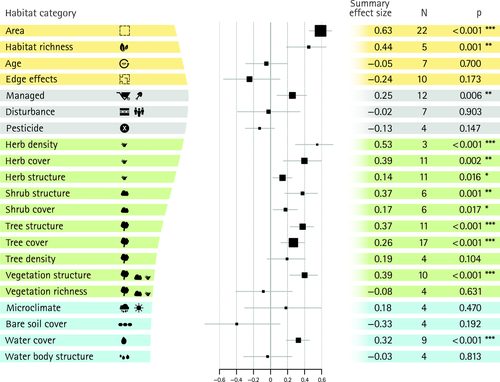
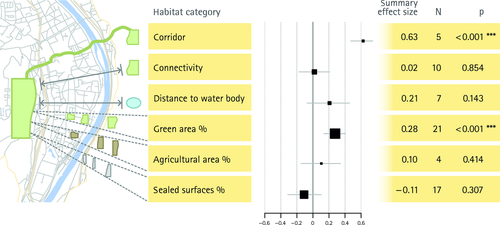
Across all cities and taxonomic groups, the random-effects models showed the strongest summary effect estimate for patch area and corridors, with correlation coefficients above 0.6 in both cases (Figs. 3 and 4). Overall, the random-effects models revealed 15 positively significant and no negatively significant summary effect size estimates for factors on measures of biodiversity. Fourteen significant summary effects were found for species richness (Figs. 3 and 4) and one significant summary effect for species diversity (Fig. 5). For species richness, one management factor (managed), two design factors (corridor & patch area) and one abiotic factor (water cover) were significant, with corridor and patch area yielding the strongest summary effect estimates. The remaining eleven factors significant for species richness were all biotic factors, including vegetation cover, density, structure or a subset thereof, i.e. herbaceous plants, shrubs, or trees. No significant effect on species richness was found for connectivity, age, pesticides, edge effects, tree density, vegetation richness, disturbance, microclimate, bare soil cover and water body structure (Figs 3 and 4), but for most of these variables the number of studies was relatively low (n = 4–10).
As expected, there was significant heterogeneity in the dataset in models for 18 factors, Q − d.f. < 0.05 (Table 1). I² ranged from 51% to 84%, indicating that a substantial amount of between-study variance could be attributed to real differences in true effect sizes of studies (Borenstein et al. 2009). A correlation of heterogeneity of data corrected for sample size (Q − d.f.) and the number of different taxa analysed was highly significant (Pearson's product-moment correlation; r = 0.529, n = 45, P-value < 0.001). Looking at the Tau² values, water cover (for species diversity) and edge effects as well as soil cover (for species richness) showed strongest heterogeneity in effect sizes. The summary effect for edge effects was computed with three added Trim-and-Fill values, which altered the summary effect to become non-significant. No result for any other factor changed in significance due to this correction for publication bias, although effect estimates were added for seven factors due to asymmetry (Table 2). Much of the heterogeneity is due to differences in the true effect sizes, with I² values above 50% in 19 models.
| Habitat variable | P-value of mixed-effects model | Taxa levels | Summary effect size | P-value of taxa level | n |
|---|---|---|---|---|---|
| Age | 0.0001 | Insects | −0.30 | <0.001*** | 4 |
| Vegetation structure | 0.0029 | Birds | 0.60 | <0.001*** | 3 |
| Insects | 0.36 | <0.05* | 4 | ||
| Area | 0.0001 | Birds | 0.76 | <0.0001**** | 10 |
| Insects | 0.62 | <0.0001**** | 6 | ||
| Plants | 0.52 | <0.01** | 4 | ||
| Herbaceous cover | 0.0204 | Birds | 0.35 | <0.01** | 6 |
| Insects | 0.52 | <0.01** | 4 | ||
| Herbaceous structure | 0.0366 | Birds | 0.12 | 4 | |
| Insects | 0.07 | 4 | |||
| % green | 0.0293 | Birds | 0.24 | <0.05* | 8 |
| Insects | 0.34 | <0.01** | 9 | ||
| Shrub structure | 0.0132 | No taxa level with n > 2 | |||
| Tree cover | 0.0093 | Birds | 0.30 | <0.001*** | 9 |
| Insects | 0.18 | 5 | |||
| Tree structure | 0.0001 | Birds | 0.33 | <0.0001**** | 7 |
| Water body cover | 0.0093 | Birds | 0.35 | <0.01** | 3 |
| Insects | 0.27 | <0.05* | 3 | ||
- Signif. codes: < 0.0001 **** < 0.001 *** < 0.01 ** < 0.05 *.
Taxon-specific effects
For models of 10 factors explaining variation in species richness, taxonomic group showed a significant effect when tested as a moderator in a mixed-effects model (Table 2). Only for ‘age’ the effect size of one taxonomic group (insects) differed significantly from the common summary effect. All taxa responded positively to area, with the strongest effects on birds followed by insects and plants, which showed a weaker effect than the common summary effect. Birds also responded much stronger to vegetation structure (particularly trees) than any other or all combined taxonomic groups. Insects showed the strongest effects to herbaceous cover. However, the number of available studies for single factors was often higher for birds than for other taxa. The effect of taxonomic group as a moderator was independent of heterogeneity (T²; I²) observed across models (Wilcoxon test, P > 0.25 in both cases). Also, the number of different taxonomic groups included in the random-effects models did not significantly affect the results of the moderator (Wilcoxon test, P = 0.24). The results of the moderator were significantly affected by the number of studies (n) included in the model (Wilcoxon test, W = 25, P = 0.006).
Generalised data set
Species richness was significantly more affected by biotic factors than by abiotic factors (Fisher's Exact Test; d.f. = 1, P = 0.032), by management factors than by design factors (Fisher's Exact Test; d.f. = 1, P = 0.01) and by local factors compared with landscape factors (Fisher's Exact Test; d.f. = 1, P = 0.025). No significant differences were found for the effects of the three dichotomies on species diversity: abiotic/biotic (Fisher's Exact Test; d.f. = 1, P = 0.51), design/management (Fisher's Exact Test; d.f. = 1, P = 0.20), landscape/local (Fisher's Exact Test; d.f. = 1, P = 0.70). However, the sample size for species diversity was also lower than for species richness.
Area thresholds
Threshold values for species richness computed for areas of study sites ranged from 1 to 140 ha, with an average threshold area of 26.7 ha ± 7.4 SE (n = 24; Table 3). Threshold values differed significantly between the different approaches to identify the threshold (Wilcoxon test, W = 143, P < 0.001). If authors aimed to identify areas necessary to include a significant number of area-sensitive species (or a high level of diversity) the average threshold was 53.3 ha ± 12.1 SE (n = 11). Although, if a threshold was identified describing the loss of urban-adapter species (or a low level of diversity) the average was 4.4 ha ± 0.85 SE (n = 13).
| Threshold value (ha) | Goal | Author | Taxa level |
|---|---|---|---|
| 1 | Low | Arca et al. (2012) | Birds |
| 4 | Low | Drinnan (2005) | Birds |
| 3 | Low | Drinnan (2005) | Frogs |
| 2 | Low | Drinnan (2005) | Plants |
| 2 | Low | Drinnan (2005) | Fungus |
| 1 | Low | Germaine et al. (1998) | Birds |
| 8 | Low | Hinners et al. (2012) | Pollinators |
| 1 | Low | Loss et al. (2009) | Birds |
| 10 | Low | Natuhara & Imai (1999) | Birds |
| 8 | Low | Sadler et al. (2006) | Carabids |
| 5 | Low | Sewell & Catterall (1998) | Birds |
| 6.5 | Low | Smith (2007) | Birds |
| 5 | Low | Tilghman (1987) | Birds |
| 140 | High | Bickford et al. (2010) | Amphibians |
| 42.2 | High | Donnelly & Marzluff (2004) | Birds |
| 50 | High | Drinnan (2005) | Birds |
| 50 | High | Drinnan (2005) | Frogs |
| 20 | High | Hinners et al. (2012) | Pollinators |
| 20 | High | Natuhara & Imai (1999) | Birds |
| 20 | High | Smith (2007) | Birds |
| 25 | High | Tilghman (1987) | Birds |
| 50 | High | Vignoli et al. (2009) | Reptiles |
| 50 | High | Vignoli et al. (2009) | Amphibians |
| 118.9 | High | Watson et al. (2005) | Birds |
Discussion
Key determinants of urban species richness
Our study substantiates the overarching positive effect of patch size on biodiversity in cities, which has often been postulated based on the general validity of species-area relationships (Arrhenius 1921). A positive impact of area on urban biodiversity levels has been found previously (Faeth & Kane 1978; Pacheco & Vasconcelos 2007; Basham et al. 2010; Shanahan et al. 2011). However, many studies showed equivocal results, with other predictive variables than area found to have more explanatory power for urban species richness globally and across taxonomic groups, i.e. amount of forest vegetation and soil moisture (Clarke et al. 2008), amount of green space in 1-km surrounding (Ockinger et al. 2009), isolation (Weller & Ganzhorn 2004), proportion of impervious surfaces (Su et al. 2011) or barren ground, vegetation density, distance to buildings and vegetation patchiness (Dickman 1987). In summary, our results corroborate the validity of species-area relationships also in urban landscapes.
The effect of corridors was equally strong as for patch size, but is based on data from two cities only, even though five taxonomic groups were covered. Corridors are defined as functional habitat connecting two habitat patches. Despite the small sample size, they had a markedly stronger effect than the distance between patches (factor ‘connectivity’ in our analysis). This suggests that corridors can be much more effective in promoting urban species richness than stepping-stone habitats. Although stepping-stone habitats are often proposed as a means to increase permeability of a matrix (Saura et al. 2014), they simply decrease the distance between patches (Fahrig 2003) without providing a functional corridor. Nevertheless, they may enfold a beneficial effect on biodiversity through their provisioning of additional vegetation cover in cities. When overall biodiversity within cities is considered (Aronson et al. 20142015), the effects of area and corridors are difficult to disentangle, since corridors themselves attribute to the amount of habitat as well as to vegetation cover (Tewksbury et al. 2002). However, our study design focused on within-patch biodiversity and thus these two effects can be analysed separately. In a large-scale experimental setup (albeit not in an urban setting), controlling for the increase in area by corridors, Tewksbury et al. (2002) also found strong additional benefits of corridors for the dispersal of plants and animals. This highlights that equating the effect of corridors solely with an increase in area that they provide, could neglect a strong positive effect enfolded by their functional component. However, when drawing this conclusion, one has to consider that, despite being beneficial for species richness per se, corridors can also facilitate the spread of unintended aspects of biodiversity, such as pathogens or invasive alien species (Haddad et al. 2014).
Interestingly, only factors with a positive effect on biodiversity measures had significant summary effect size estimates. Apparently, fragmentation and edge effects are not as consistently negative in impact on urban biodiversity as often assumed, a finding also made for other ecosystems (Fahrig 2003). However, if the effect size estimate for edge-effects was not corrected using the Trim-and-Fill procedure, this factor would have been significantly negative in effect. This indicates that positive effects of edges, despite being predictable (Ries et al. 2004), still remain unpublished. These variable responses to edges, fragmentation and connectivity (Fischer & Lindenmayer 2007) emphasise the much stronger effect of area for biodiversity (Fahrig 2003).
Apart from water cover and management intensity, all other significant effects on species richness were caused by biotic factors: herbaceous, shrub, tree or total vegetation density, cover or structure as well as the proportion of green spaces in the surrounding area. It is interesting that all but one of the local and biotic factors had positive effect estimates on species richness and eight of 10 of these factors were significant. This propensity is confirmed by the generalised data set, which showed a higher likelihood for biotic, local and management variables to affect species richness. The strong and positive effects of most of the vegetation factors could be utilised in conservation practice to enhance species richness in those urban landscapes where extending the size of green spaces is not an option. If such an approach is selected, it could be complemented by biodiversity friendly management (Shwartz et al. 2013). Interestingly, plant species number (factor ‘vegetation richness’) and tree density were the only non-significant vegetation factors, whereas most factors of vegetation structure had a significant positive effect. When we tested for taxon-specific effects, it turned out that these effects were mainly driven by birds (vegetation structure, herb cover, tree cover, tree structure) and insects (vegetation structure, herb cover). This suggests that a heterogenic vegetation structure would be ideal to promote biodiversity in urban green spaces.
How much area is needed for maintaining high levels of urban biodiversity?
Despite measuring strong effects of patch size on biodiversity in the random-effects model, these results do not inform the amount of area required to maintain high levels of biodiversity. We addressed this question by summarising threshold values for species richness of local habitat patches as well as by looking at the total proportion of vegetation cover in cities at a landscape level.
Species richness declined rapidly at an average of ca. 27 ha, but with strong variation which cannot be attributed to the different taxa under investigation (Kruskal–Wallis P > 0.7), rather it reflects the conservation value authors assigned a priori to species or species groups within urban landscapes. Smaller areas are often considered sufficient, if the declared goal is to minimise a loss of urban-adapter species (Germaine et al. 1998; Drinnan 2005; Ehrenfeld 2008), resulting in an emphasis of thresholds of small habitat patches at an average of 4.4 ha. By contrast, larger areas are usually considered necessary if the conservation of urban avoider species was the objective (Drinnan 2005) and an average of 53.3 ha or more were necessary to conserve also threatened or urban-avoider species. Determination of a threshold for patch area is thus only possible if the conservation objective is clearly defined.
The city-wide vegetation cover is commonly assessed to derive conclusion on a city's species richness or its capacity therefore. Vegetation cover below 10% has been found to cause rapid declines in species richness (Radford et al. 2005), and some authors (e.g. Andrén 1994) suggest that a landscape-level threshold of 20–30% of a specific habitat has to remain to prevent the combined effects of habitat loss and fragmentation to exacerbate the loss of species or populations (Hedblom & Soderstrom 2010). These threshold values have not been identified specifically for urban landscapes, but recently Aronson et al. (20142015) concluded that intact vegetation cover is the strongest explanatory variable for variation in species density among cities worldwide. Our results confirm that the proportion of green surroundings as well as many other biotic habitat categories have a significant positive effect on urban species richness. Green-space cover of European cities, for example, varies between 2 and 46% (Fuller & Gaston 2009) and indicates that the amount of green space may not always be suitable to maintain high levels of biodiversity, even if a 10% threshold at the landscape level is assumed sufficient.
It is important to keep in mind that this landscape level approach generalises the landscape and assumes vegetation to be the major determinant of biodiversity. Important for the long-term persistence of a species are also its population dynamics, genetic diversity, adaptability or interspecific interactions (Fischer & Lindenmayer 2007). To maintain populations of individual species, i.e. to ensure reproductive success, even larger areas may be needed than those predicted by thresholds of patch area or city-wide vegetation cover derived from species richness alone (Smith 2007).
A multi-species approach for future urban planning
More than half of the world's inhabitants currently live in cities, and global urbanisation forecasts predict the urban population to reach 70% by 2050 (United Nations 2012). Expressed in terms of growth, this increase in the urban population translates to a doubling of the current extent of urban areas by 2030, with the fastest growing cities being located in Asia and Africa (Seto et al. 2011). Given that most of the studies on urban biodiversity were conducted in Europe and North America, inferences for those regions which will face the largest future increase in the urban landscape have to be drawn with caution. Generally, due to their frequent alterations, designing and planning urban landscapes to maximise biodiversity levels is a challenge. Nonetheless, if urban planning focuses on preserving also large areas of habitats (> 50 ha) and a network of corridors between them, cities may even develop into refuges for species conservation. Designing future cities in a way that increases biodiversity can create a more sustainable setting for urban biodiversity and the ecosystem services they provide (Son et al. 2012). Losing species in an urban landscape might seem less substantial than losing it in natural or near-natural habitats. However, urban biodiversity delivers many services to humans, and the majority of nature-citizen interactions take place within cities (Luck et al. 2011). A benefit for mental health was recently attributed to natural areas with less management, e.g. less mowing, (Clark et al. 2014), as well as increases of human health through benefits in the regulation of the immune system by contact with microbiota of green spaces (Rook 2013). This highlights the strong interest and need for nature in the city. Urban nature reserves could be established to help cities create space for biodiversity to flourish and to help citizens to enjoy nature and find a place for contemplation and relaxation (Niemela 1999).
Future research needs
Our results are unambiguous in identifying area, corridors and vegetation factors as key determinants for species richness. Nonetheless, many ecological questions could not be fully answered. The currently available studies on factors influencing urban biodiversity are strongly biased towards ornithological studies in temperate regions. It is thus obvious that the outcomes of our meta-analysis are biased towards the habitat requirements of temperate bird species. Therefore,studies on urban biodiversity in tropical regions focusing on other taxa than birds are needed. Our taxon-specific analysis suggests differential responses of some taxa, while for many taxa the number of studies was too low. Furthermore, there are probably multiple sources of heterogeneity in the data set, such as the type of sample sites (e.g. green spaces, remnants, cemeteries, random sites etc.), biogeographical locations of the cities under investigation, amount of variation allowed in sample sites (e.g. controlled for individual factors), or simple differences in measurement procedure. To obtain more information on the importance of these factors, a greater data set is needed or a more standardised approach of urban ecological studies. Also, with an adequately sized data set, other interesting ecological questions could be answered, e.g. regarding the differences in invasive alien and native species in their response to the urban landscape (Aronson et al. 2015), or the best way to design a city, i.e. compact vs. sprawling (Sushinsky et al. 2013).
Considering the commonness of invasive species in cities (Loram et al. 2008) it also becomes apparent that it will be important to develop more informative measures of biodiversity. Species richness and standard alpha diversity indices do not distinguish among species, but from a conservation point of view the maintenance of threatened species in a city is more valuable than the occurrence of non-native invasive species. It would thus be useful to develop an index that considers the conservation ‘quality’ of species rather than their quantity and abundances alone.
Apart from these overarching questions, the importance of functional corridors needs to be tested more thoroughly in the urban landscape. It would be particularly important to study biodiversity and the occurrence of rare or threatened species in corridors compared with larger habitat patches. Thresholds of patch area are certainly useful for urban planning, but there needs to be a clearer definition of conservation goals for these purposes.
Authorship
All authors designed the study, JB collected data, performed the meta-analysis and wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Acknowledgments
We thank all authors who contributed data to this meta-analysis and two anonymous reviewers for useful comments on a previous draft of the manuscript. All authors are members of the interdisciplinary graduate school “Cooperation of Science and Jurisprudence in Improving Development and Use of Standards for Environmental Protection – Strategies for Risk Assessment and Management” funded by the German Science Foundation (DFG, GRK 1319).




