Neuroanatomy of dyslexia: An allometric approach
Abstract
Despite evidence for a difference in total brain volume between dyslexic and good readers, no previous neuroimaging study examined differences in allometric scaling (i.e. differences in the relationship between regional and total brain volumes) between dyslexic and good readers. The present study aims to fill this gap by testing differences in allometric scaling and regional brain volume differences in dyslexic and good readers. Object-based morphometry analysis was used to determine grey and white matter volumes of the four lobes, the cerebellum and limbic structures in 130 dyslexic and 106 good readers aged 8–14 years. Data were collected across three countries (France, Poland and Germany). Three methodological approaches were used as follows: principal component analysis (PCA), linear regression and multiple-group confirmatory factor analysis (MGCFA). Difference in total brain volume between good and dyslexic readers was Cohen's d = 0.39. We found no difference in allometric scaling, nor in regional brain volume between dyslexic and good readers. Results of our three methodological approaches (PCA, linear regression and MGCFA) were consistent. This study provides evidence for total brain volume differences between dyslexic and control children, but no evidence for differences in the volumes of the four lobes, the cerebellum or limbic structures, once allometry is taken into account. It also finds no evidence for a difference in allometric relationships between the groups. We highlight the methodological interest of the MGCFA approach to investigate such research issues.
Abbreviations
-
- ADHD
-
- Attention Hyperactivity Deficit Disorder
-
- IQ
-
- Intelligence Quotient
-
- MGCFA
-
- Multiple-group Confirmatory Factor Analysis
-
- MRI
-
- Magnetic Resonance Imaging
-
- OBM
-
- Object-Based Morphometry
-
- PCA
-
- Principal Component Analysis
-
- VBM
-
- Voxel-Based Morphometry
-
- WISC
-
- Wechsler Intelligence Scale for Children
1 INTRODUCTION
Dyslexia is characterized by persistent difficulties in learning the written language code that cannot be accounted for by another disorder and by lack of education or sensory deficits (American Psychiatric Association, 2013). Like other neurodevelopmental disorders, the aetiology of dyslexia involves complex interactions between multiple genetic and environmental risk factors (Bishop, 2015; Mascheretti et al., 2013; Oliver & Plomin, 2007). At the cognitive level, deficits in phonological processes are thought to be central to the development of dyslexia in most cases (Ramus et al., 2003; Saksida et al., 2016).
There is a vast literature on the neuroanatomical correlates of dyslexia, most of them relying on voxel-based morphometry (VBM) and reporting regional brain volume differences between dyslexic and good readers. However, meta-analyses of those studies reported remarkably few consistent findings after proper statistical corrections (Eckert, Berninger, Vaden, Gebregziabher, & Tsu, 2016; Linkersdörfer, Lonnemann, Lindberg, Hasselhorn, & Fiebach, 2012; Richlan, Kronbichler, & Wimmer, 2013). Indeed, the validity of these results has been questioned due to several methodological limitations (Ramus, Altarelli, Jednoróg, Zhao, & Scotto di Covella, 2018). The problem of multiple testing is particularly acute in cluster-based VBM studies with small samples, as it is the case for most studies on dyslexia. Comparatively, neuroanatomical studies using predefined segmentations, which reduce the number of multiple comparisons (known as Object-Based Morphometry [OBM] (Mangin et al., 2004)) of tissues and lobes, are less concerned by false-positive results (Scarpazza, Sartori, Simone, & Mechelli, 2013). Ramus and colleagues (2018) argued that the only robust result emerging from this literature was a smaller total brain volume in dyslexics compared to good readers (Cohen's d = −0.58 [CI-95%: −0.32; −0.85]) (Ramus et al., 2018). Yet, these global differences are only taken into account when investigating of regional differences in about half of the studies. This is particularly problematic when examining cerebral differences between groups with differing total brain volumes. For instance, previous findings suggest that sex differences and sex by age interactions in local brain volumes practically disappeared when taking into account brain size (Jäncke, Mérillat, Liem, & Hänggi, 2015; Sanchis-Segura et al., 2019). Brain size should thus be considered when examining volumetric differences across brain regions, as it accounts for more interindividual differences than sex or age.
Given that dyslexic and normal readers are thought to differ in terms of total brain volumes, the question now arises whether they also differ in the relationship between any given regional volume and total brain volume. This scaling relationship between a regional volume (y) and total brain volume (x) can be investigated with the commonly used power equation y = b xa (Finlay, Darlington, & Nicastro, 2001). If a = 1, the volumes x and y are directly proportional (isometric). If a ≠ 1, they are not (they are allometric). The allometric scaling coefficient “a” can be easily estimated in a linear regression using a log–log transformation {log(y) = a log(x) + log(b)}. When the regional volume grows disproportionately with total brain volume (a > 1), this is called positive allometry or hyperallometry. When the regional volume grows more slowly than total brain volume (a < 1), this is called negative allometry or hypoallometry. In many cases, “a” has been shown to differ from 1, non-linear relationships being the rule more than the exception between regional and total brain volumes (Jäncke et al., 2015; de Jong et al., 2017; Reardon et al., 2018). Therefore, linearly adjusting for total brain volume when comparing regional volumes is theoretically inappropriate. Indeed, recent studies have shown that omitting brain allometry can lead to overestimating or underestimating regional volumetric group differences and recommend that studies adjust for total brain volume differences using allometric scaling (Germanaud et al., 2014; Jäncke, Liem, & Merillat, 2019; de Jong et al., 2017; Mankiw et al., 2017; Reardon et al., 2016; Sanchis-Segura et al., 2019).
In the present study, we examined differences in allometric scaling and regional brain volume differences in dyslexic and good readers. Our analyses focused on 24 regional brain volumes (two hemispheres × six regions (frontal, temporal, parietal, cerebellum, limbic and occipital) × two tissue compartments (grey and white matter)) of 130 dyslexic and 106 good readers. Data were collected across three countries (France, Poland and Germany). Since several sex differences in clinical and neuroanatomical characteristics of dyslexic readers have previously been reported (Altarelli et al., 2014; Arnett et al., 2017; Evans, Flowers, Napoliello, & Eden, 2014), our analyses were performed on the whole sample as well as in boys and girls separately. We addressed the following research questions: Do allometric scaling and regional brain volumes differ between dyslexic and good readers? Do these observed differences (if any) between dyslexic and good readers depend on sex? We had no specific a priori hypotheses.
Three methodological approaches were applied to address our research questions (principal component analysis (PCA), linear regression and multiple-group confirmatory factor analysis (MGCFA)). In theory, MGCFA is more advantageous than PCA since it tests regional allometric scaling group differences as well as global and regional volumetric group differences (Jolicoeur, 1963; Toro et al., 2009), and unlike linear regression, MGCFA also considers the mutual relationship between regional brain structures and evaluates global group differences in allometric scaling (de Jong et al., 2017; Toro et al., 2009). However, since MGCFA is rarely conducted in this literature, the present study compared the results of the three approaches to determine whether the results of the less commonly used MGCFA were consistent with the results of the classical linear regression and PCA approaches.
2 METHODS
2.1 Participants
Dyslexic and good readers were recruited in three countries (France, Poland and Germany). Reading accuracy and speed were assessed using different language-appropriate standardized reading tests (see Jednoróg et al., 2015; Płoński et al., 2017 for details). Participants came from diverse social backgrounds and had at least one and a half year of formal reading instruction to differentiate serious problems in reading acquisition from early delays that are not always persistent. They were recruited based on the following criteria: age was between 8.5 and 13.7 years old, intelligence quotient (IQ) higher than 85 or an age-appropriate scaled score of at least 7 on the Wechsler Intelligence Scale for Children (WISC) Block Design and 6 on the WISC Similarities, no spoken language disorders, no formal diagnosis of attention hyperactivity deficit disorder (ADHD), and no reported hearing, sight or other neurological problems.
Dyslexic readers were either identified in school, through clinics, or were specifically requesting a clinical assessment of their reading problems. Most of the studied children already had a clinical diagnosis of dyslexia, and all were screened for inattention/hyperactivity symptoms and language disorders. The inclusion criterion for dyslexic readers was defined as more than 1.5 SD below grade level on different appropriate standardized tests of reading, whereas good readers were less than 0.85 SD below grade level.
All studies were approved by local ethics committees (CPP Bicêtre in France; Medical University of Warsaw in Poland; Uniklinik RWTH Aachen in Germany) in compliance with the Code of Ethics of the World Medical Association—Declaration of Helsinki. The children and their parents gave informed written consent to participate in the study.
Together, 130 dyslexic (56 girls) and 106 good readers (55 girls) were included in the study.
2.2 Imaging procedure
High-resolution T1w images were acquired in five different studies:
2.2.1 French group (studies 1 & 2)
Whole brain T1w images were acquired for the total sample on the same 3 Tesla (3T) Siemens Trio Tim MRI platform.
Study 1 (13 good and 11 dyslexic readers): The MRI had a 12-channel head coil with the following parameters: acquisition matrix: 256 × 256 × 176, TR = 2,300 ms, TE = 4.18 ms, flip angle = 9°, FOV = 256 mm and voxel size: 1 × 1 × 1 mm.
Study 2 (32 good and 28 dyslexic readers): MRI platform used a 32-channel head coil with the following parameters: acquisition matrix = 230 × 230 × 202, TR = 2,300 ms, TE = 3.05 ms, flip angle = 9°, FOV = 230 mm and voxel size = 0.9 × 0.9 × 0.9 mm.
2.2.2 German group (studies 3 & 4)
Study 3 (10 good and 35 dyslexic readers): Whole brain images were acquired on a 3T Siemens Trio Tim scanner using a standard birdcage head coil. T1w images had the following specifications: acquisition matrix: 256 × 256 × 176, TR = 1,900 ms, TE = 2.52 ms, flip angle = 9°, FOV = 256 mm and voxel size: 1 × 1 × 1 mm.
Study 4 (16 good and 10 dyslexic readers): Whole brain images were acquired on a 1.5T Siemens Avanto scanner using a standard birdcage head coil with the following parameters: acquisition matrix: 256 × 256 × 170, TR = 2,200 ms, TE = 3.93 ms, flip angle = 15°, FOV = 256 mm and voxel size: 1 × 1 × 1 mm.
2.2.3 Polish group (Study 5)
Study 5 (35 good and 46 dyslexic readers): Whole brain images were acquired for the total sample on a 1.5T Siemens Avanto platform equipped with 32-channel phased array head coil. T1w images had the following specifications: acquisition matrix: 256 × 256 × 192, TR = 1,720 ms, TE = 2.92 ms, flip angle = 9°, FOV = 256 and voxel size 1 × 1 × 1 mm.
2.3 Morphometric analysis
Image processing and analyses were carried out using SPM8 (http://ww.fil.ion.ucl.ac.uk/spm/) run in MATLAB7.11 (Mathworks, Sherborn, MA). T1w images were automatically segmented into different tissue classes (grey matter, white matter and nonbrain [cerebrospinal fluid, skull]), using the “New Segmentation” option in SPM8 (Ashburner & Friston, 2005). Tissue probability maps were taken from a customized paediatric brain generated using Template-O-Matic toolbox (http://dbm.neuro.uni-jena.de/software/tom/). The Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm was then used to create a study-specific template (Ashburner, 2007; Marchewka et al., 2014). This step was followed by an affine registration of the GM maps to the Montreal Neurological Institute (MNI) space, scaling the GM probability values with the Jacobian determinants to ensure that the total signal in each tissue class remained constant (i.e. “modulation”) (Ashburner & Friston, 2000). Binary masks for the main lobes (frontal, temporal, parietal, cerebellum, limbic and occipital) were derived from a cerebral lobe atlas defined in the MNI template space and published by Fonov et al. (Fonov et al., 2011).
2.4 Statistical analysis
Three different methodological approaches were used to investigate differences in allometric scaling and regional brain volume differences in dyslexic and good readers. The project was preregistered on OSF (https://osf.io/t2v5h/). All brain volumes were log-transformed because the relation between a regional volume and the total volume is not linear (de Jong et al., 2017).
2.5 ANOVA
First, we performed an ANOVA to examine the main effects of group (dyslexic and good readers), sex (girls and boys), hemisphere (left and right), tissue (grey matter and white matter), brain regions (frontal, temporal, parietal, cerebellum, limbic and occipital) and the interactions between group, sex and the other variables (hemisphere, tissue, lobe). Group and sex were between-subject variables, and hemisphere, tissue and lobe were within-subjects variables.
2.6 Linear regression models
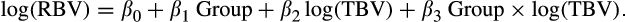 (1)
(1)Linear regression models were performed using SAS 9.2 software (SAS Institute, Cary, NC). To reduce type 1 error inflation due to multiple testing, we set the alpha threshold at 0.002 [0.05/24, 24 being the number of observed brain regions]. We also tested the interaction between sex and group (dyslexic and good readers) in linear regression models. Moreover, we performed linear regression models in dyslexic versus good readers in girls and boys separately, to follow the same analytic plan as the MGCFA.
2.7 Principal component analysis
The second method used to investigate differences in allometric scaling between groups was PCA. A PCA of the log-transformed regional brain volumes was performed separately in dyslexics and good readers. The loadings of the first principal components of the groups were considered as the coordinates of two vectors (Krzanowski, 1979). The angle between these two vectors served to test global differences in allometric scaling across groups (Jolicoeur, 1963). PCA and permutation tests (10,000 iterations) of the angle between vectors were performed by running the psych package in R software (Revelle, 2019). The statistical significance of the difference in angle was estimated by comparing it to a null distribution obtained from 10,000 random permutations. In each of these permutations, two groups of subjects were created with sample sizes corresponding to the number of dyslexic and control participants, independently of their diagnostic status. A p-value was estimated by counting the proportion of random permutations where the angle difference was more extreme than the one observed in the original groups (Toro et al., 2009). If the proportion was smaller than alpha = 0.05, then there was a significant group difference and follow-up analyses were conducted with a post hoc examination of the individual exponents. Differences in allometric scaling in dyslexic versus good readers in girls and boys were compared separately, and the interaction between sex and group (dyslexic and good readers) was tested.
2.8 Multiple-group confirmatory factor analysis
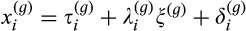 (2)
(2)The MGCFA starts with the determination of a well-fitting multi-group baseline model and continues by testing, in a hierarchical fashion, the metric invariance (i.e. equality of factor loadings) and the scalar invariance (i.e. equality of intercepts) between groups. To do this, some parameters are constrained to be the same across groups and this model is compared to a model that is unconstrained on these parameters, in terms of fit, by computing a chi-square difference test. Metric invariance is tested by constraining the loadings of the factor across groups (intercept being unconstrained) (Steenkamp & Baumgartner, 1998). If a global test (comparison of the fit of a model with all loadings constrained to be equal across groups to an unconstrained model) indicates that the metric invariance hypothesis is rejected, then each regional brain volume is examined one after the other (comparison of the fit of a model with all loadings constrained to be equal across groups with that of a model with the loading of one regional brain volume unconstrained). Similarly, scalar invariance is tested by constraining the intercepts across groups and comparing this model with an unconstrained model (loadings are also constrained if metric invariance was found in the previous step) (Davidov et al., 2014; Hong, Malik, & Lee, 2003; Steenkamp & Baumgartner, 1998). Again, if this global test indicates that the scalar invariance hypothesis is rejected, then each regional brain volume is examined one after the other (comparison of the fit of a model with all intercepts constrained to be equal across groups to a model with the intercept of one regional brain volume unconstrained).
MGCFA models were applied using procedures implemented in Mplus (Schnabel, Kelava, Vijver, & Seifert, 2015). The maximum likelihood estimation with robust standard errors (MLMV) estimator was used for parameter estimation (Asparouhov & Muthén, 2010). MGCFA models were also performed on four groups: dyslexic boys, dyslexic girls, non-dyslexic boys and non-dyslexic girls. We additionally compared dyslexic versus good readers in girls and boys separately and in non-dyslexic girls and dyslexic boys versus dyslexic girls and non-dyslexic boys to identify an interaction between sex and group (dyslexic and good readers). The sample sizes were sufficiently large to perform MGCFA on these subgroups (Mundfrom, Shaw, & Ke, 2005).
In order to evaluate the concordance between linear regression and MGCFA approaches, we estimated, using both methods, the slope and intercept differences between groups in the 24 regional brain volumes and then computed a Spearman rank order correlation.
3 RESULTS
3.1 ANOVA
The analysis of variance showed a main effect of group (F(1, 5,400) = 10.5, p-value = .001, η2 = 0.1%; good > dyslexic readers), sex (F(1, 5,400) = 45.6, p-value < 0.001, η2 = 0.4%; boys > girls), hemisphere (F(1, 5,400) = 5.5, p-value = .019, η2 < 0.1%; left > right hemisphere), tissue (F(1, 5,400) = 27,832.7, p-value < 0.001, η2 = 31.6%; grey > white matter) and brain regions (F(5, 5,400) = 10,542.5, p-value < 0.001, η2 = 59.8%; frontal > temporal>parietal > occipital>cerebellum > limbic region). There was no significant interaction between group and sex and hemisphere, tissue and brain regions.
3.2 Linear regression
In linear regression models, we found no difference in allometric scaling (Table 1) nor in regional brain volume (Table 2) between dyslexic and good readers, even in the subsamples of boys and girls or when the interaction between sex and group was considered. In Figure S1, we present, as an example, the relationship between the left temporal lobe grey matter and the total brain volumes in dyslexic and good readers. Together, these results indicate no differences in allometric scaling nor regional brain volumes between dyslexic and good readers.
| Regional brain volumesa | Group differences in the total sample | Group differences in girls | Group differences in boys | Group × sex interaction in the total sample | ||||
|---|---|---|---|---|---|---|---|---|
| N = 236 (N = 106 good readers & N = 130 dyslexic readers) | N = 111 (N = 55 good readers & N = 56 in dyslexic readers) | N = 125 (N = 51 good readers & N = 74 dyslexic readers) | N = 236 (N = 55 non-dyslexic girls and N = 74 dyslexic boys & N = 51 non-dyslexic boys and N = 56 dyslexic girls) | |||||
| Δ slopes (good readers – dyslexic readers) | p-value | Δ slopes (good readers – dyslexic readers) | p-value | Δ slopes (good readers – dyslexic readers) | p-value | Δ slopes (good readers girls and non-dyslexic boys) – (non-dyslexic boys and dyslexic girls) | p-value | |
| Left Cerebellum (white matter) | −0.01 | .9321 | −0.09 | .5710 | 0.19 | .2190 | −0.12 | .2303 |
| Right Cerebellum (white matter) | 0.01 | .8913 | 0.00 | .9906 | 0.14 | .3844 | −0.05 | .5962 |
| Left Cerebellum (grey matter) | 0.04 | .6363 | −0.03 | .7908 | 0.20 | .1685 | −0.13 | .1398 |
| Right Cerebellum (grey matter) | 0.02 | .8534 | −0.14 | .2936 | 0.21 | .1664 | −0.17 | .0594 |
| Left Frontal lobe (white matter) | −0.04 | .4846 | −0.01 | .9324 | −0.08 | .3788 | 0.04 | .4964 |
| Right Frontal lobe (white matter) | 0.01 | .9203 | 0.06 | .4698 | −0.05 | .5367 | 0.04 | .4705 |
| Left Frontal lobe (grey matter) | −0.03 | .5841 | 0.05 | .5783 | −0.10 | .2879 | 0.09 | .1181 |
| Right Frontal lobe (grey matter) | −0.02 | .7229 | 0.04 | .6349 | −0.03 | .6943 | 0.05 | .3627 |
| Left Limbic structures (white matter) | 0.02 | .7145 | −0.03 | .6775 | −0.01 | .8736 | −0.02 | .6943 |
| Right Limbic structures (white matter) | 0.05 | .3807 | 0.02 | .7742 | 0.02 | .8370 | −0.04 | .4838 |
| Left Limbic structures (grey matter) | −0.03 | .4952 | −0.11 | .1367 | 0.11 | .1490 | −0.11 | .0195 |
| Right Limbic structures (grey matter) | −0.02 | .7115 | −0.06 | .4848 | 0.08 | .2907 | −0.07 | .1412 |
| Left Occipital lobe (white matter) | 0.01 | .9092 | 0.15 | .1910 | −0.13 | .3012 | 0.12 | .1058 |
| Right Occipital lobe (white matter) | −0.05 | .5394 | −0.01 | .9051 | −0.10 | .4165 | 0.05 | .5209 |
| Left Occipital lobe (grey matter) | 0.04 | .5633 | 0.11 | .3242 | −0.12 | .2841 | 0.07 | .3343 |
| Right Occipital lobe (grey matter) | 0.05 | .4916 | 0.08 | .4025 | 0.00 | .9932 | 0.01 | .9159 |
| Left Parietal lobe (white matter) | −0.06 | .3291 | 0.04 | .6554 | −0.14 | .1408 | 0.09 | .1203 |
| Right Parietal lobe (white matter) | 0.00 | .9790 | 0.06 | .5013 | −0.05 | .5984 | 0.09 | .1149 |
| Left Parietal lobe (grey matter) | −0.02 | .6889 | −0.01 | .8835 | −0.08 | .4657 | 0.06 | .2952 |
| Right Parietal lobe (grey matter) | −0.04 | .5342 | −0.06 | .5216 | −0.01 | .9250 | 0.03 | .5826 |
| Left Temporal lobe (white matter) | 0.04 | .4801 | −0.03 | .6841 | 0.03 | .7414 | −0.03 | .5343 |
| Right Temporal lobe (white matter) | 0.02 | .6550 | −0.04 | .6511 | 0.01 | .9496 | −0.03 | .6205 |
| Left Temporal lobe (grey matter) | 0.01 | .9295 | −0.03 | .7684 | 0.05 | .5542 | −0.05 | .3407 |
| Right Temporal lobe (grey matter) | 0.01 | .8715 | −0.06 | .5175 | 0.01 | .8774 | −0.01 | .7714 |
- a All regional volumes were log-transformed.
| Regional brain volumesa | Group differences in the total sample | Group differences in girls | Group differences in boys | Group x sex interaction in the total sample | Sex differences in the total sample | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 236 (N = 106 good readers & N = 130 dyslexic readers) | N = 111 (N = 55 good readers & N = 56 dyslexic readers) | N = 125 (N = 51 good readers & N = 74 dyslexic readers) | N = 236 (N = 55 non-dyslexic girls and N = 74 dyslexic boys & N = 51 non-dyslexic boys and N = 56 dyslexic girls) | N = 236 (N = 111 girls & 125 boys) | ||||||
| Δ intercepts (good readers – dyslexic readers) | p-value | Δ intercepts (good readers – dyslexic readers) | p-value | Δ intercepts (good readers – dyslexic readers) | p-value | Δ intercepts (good readers girls and dyslexic readers boys) – (non-dyslexic boys and dyslexic girls) | p-value | Δ intercepts (girls – boys) | p-value | |
| Left Cerebellum (white matter) | 0.05 | .6444 | 0.13 | .3927 | −0.05 | .6839 | 0.09 | .3655 | 0.09 | .3839 |
| Right Cerebellum (white matter) | 0.02 | .8206 | 0.10 | .5239 | −0.07 | .6286 | 0.08 | .4302 | 0.07 | .5091 |
| Left Cerebellum (grey matter) | −0.01 | .9115 | 0.10 | .4034 | −0.06 | .6643 | 0.10 | .2360 | −0.18 | .0585 |
| Right Cerebellum (grey matter) | −0.03 | .7273 | 0.04 | .7190 | −0.04 | .7487 | 0.07 | .4178 | −0.18 | .0520 |
| Left Frontal lobe (white matter) | 0.05 | .3954 | 0.01 | .8949 | 0.04 | .6495 | −0.03 | .6077 | 0.15 | .0080 |
| Right Frontal lobe (white matter) | 0.07 | .1887 | 0.02 | .7444 | 0.07 | .3270 | −0.04 | .4698 | 0.13 | .0169 |
| Left Frontal lobe (grey matter) | −0.02 | .7355 | −0.03 | .7705 | −0.06 | .4779 | 0.01 | .9301 | 0.14 | .0249 |
| Right Frontal lobe (grey matter) | −0.01 | .8101 | 0.02 | .8467 | −0.07 | .3586 | 0.04 | .4935 | 0.10 | .1037 |
| Left Limbic structures (white matter) | 0.03 | .5658 | −0.05 | .4722 | 0.11 | .1323 | −0.09 | .0912 | −0.03 | .5919 |
| Right Limbic structures (white matter) | 0.06 | .3172 | 0.01 | .9336 | 0.13 | .1023 | −0.05 | .3317 | −0.09 | .1275 |
| Left Limbic structures (grey matter) | 0.00 | .9270 | 0.08 | .2779 | −0.05 | .4482 | 0.09 | .0731 | −0.01 | .8010 |
| Right Limbic structures (grey matter) | 0.01 | .8216 | 0.07 | .3704 | −0.04 | .5620 | 0.07 | .1574 | 0.01 | .7771 |
| Left Occipital lobe (white matter) | 0.01 | .8852 | 0.04 | .6910 | −0.01 | .9160 | 0.00 | .9646 | −0.05 | .5470 |
| Right Occipital lobe (white matter) | −0.04 | .6255 | 0.02 | .8599 | −0.06 | .6059 | 0.02 | .7711 | −0.15 | .0713 |
| Left Occipital lobe (grey matter) | 0.07 | .3305 | −0.05 | .6384 | 0.17 | .0893 | −0.11 | .1049 | 0.03 | .7089 |
| Right Occipital lobe (grey matter) | 0.04 | .5216 | 0.04 | .6612 | 0.07 | .4868 | −0.02 | .8051 | −0.08 | .2620 |
| Left Parietal lobe (white matter) | −0.02 | .7767 | 0.00 | .9785 | −0.06 | .5231 | 0.02 | .6881 | 0.07 | .2752 |
| Right Parietal lobe (white matter) | −0.07 | .2118 | −0.09 | .3069 | −0.09 | .2539 | −0.01 | .9111 | 0.10 | .1052 |
| Left Parietal lobe (grey matter) | −0.13 | .0311 | −0.17 | .0338 | −0.09 | .3049 | −0.02 | .7647 | 0.00 | .9599 |
| Right Parietal lobe (grey matter) | −0.11 | .0719 | −0.13 | .1190 | −0.12 | .1492 | 0.00 | .9805 | 0.13 | .0494 |
| Left Temporal lobe (white matter) | 0.00 | .9706 | −0.08 | .3251 | 0.09 | .2107 | −0.08 | .1103 | −0.07 | .2266 |
| Right Temporal lobe (white matter) | 0.01 | .8251 | −0.05 | .5272 | 0.09 | .2489 | −0.07 | .1669 | −0.09 | .1114 |
| Left Temporal lobe (grey matter) | 0.04 | .4490 | 0.07 | .4292 | 0.03 | .6357 | 0.02 | .7676 | −0.06 | .3259 |
| Right Temporal lobe (grey matter) | −0.06 | .2310 | −0.11 | .1584 | 0.00 | .9774 | −0.05 | .3509 | −0.06 | .2492 |
- a All regional volumes were log-transformed.
3.3 PCA
In PCA models, we found no differences in allometric scaling (Table 3) between dyslexic and good readers, even in the subsamples of boys and girls or when the interactions between sex and group were considered.
| Angle (degrees) | p-value | |
|---|---|---|
| Total sample (N = 236): good readers (N = 106) vs dyslexic readers (N = 130) | 1.45 | .92 |
| Girls (N = 111): good readers (N = 55) vs dyslexic readers (N = 56) | 2.60 | .86 |
| Boys (N = 125): good readers (N = 51) vs dyslexic readers (N = 74) | 5.14 | .17 |
| Total sample (N = 236): non-dyslexic girls (N = 55) and dyslexic boys (N = 74) vs non-dyslexic boys (N = 51) and dyslexic girls (N = 56) | 3.79 | .10 |
3.4 MGCFA
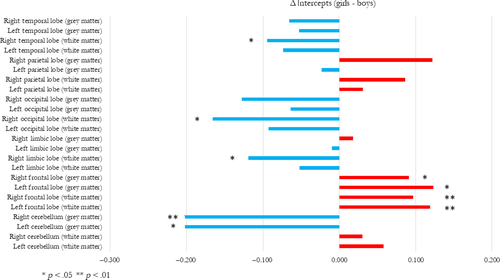
Group differences were reported with Cohen's d and uncorrected p-values. Differences in total brain volume between good and readers were 0.39 in the whole sample, 0.34 in girls, and 0.43 in boys. Differences in total brain volume between girls and boys were −0.88 in the whole sample, −0.94 in good readers, and −0.72 in dyslexic readers. No differences between dyslexic and good readers were found with the test of equality of all loadings (metric invariance), in the entire sample and each subgroup (dyslexic boys, dyslexic girls, non-dyslexic boys and non-dyslexic girls; Table 4). No differences between dyslexic and good readers were reported from the test of equality of all intercepts (scalar invariance) in the entire sample. Interestingly, the test of equality of all intercepts in the four groups was significant (p-value = 0.004; Table 5). Further analyses revealed scalar invariance in boys (good readers and dyslexic readers; p-value = 0.3), girls (p-value = 0.7), and when the interaction between sex and group was considered (p-value = 0.5). A supplementary test was performed to confirm that scalar invariance between the four groups was rejected because of sex (p-value < 0.001). These results indicated sex differences in regional brain volumes) between girls and boys, after the proper adjustment (in log–log scale) of total brain volume differences (see Figure 1). Girls had larger frontal grey and white matter (d = 0.1) volumes and a smaller cerebellar grey matter (d = −0.2) volume than boys, relative to total brain volume.
| Group differences in the total sample | Group differences in girls | Group differences in boys | Group × sex interaction in the total sample | |||||
|---|---|---|---|---|---|---|---|---|
| Regional brain volumesa | N = 236 (N = 106 good readers & N = 130 dyslexic readers)*1,2 | N = 111 (N = 55 good readers & N = 56 in dyslexic readers)*3 | N = 125 (N = 51 good readers & N = 74 dyslexic readers)*4 | N = 236 (N = 55 non-dyslexic girls and N = 74 dyslexic boys & N = 51 non-dyslexic boys and N = 56 dyslexic girls)*5 | ||||
| Δ loadings (good readers – dyslexic readers) | p-value | Δ loadings (good readers – dyslexic readers) | p-value | Δ loadings (good readers – dyslexic readers) | p-value | Δ loadings (non-dyslexic girls and dyslexic boys) – (non-dyslexic boys and dyslexic girls) | p-value | |
| Left Cerebellum (white matter) | 0.00 | ns | −0.10 | ns | 0.21 | ns | −0.16 | 0.0726 |
| Right Cerebellum (white matter) | 0.02 | ns | −0.02 | ns | 0.17 | ns | −0.10 | ns |
| Left Cerebellum (grey matter) | 0.04 | ns | −0.07 | ns | 0.20 | ns | −0.13 | ns |
| Right Cerebellum (grey matter) | 0.01 | ns | −0.17 | ns | 0.21 | ns | −0.19 | 0.0346 |
| Left Frontal lobe (white matter) | −0.04 | ns | −0.02 | ns | −0.06 | ns | 0.02 | ns |
| Right Frontal lobe (white matter) | 0.00 | ns | 0.04 | ns | −0.03 | ns | 0.03 | ns |
| Left Frontal lobe (grey matter) | 0.00 | ns | 0.07 | ns | −0.08 | ns | 0.07 | ns |
| Right Frontal lobe (grey matter) | −0.01 | ns | 0.06 | ns | −0.02 | ns | 0.04 | ns |
| Left Limbic structures (white matter) | 0.01 | ns | −0.05 | ns | 0.02 | ns | −0.03 | ns |
| Right Limbic structures (white matter) | 0.04 | ns | −0.01 | ns | 0.04 | ns | −0.02 | ns |
| Left Limbic structures (grey matter) | −0.03 | ns | −0.11 | ns | 0.12 | ns | −0.12 | 0.0041 |
| Right Limbic structures (grey matter) | −0.02 | ns | −0.08 | ns | 0.10 | ns | −0.09 | 0.0476 |
| Left Occipital lobe (white matter) | −0.01 | ns | 0.10 | ns | −0.12 | ns | 0.11 | 0.0421 |
| Right Occipital lobe (white matter) | −0.05 | ns | −0.05 | ns | −0.08 | ns | 0.02 | ns |
| Left Occipital lobe (grey matter) | 0.03 | ns | 0.08 | ns | −0.12 | ns | 0.10 | ns |
| Right Occipital lobe (grey matter) | 0.05 | ns | 0.06 | ns | 0.02 | ns | 0.02 | ns |
| Left Parietal lobe (white matter) | −0.07 | ns | 0.01 | ns | −0.13 | ns | 0.07 | ns |
| Right Parietal lobe (white matter) | −0.01 | ns | 0.03 | ns | −0.03 | ns | 0.03 | ns |
| Left Parietal lobe (grey matter) | −0.03 | ns | −0.01 | ns | −0.08 | ns | 0.03 | ns |
| Right Parietal lobe (grey matter) | −0.04 | ns | −0.07 | ns | −0.01 | ns | −0.03 | ns |
| Left Temporal lobe (white matter) | 0.03 | ns | −0.06 | ns | 0.05 | ns | −0.05 | ns |
| Right Temporal lobe (white matter) | 0.01 | ns | −0.06 | ns | 0.01 | ns | −0.04 | ns |
| Left Temporal lobe (grey matter) | 0.00 | ns | −0.03 | ns | 0.05 | ns | −0.04 | ns |
| Right Temporal lobe (grey matter) | 0.01 | ns | 0.00 | ns | 0.01 | ns | −0.01 | ns |
Note
- Δ Brain volume (good readers – dyslexic readers) = 0.415 (total sample); 0.355 (girls); 0.475 (boys). Δ Brain volume (girls – boys) = −0.876 (total sample); −0.936 (good readers); −0.716 (dyslexic readers). *1 Test of equality of all loadings between good readers and dyslexic readers: chi-square test for difference testing: value = 8.723; DF = 24; p-value = .9981. *2 Test of equality of all loadings between dyslexic boys, dyslexic girls, non-dyslexic boys and non-dyslexic girls: chi-square test for difference testing: value = 60.085; DF = 72; p-value = .8407. *3 Test of equality of all loadings between non-dyslexic and dyslexic girls: chi-square test for difference testing: value = 15.911; DF = 24; p-value = .8913. *4 Test of equality of all loadings between non-dyslexic and dyslexic boys: chi-square test for difference testing: value = 22.426; DF = 24; p-value = .5539. *5 Test of equality of loadings between non-dyslexic girls & dyslexic boys versus dyslexic girls & non-dyslexic boys: chi-square test for difference testing: value = 32.958; DF = 24; p-value = .1050.
- a All regional volumes were log-transformed. ns, non-significant.
| Regional brain volumesa | Group differences in the total sample | Group differences in girls | Group differences in boys | Group x sex interaction in the total sample | Sex differences in the total sample | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 236 (N = 106 good readers & N = 130 dyslexic readers)*1,2 | N = 111 (N = 55 good readers & N = 56 dyslexic readers)*3 | N = 125 (N = 51 good readers & N = 74 dyslexic readers)*4 | N = 236 (N = 55 non-dyslexic girls and N = 74 dyslexic boys & N = 51 non-dyslexic boys and N = 56 dyslexic girls)*5 | N = 236 (N = 111 girls & 125 boys)*6 | ||||||
| Δ intercepts (good readers – dyslexic readers) | p-value | Δ intercepts (good readers – dyslexic readers) | p-value | Δ intercepts (good readers – dyslexic readers) | p-value | Δ intercepts (good readers girls and dyslexic readers boys) – (non-dyslexic boys and dyslexic girls) | p-value | Δ intercepts (girls – boys) | p-value | |
| Left Cerebellum (white matter) | 0.07 | ns | 0.15 | ns | −0.17 | ns | 0.16 | .3882 | 0.06 | .7367 |
| Right Cerebellum (white matter) | 0.05 | ns | 0.14 | ns | −0.16 | ns | 0.15 | .4493 | 0.03 | .8613 |
| Left Cerebellum (grey matter) | 0.01 | ns | 0.12 | ns | −0.17 | ns | 0.15 | .2518 | −0.20 | .0123 |
| Right Cerebellum (grey matter) | −0.01 | ns | 0.05 | ns | −0.17 | ns | 0.11 | .1774 | −0.20 | .0096 |
| Left Frontal lobe (white matter) | 0.04 | ns | 0.01 | ns | 0.09 | ns | −0.04 | .8639 | 0.12 | .0034 |
| Right Frontal lobe (white matter) | 0.07 | ns | 0.04 | ns | 0.10 | ns | −0.03 | .2835 | 0.10 | .0070 |
| Left Frontal lobe (grey matter) | −0.02 | ns | −0.01 | ns | 0.01 | ns | −0.01 | .3441 | 0.12 | .0109 |
| Right Frontal lobe (grey matter) | −0.02 | ns | 0.03 | ns | −0.06 | ns | 0.04 | .2144 | 0.09 | .0277 |
| Left Limbic structures (white matter) | 0.03 | ns | −0.05 | ns | 0.11 | ns | −0.08 | .1106 | −0.05 | .4050 |
| Right Limbic structures (white matter) | 0.06 | ns | 0.02 | ns | 0.11 | ns | −0.05 | .1580 | −0.12 | .0339 |
| Left Limbic structures (grey matter) | 0.00 | ns | 0.07 | ns | −0.14 | ns | 0.10 | .0185 | −0.01 | .3013 |
| Right Limbic structures (grey matter) | 0.01 | ns | 0.07 | ns | −0.11 | ns | 0.09 | .0959 | 0.02 | .3136 |
| Left Occipital lobe (white matter) | 0.02 | ns | 0.08 | ns | 0.10 | ns | −0.01 | .3067 | −0.09 | .1713 |
| Right Occipital lobe (white matter) | −0.03 | ns | 0.03 | ns | 0.03 | ns | 0.00 | .3070 | −0.17 | .0312 |
| Left Occipital lobe (grey matter) | 0.08 | ns | −0.01 | ns | 0.28 | 0.0866 | −0.15 | .1531 | −0.06 | .3027 |
| Right Occipital lobe (grey matter) | 0.06 | ns | 0.07 | ns | 0.08 | ns | −0.01 | .8969 | −0.13 | .0774 |
| Left Parietal lobe (white matter) | −0.03 | ns | 0.01 | ns | 0.05 | ns | −0.02 | .2907 | 0.03 | .3103 |
| Right Parietal lobe (white matter) | −0.07 | ns | −0.08 | ns | −0.06 | ns | −0.01 | .9357 | 0.09 | .0651 |
| Left Parietal lobe (grey matter) | −0.13 | 0.0232 | −0.16 | 0.0327 | −0.03 | ns | −0.07 | .2660 | −0.02 | .3064 |
| Right Parietal lobe (grey matter) | −0.11 | ns | −0.13 | ns | −0.11 | ns | −0.01 | .8946 | 0.12 | .0708 |
| Left Temporal lobe (white matter) | 0.01 | ns | −0.08 | ns | 0.07 | ns | −0.07 | .1039 | −0.07 | .0741 |
| Right Temporal lobe (white matter) | 0.01 | ns | −0.05 | ns | 0.09 | ns | −0.07 | .1128 | −0.09 | .0316 |
| Left Temporal lobe (grey matter) | 0.04 | ns | 0.07 | ns | 0.00 | ns | 0.03 | .8233 | −0.05 | .1608 |
| Right Temporal lobe (grey matter) | −0.06 | ns | −0.11 | ns | −0.01 | ns | −0.05 | .3884 | −0.07 | .1512 |
Note
- Δ Brain volume (good readers – dyslexic readers) = 0.415 (total sample); 0.355 (girls); 0.475 (boys). Δ Brain volume (girls – boys) = −0.876 (total sample); −0.936 (good readers); −0.716 (dyslexic readers). *1 Test of equality of all thresholds between good readers and dyslexic readers: chi-square test for difference testing: value = 19.783; DF = 24; p-value = .7091. *2 Test of equality of all intercepts between dyslexic boys, dyslexic girls, non-dyslexic boys and non-dyslexic girls: chi-square test for difference testing: value = 108.107; DF = 72; p-value = .0038. *3 Test of equality of all intercepts between non-dyslexic and dyslexic girls: chi-square test for difference testing: value = 20.489; DF = 24; p-value = .6687. *4 Test of equality of all intercepts between non-dyslexic and dyslexic boys: chi-square test for difference testing: value = 26.365; DF = 24; p-value = .3349. *5 Test of equality of intercepts between non-dyslexic girls & dyslexic boys versus dyslexic girls & non-dyslexic boys: chi-square test for difference testing: value = 23.666; DF = 24; p-value = .4808. *6 Test of equality of intercepts between non-dyslexic girls & dyslexic girls and non-dyslexic boys & dyslexic boys: chi-square test for difference testing: value = 62.157; DF = 24; p-value < .001.
- a All regional volumes were log-transformed. ns, non-significant.
3.5 Comparison of the three methodological approaches
Results of the three methodological approaches (linear regression, PCA and MGCFA) were largely consistent. The ranking of Δ slopes (good readers – dyslexic readers) and Δ intercepts (good readers - dyslexic readers) between linear regression and MGCFA methods in the 24 regional brain volumes using Spearman's correlation coefficients was 0.96 and 0.97, respectively. Moreover, the PCA method was concordant with the two other approaches since it did not detect differences in allometric scaling between dyslexic and good readers (even in the subsamples of boys and girls or when the interaction between sex and group was considered).
4 DISCUSSION
In this study, we investigated differences in allometric scaling and regional brain volume between dyslexic and control children using three methodological approaches (linear regression, PCA and MGCFA). We replicated the well-established finding that total brain volume differs between dyslexic and good readers. Although we did not find differences in allometric scaling or regional brain volume between dyslexic and good readers, the present study highlights the methodological advantage of the MGCFA approach to investigate allometric scaling and volumetric differences between groups.
Since previous reports on the brain anatomy of dyslexia overlooked brain allometry, this study is the first to investigate allometric scaling differences in dyslexia. Such a study was warranted given that the differences in total brain volume between dyslexic and good readers are robustly established (Ramus et al., 2018) and were replicated in the present study (Cohen's d = 0.39; delta = 28 cm3). Omitting allometric scaling has been found to overestimate or underestimate some volumetric group differences (Mankiw et al., 2017; Reardon et al., 2016). Adjusting for total brain volume using allometry is thus crucial to reduce the odds of false-positive or false-negative results. In light of the lack of allometric scaling group differences, our results support the idea that the brain of dyslexic readers follows the same structural organization as the typical brain, despite being slightly smaller. A smaller brain volume is clearly not specific to dyslexia, as it is also found in many but not all neurodevelopmental disorders. On the contrary, a larger brain volume is reported in neurodevelopmental disorders such as fragile X syndrome (Hazlett et al., 2012) or in autism during the first 2–4 years of life (Redcay & Courchesne, 2005). Thus, the interpretation of such global differences remains unclear. One prominent hypothesis was that global brain differences in dyslexia may stem from the correlation between total brain volume and IQ, and to a lower mean IQ in children with dyslexia. However, this hypothesis can be refuted considering that total group differences remain significant after adjusting for IQ in the present data (Ramus et al., 2018). Perhaps lower brain volume is a general risk factor for neurodevelopmental disorders or perhaps it is a secondary consequence of certain types of early regional disruption.
In line with the recent literature review by Ramus et al. (2018), we did not find regional brain volume differences between dyslexic and good readers at the “lobar level.” Studies that reported differences in regional brain volumes between dyslexics and controls have mainly been conducted using a VBM approach in relatively small samples. However, in the present study, we conducted an OBM approach (i.e. predefined segmentation of tissues and lobes vs. cluster-based in VBM) and adjusted for brain allometry which are thought to reduce the rate of false positives (Smith & Nichols, 2009). Since several studies reported an increased gyrification of the brain of dyslexic readers (Im et al., 2016; Płoński et al., 2017; Williams et al., 2018), other structural measures besides volume may be associated with dyslexia and could be further investigated using an OBM approach (for instance, the folds as segmented by Morphologist (Fischer et al., 2012)).
The present study additionally highlights the methodological advantage of the MGCFA approach to investigate allometric scaling and volumetric group differences by summarizing the benefits of MGCFA over the more frequently used methodological approaches (linear regression and PCA; Table 6).
| Linear regression | PCA | MGCFA | |
|---|---|---|---|
| Global test of allometric differences | No | Yes (Table S3) | Yes (Table S4) |
| Test of allometric differences at a regional level | Yes (Table S1) | No | Yes (Table S4) |
| Global test of regional brain volume differences | No | No | Yes (Table S5) |
| Test of regional brain volumes differences | Yes (Table S2) | No | Yes (Table S5) |
| Adjusting for potential confounding variables | Yes | No | No |
| Take into account the mutual relationships between regional brain volumes | No | Yes | Yes |
Finally, we incidentally found that girls had larger frontal grey and white matter (d = 0.1) and smaller cerebellar grey matter (d = −0.2) than boys (relative to total brain volume; see Figure 1), which is largely inconsistent with the majority of adult neuroanatomical studies (Chen, Sachdev, Wen, & Anstey, 2007; Lotze et al., 2019; Ritchie et al., 2018; Ruigrok et al., 2014). To our knowledge, Lotze et al. were the only to report a larger prefrontal grey matter in women (Lotze et al., 2019) and Chen et al. were the only to report smaller cerebellar hemispheres grey matter in men (Chen et al., 2007). The study by Ritchie et al. which examined a much larger sample of 5,216 UK Biobank participants did not found sex differences in these brain regions (as well as the meta-analysis by Ruigrok et al. (2014)). Therefore, our incidental results regarding sex dimorphism in brain structure should be considered with great caution.
4.1 Strengths and limitations
The two major strengths of this study are the large sample of female and male dyslexic and good readers and the comparison of three methods (linear regression, PCA and MGCFA) used to examine volumetric group differences. However, the current study also has several limitations that must be considered when evaluating the results. First, total brain volume estimates were not identical across methods. While it remains a measured variable in the linear regressions, total brain volume is a latent variable estimated by the shared variance of lobar volumes in the MGCFA and corresponds to the first principal component in PCA, which reflects the component with the most shared and unshared variance of the lobar volumes. While estimates may differ across methods, the consistency of our findings suggests that the MGCFA and PCA are nonetheless advantageous methods to investigate overall neuroanatomical group differences in future studies.
Second, our total sample gathered participants from 5 studies conducted in 3 different countries with different languages and different school systems, although good and dyslexic readers were recruited in comparable proportions in each of these studies. Since the PCA and MGCFA cannot simultaneously examine the effect of group, sex, scanner site and language, we were not able to correct for the non-independency of data collected per scanning site nor could we investigate how different languages influence the present findings (Table 6). Future large-scale studies should nevertheless investigate the impact of different cultures and languages to obtain a more precise estimate of volumetric group differences in dyslexia.
Third, in theory, the relationship between total brain volume and each lobar volume could correspond to a more complex equation than the power function we employ. However, there is an extensive literature on brain allometry, and the power equation is widely considered as a sufficiently good fit and is the current state of the art (Sanchis-Segura et al., 2019).
Finally, the brain region segmentations used in this study were quite coarse. It remains entirely possible that allometric scaling and regional brain volume differences between dyslexic and good readers might emerge when considering smaller brain regions (e.g. superior temporal gyrus of the left temporal lobe). Of course, the smaller the brain regions considered, the more numerous they are, the higher the risk of false-positive results and the more stringent corrections for multiple tests should be. We suggest that MGCFA is a powerful approach to the study general group differences across a large number of brain regions since it allows for a global test of allometric differences, which does not necessitate correction for multiple comparisons. Our study therefore paves the way for more fine-grain investigations of regional brain volume differences in dyslexia, by taking allometry into account.
5 CONCLUSIONS
This study provides further evidence that the brain of dyslexic readers has the same structural organization than a typical brain at the “lobar” spatial resolution, despite being slightly smaller. It also emphasizes the methodological advantages of the MGCFA approach to investigate differences in allometric scaling.
ACKNOWLEDGEMENTS
This work was supported by Agence Nationale de la Recherche (grant numbers: ANR-06-NEURO-019-01, ANR-11-BSV4-014-01, ANR-17-EURE-0017 and ANR-10-IDEX-0001-02 PSL); Ecole des Neurosciences de Paris, the Bettencourt-Schueller Foundation, and Polish Ministry of Science and Higher Education (grant numbers: IP2010 015170, IP2011 020271 (Ministry of Science and Higher Education) and 2011/03/D/HS6/05584, 2016/22/E/HS6/00119 (National Science Center)); National Science Center (grant number: DEC-2011/03/D/HS6/05584); German Bundesministerium für Bildung und Forschung (grant numbers: BMBF 01GJ0613, 01GJ0614 and 01GJ0804); and Swiss National Science Foundation (grant number: 320030_135679).
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
KJ, SH, MG, MvEM, KM and FR collected the data. HP, NM, CW, DG, RT and FR analysed and interpreted the data. HP, CW, DG, RT and FR drafted the manuscript. All authors critically revised the manuscript and gave final approval of the version to be published.
ETHICAL APPROVAL
All studies were approved by regional ethics committees (CPP Bicêtre in France; Medical University of Warsaw in Poland; Uniklinik RWTH Aachen in Germany) in compliance with the Code of Ethics of the World Medical Association—Declaration of Helsinki. The children and their parents gave informed written consent to participate in the study.
Open Research
DATA AVAILABILITY STATEMENT
Anonymized data and details about preprocessing/analyses will be made available to colleagues if requested.




