The highly selective mGlu2 receptor positive allosteric modulator LY-487,379 alleviates l-DOPA-induced dyskinesia in the 6-OHDA-lesioned rat model of Parkinson's disease
Abstract
l-3,4-Dihydroxyphenylalanine (l-DOPA) is the most effective treatment for Parkinson's disease (PD), but its use over a long period is marred by motors complications such as dyskinesia. We previously demonstrated that selective metabotropic glutamate 2/3 (mGlu2/3) receptor activation with LY-354,740 alleviates dyskinesia in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned marmoset and the 6-hydroxydopamine (6-OHDA)-lesioned rat. Here, we sought to determine the role played by selective mGlu2 activation in the anti-dyskinetic effect of mGlu2/3 stimulation and have investigated the effect of the highly selective mGlu2 positive allosteric modulator LY-487,379 at alleviating established, and preventing the development of, l-DOPA-induced dyskinesia in the 6-OHDA-lesioned rat. First, dyskinetic 6-OHDA-lesioned rats were administered l-DOPA in combination with LY-487,379 (0.1, 1 and 10 mg/kg) or vehicle, and the severity of dyskinesia was determined. Second, 6-OHDA-lesioned rats were administered LY-487,379 (0.1 or 1 mg/kg), started concurrently with l-DOPA, once daily for 22 days, and dyskinesia severity was evaluated weekly for four consecutive weeks. We also assessed the effect of LY-487,379 on l-DOPA anti-parkinsonian effect. We found that acute challenges of LY-487,379 0.1 mg/kg in combination with l-DOPA, significantly diminished dyskinesia severity, by ≈54% (p < .01), when compared to vehicle. Moreover, animals treated with l-DOPA/LY-487,379 0.1 and 1 mg/kg during the dyskinesia induction phase exhibited milder dyskinesia, by ≈74% and ≈61%, respectively (both p < .01), when compared to l-DOPA/vehicle. LY-487,379 did not impair l-DOPA anti-parkinsonian activity. These results suggest that mGlu2 activation may be an effective and promising therapeutic strategy to alleviate the severity and prevent the development of dyskinesia.
Abbreviations
-
- 3-MT
-
- 3-methoxytyramine
-
- 6-OHDA
-
- 6-hydroxydopamine
-
- AIMs
-
- abnormal involuntary movements
-
- ALO
-
- axial, limbs and oro-lingual
-
- DOPAC
-
- 3,4-dihydroxyphenylacetic acid
-
- HVA
-
- homovanillic acid
-
- l-DOPA
-
- l-3,4-dihydroxyphenylalanine
-
- mGlu2
-
- metabotropic glutamate 2
-
- mGlu2/3
-
- metabotropic glutamate 2/3
-
- MPTP
-
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
-
- NMDA
-
- N-methyl-d-aspartate
-
- PAM
-
- positive allosteric modulator
-
- PD
-
- Parkinson's disease
-
- PK
-
- pharmacokinetic
1 INTRODUCTION
l-3,4-Dihydroxyphenylalanine (l-DOPA) is the main symptomatic treatment for Parkinson's disease (PD), but long-term administration induces motor complications such as dyskinesia (Fabbrini, 2007; Jankovic, 2005). Dyskinesia is a debilitating complication that affects approximately 40% of PD patients after 5 years of treatment, 95% of patients after 10 years of treatment, and negatively impacts their quality of life (Ahlskog & Muenter, 2001; Hauser, McDermott, & Messing, 2006).
Excessive glutamatergic transmission is implicated in the pathophysiology of dyskinesia (Huot, Johnston, Koprich, Fox, & Brotchie, 2013; Sgambato-Faure & Cenci, 2012). Amantadine, an N-methyl-d-aspartate (NMDA) antagonist (Blanpied, Clarke, & Johnson, 2005), was recently approved by the Food and Drug Administration for the treatment of l-DOPA-induced dyskinesia (Perez-Lloret & Rascol, 2018), following positive randomised-controlled trials (Oertel et al., 2017; Pahwa & Hauser, 2017). However, risks of tolerance over long-term administration (Thomas et al., 2004) and possible psychiatric adverse effects (Postma & Van Tilburg, 1975) limit its therapeutic potential.
Metabotropic glutamate 2 (mGlu2) receptors regulate glutamate transmission and, upon activation, diminish glutamate release (Crupi, Impellizzeri, & Cuzzocrea, 2019). As such, mGlu2 activation could act in a way similar to NMDA blockade, suggesting that it could elicit an anti-dyskinetic effect. Accordingly, we have recently shown that orthosteric activation of metabotropic glutamate 2/3 (mGlu2/3) receptors with LY-354,740 reduces l-DOPA-induced dyskinesia in both the 6-hydroxydopamine (6-OHDA)-lesioned rat and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned marmoset (Frouni et al., 2019). In addition, LY-354,740 attenuated the development of dyskinesia in the context of a de novo study, that is, when begun concurrently with l-DOPA treatment (Frouni et al., 2019). However, LY-354,740 does not allow to separate an mGlu2-mediated from an mGlu3-mediated effect, as it is only modestly selective for mGlu2 over mGlu3 receptors (Monn et al., 1997).
Here, we investigated the contribution of mGlu2 receptors to the anti-dyskinetic benefit achieved through mGlu2/3 activation. To this end, we have assessed the effect of LY-487,379 (also known as (N-(4-(2-methoxyphenoxy)-phenyl-N-(2,2,2-trifluoroethylsulfonyl)-pyrid-3-ylmethylamine), a highly selective mGlu2 positive allosteric modulator (PAM) that does not interact with off-target receptors (Johnson et al., 2003), on the expression and development of dyskinesia in the 6-OHDA-lesioned rat.
2 MATERIALS AND METHODS
2.1 Animals
Adult female Sprague-Dawley rats (250–275 g; Charles River) were housed in groups of three under conditions of controlled temperature (21 ± 1°C), humidity (55%) and light (12-hr light/dark cycle, lights on at 7.00), with unrestricted access to food and water. Upon arrival, rats were left undisturbed for 1 week to acclimatise. Animal care and experimental procedures were carried in agreement with a protocol approved by the Montreal Neurological Institute Animal Care Committee, in accordance with the guidelines established by the Canadian Council on Animal Care.
2.2 Unilateral 6-OHDA lesion
6-OHDA lesion was performed as previously described (Frouni et al., 2019, 2018). Briefly, rats were pre-treated with pargyline (5 mg/kg sub-cutaneously [s.c.]; MilliporeSigma) and desipramine (10 mg/kg s.c.; MilliporeSigma), to prevent damage to noradrenergic neurons (Ungerstedt, 1968). Thirty minutes later, they were anaesthetised using isoflurane (2%–4%; MilliporeSigma) in 100% oxygen (1 L/min) and placed into a stereotaxic frame (David Kopf Instruments) with the incisor bar set 3.3 mm below ear bars (Huot et al., 2015). Then, rats were injected with 2.5 μl of 6-OHDA hydrobromide (7 μg/μl in 0.02% ascorbic acid dissolved in 0.9% NaCl; MilliporeSigma) in the right medial forebrain bundle at the following co-ordinates, according to the Paxinos and Watson, (2017) rat brain Atlas: (antero-posterior:–2.8, medio-lateral:–2.0, dorso-ventral:–9.0) relative to Bregma, using a 10-μl Hamilton Syringe (MilliporeSigma). 6-OHDA was injected at a rate of 0.5 μl/min, and the syringe was left in place for 5 min after injection before being drawn back. At the end of the surgery, rats received s.c. injection of carprofen (10 mg/kg) and 0.9% NaCl (10 ml), to minimise post-surgical pain and avoid dehydration.
2.3 Assessment of parkinsonism
The degree of parkinsonism was assessed using the cylinder test 3 weeks after surgery, for both acute challenge and de novo experiments (Schallert, 2000; Schallert & Tillerson, 2000; Frouni et al., 2018). The cylinder test was performed in a transparent cylinder (14 cm diameter × 28 cm height) for 10 min during which animals were recorded for post hoc behavioural analysis whereby the number of rears on the cylinder wall would be counted by a blinded rater. Briefly, during a rear, the first limb to contact the wall was scored as an independent wall placement for that limb. If a subsequent placement of the other limb on the wall occurred while the initial placement was maintained, a score of “bilateral” was attributed. A simultaneous placement of both forepaws on the walls was also scored as a “bilateral” movement. Only animals exhibiting preferential use of the un-lesioned forepaw in ≥70% of the rears, which is indicative of ≥88% striatal dopamine depletion, were selected to undergo further testing (Schallert et al., 2000).
2.4 ALO AIMs induction and assessment
Axial, limbs and oro-lingual (ALO) abnormal involuntary movements (AIMs) were scored according to validated rating scales (Cenci, 2007). ALO AIMs were rated by an observer blinded to treatment, according to scales encompassing both time-based, that is, “duration” and severity-based, that is, “amplitude” assessment of abnormal movements. ALO AIMs were rated for 2 min, starting at baseline and then every 20 min for 180 min. ALO AIMs duration was rated according to the following scale: 0 = no dyskinesia; 1 = occasional signs of dyskinesia, present for less than 50% of the observation period; 2 = frequent signs of dyskinesia, present for more than 50% of the observation period; 3 = dyskinesia present during the entire observation period, but suppressible by external stimuli and 4 = continuous dyskinesia not suppressible by external stimuli.
Axial AIMs amplitude was rated according to the following scale: 1 = sustained deviation of the head and neck at ~30° angle; 2 = sustained deviation of the head and neck at an angle between 30° and 60°; 3 = sustained twisting of the head, neck and upper trunk at an angle between 60°and 90°; and 4 = sustained twisting of the head, neck and trunk at an angle greater than 90°, causing the rat to lose balance from a bipedal position. Limbs AIMs amplitude was rated according to the following scale: 1 = tiny movements of the paw around a fixed position; 2 = movements leading to a visible displacement of the whole limb; 3 = large displacement of the whole limb with visible contraction of shoulder muscles and 4 = vigorous limb displacement of maximal amplitude, with concomitant contraction of shoulder and extensor muscles. Oro-lingual AIMs amplitude was rated according to the following scale: 1 = twitching of facial muscles accompanied by small masticatory movements without jaw opening; 2 = twitching of facial muscles accompanied by masticatory movements that occasionally result in jaw opening; 3 = movements with broad involvement of facial muscles and masticatory muscles, with frequent jaw opening and occasional tongue protrusions and 4 = involvement of all of the above muscles to the maximal possible degree.
Integrated ALO AIMs were defined as the product of ALO AIMs amplitude × ALO AIMs duration, as previously described (Frouni et al., 2018; Ohlin et al., 2011), while cumulative ALO AIMs indicates the sum of ALO AIMs duration or ALO AIMs amplitude over different consecutive measurement time points. Peak dose ALO AIMs represent the sum of AIMs logged during the 60 and 80 min observation periods.
2.5 Drug treatments
2.5.1 Acute challenges of LY-487,379
Severely, parkinsonian rats were administered once daily injections of l-DOPA/benserazide (from now on referred to as l-DOPA, 6/15 mg/kg s.c.; MilliporeSigma) for 21 days, to induce stable and severe ALO AIMs (Frouni et al., 2018; Huot et al., 2015).
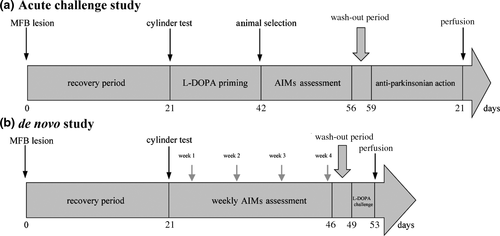
The acute challenges study of LY-487,379 was performed according to the timeline shown in Figure 1a. On days of behavioural testing, 6-OHDA-lesioned rats (N = 16) were administered an acute challenge of l-DOPA s.c., in combination with LY-487,379 (0.1, 1, 10 mg/kg s.c.) or vehicle and were then placed in a transparent container for behavioural assessment. A minimum of 48 hr was left between treatments. Drug administration schedule was randomised according to a within-subject design, in which all animals received all treatments, in a random order. Dose selection and intervals between experiments were based on pharmacokinetic (PK) experiments that we performed in a previous study (Gaudette et al., 2018).
2.5.2 Effect of LY-487,379 on l-DOPA anti-parkinsonian action
Following a 3-day washout period, rats used in the acute challenge study were administered a low dose of l-DOPA/benserazide (3/15 mg/kg s.c.), sufficiently high to produce an anti-parkinsonian effect, but without triggering ALO AIMs (Frouni et al., 2019, 2018), in combination with vehicle or LY-487,379 (0.1, 1 and 10 mg/kg s.c.). They then underwent the cylinder test 45 min later, to determine the effect of LY-487,379 on l-DOPA anti-parkinsonian action. Again, at least 48 hr separated each treatment.
2.5.3 De novo administration of LY-487,379
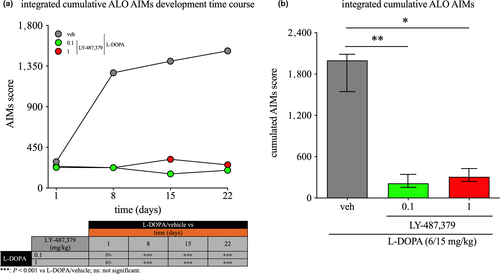
Twenty-four severely parkinsonian 6-OHDA-lesioned rats were randomised into three groups following assessment of parkinsonism (N = 8 per group): l-DOPA/vehicle, l-DOPA/LY-487,379 0.1 mg/kg and l-DOPA/LY-487,379 1 mg/kg. Treatments were started concurrently and were administered s.c. once daily for 22 days. ALO AIMs were assessed on days 1, 8, 15 and 22. Animals then underwent a 3-day washout period, after which they were all administered an acute challenge of l-DOPA (6/15 mg/kg), regardless of the group they were previously in, and the severity of ALO AIMs was then evaluated. This washout was necessary to ensure complete clearance of LY-487,379, based upon its PK profile, to make certain that any reduction of AIMs observed would not be due to a residual effect of the drug, but would rather represent an interference with the dyskinesia induction process. The timeline of the de novo study is presented in Figure 1b.
2.6 Measurement of monoamine levels
At the end of the experiments, rats were euthanised by isoflurane anaesthesia (2%–4%; MilliporeSigma), followed by trans-cardial perfusion of 0.9% NaCl, after which brains were rapidly collected. Left and right striata were dissected on ice, flash-frozen in 2-methyl-butane (−56°C) and stored at −80°C until processing. Striatal levels of dopamine and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA) and 3-methoxytyramine (3-MT) were determined by high-performance liquid chromatography coupled with electro-chemical detection, as previously described (Frouni et al., 2019, 2018; Gaudette et al., 2018; Huot, Johnston, Winkelmolen, Fox, & Brotchie, 2012).
2.7 Statistical analysis
Dopamine, DOPAC, HVA and 3-MT levels are presented as mean ± standard error (SEM) and were analysed by unpaired Welch's unequal variances t test. Cylinder test data for animal selection and the effect of LY-487,379 on l-DOPA anti-parkinsonian action are presented as the mean ± SEM and were analysed by one-way repeated measures (RM) analysis of variance (ANOVA) followed by Tukey's post hoc tests. Peak dose ALO AIMs and cumulative AIMs scores in the acute study are presented as the median with semi-interquartile range and were analysed using Friedman followed by Dunn's post hoc tests, while in the de novo study, cumulative AIMs scores are presented as the median with the semi-interquartile range and were analysed by Kruskal–Wallis followed by Dunn's post hoc tests. In the de novo study, ALO AIMs development time course data are presented as the median and were analysed, following square root transformation of data, by two-way ANOVA followed by Dunnett's post hoc tests. Statistical significance was set to p < .05. Statistical analyses were computed using graphpad prism 8.0d (GraphPad Software Inc.).
3 RESULTS
3.1 Extent of parkinsonism and dopaminergic denervation
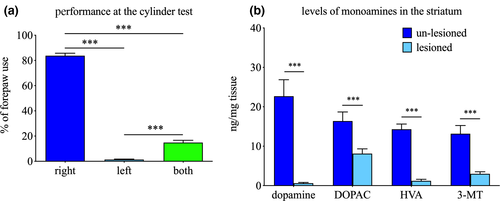
As shown in Figure 2a 6-OHDA-lesioned rats displayed significant forelimb asymmetry at the cylinder test (F(2,30) = 704.4, p < .001; one-way RM ANOVA) with preferential use of the right (un-lesioned) forepaw in 91% of rears, when compared to 0.3% for the left (lesioned) forepaw or 9% for simultaneous use of both forepaws, respectively (both p < .001, Tukey's post hoc test). Greater use of the un-lesioned forepaw was indicative of significant reductions of dopamine and its metabolites DOPAC, HVA and 3-MT in the lesioned striata, when compared to the un-lesioned striata, by 98% for dopamine (t(15.05) = 7.779, p < .001), 46% for DOPAC (t(29.39) = 4.407, p < .001), 95% for HVA (t(15.02) = 15.00, p < .001) and 78% for 3-MT (t(16) = 7.506, p < .001), as displayed in Figure 2b.
3.2 Effect of LY-487,379 on peak l-DOPA-induced ALO AIMs
Acute challenges of LY-487,379 significantly alleviated the severity of peak dose ALO AIMs duration (Friedman Statistic [FS] = 7.48, p < .05). Thus, adding LY-487,379 0.1 mg/kg to l-DOPA significantly reduced the severity of peak dose ALO AIMs duration, by 32% (p < .01, Dunn's post hoc test), when compared to l-DOPA/vehicle (Figure 3a). However, LY-487,379 1 and 10 mg/kg did not significantly reduce the severity of peak dose ALO AIMs duration, although we observed a decrease of 19% with the dose of 1 mg/kg (p = .26, Dunn's post hoc test), when compared to l-DOPA/vehicle.
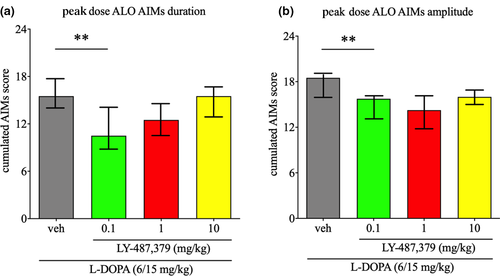
Likewise, acute challenges of LY-487,379 significantly alleviated the severity of peak dose ALO AIMs amplitude (FS = 12.66, p < .01). Thus, adding LY-487,379 0.1 mg/kg to l-DOPA significantly reduced the severity of peak dose ALO AIMs amplitude, by 15% (p < .01, Dunn's post hoc test), when compared to l-DOPA/vehicle (Figure 3b). However, LY-487,379 1 and 10 mg/kg did not significantly reduce the severity of peak dose ALO AIMs amplitude, although we observed a decrease of 22% with the dose of 1 mg/kg (p = .41, Dunn's post hoc test), and of 13% with the dose of 10 mg/kg (p = .59, Dunn's post hoc test), when compared to l-DOPA/vehicle.
3.3 Effect of LY-487,379 on global l-DOPA-induced ALO AIMs
Acute challenges of LY-487,379 significantly alleviated the severity of integrated cumulative ALO AIMs (FS = 13.88, p < .01). Thus, adding LY-487,379 0.1 mg/kg to l-DOPA significantly reduced the severity of integrated cumulative ALO AIMs, by 54% (p < .01, Dunn's post hoc test), when compared to l-DOPA/vehicle (Figure 4a). However, LY-487,379 1 and 10 mg/kg did not significantly reduce the severity of integrated cumulative ALO AIMs, although we observed a decrease of 43% and 25% (p = .170 and p > .999, respectively, Dunn's post hoc test), when compared to l-DOPA/vehicle.
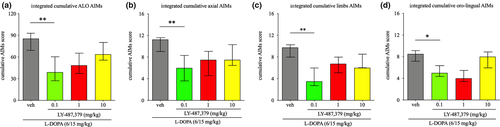
LY-487,379 also significantly alleviated the severity of integrated cumulative axial AIMs (FS = 12.56, p < .01). As shown in Figure 4b, the addition of LY-487,379 0.1 mg/kg to l-DOPA led to a significant reduction of the severity of integrated cumulative axial AIMs, by 46% (p < .01, Dunn's post hoc test), when compared to l-DOPA/vehicle. Again, LY-487,379 1 and 10 mg/kg did not significantly attenuate the severity of integrated cumulative axial AIMs (p = .422 and p > .999, respectively, Dunn's post hoc test).
LY-487,379 alleviated the severity of integrated cumulative limbs AIMs (FS = 13.05, p < .01). Thus, administration of LY-487,379 0.1 mg/kg in combination with l-DOPA diminished the severity of integrated cumulative limbs AIMs by 64% (p < .01, Dunn's post hoc test), when compared to l-DOPA/vehicle (Figure 4c), while higher doses of LY-487,379 (1 or 10 mg/kg) did not (p = .497 and p > .999, respectively, Dunn's post hoc test).
Finally, LY-487,379 alleviated the severity of integrated oro-lingual AIMs (FS = 8.87, p < .05). Thus, administration of LY-487,379 0.1 mg/kg in combination with l-DOPA reduced the severity of integrated cumulative oro-lingual AIMs, when compared to l-DOPA/vehicle (Figure 4d; p < .05, Dunn's post hoc test). Higher doses of LY-487,379 (1 and 10 mg/kg) did not have any effect on the severity of integrated oro-lingual AIMs (p = .058 and p > .999, respectively, Dunn's post hoc test).
3.4 Effect of LY-487,379 on the development of l-DOPA-induced ALO AIMs
In the de novo study, we assessed the progression of AIMs severity on a weekly basis to determine whether LY-487,379 prevents the development of dyskinesia (Figure 5). As displayed in Figure 5a, administration of LY-487,379 attenuated the induction of cumulative ALO AIMs (Ftreatment(2,20) = 17.30, p < .001; Ftime(3,60) = 4.04, p < .05, Finteraction(6,60) = 2.74 p < .05; two-way ANOVA following square root transformation). On day 1, integrated cumulative ALO AIMs were lower, by 21% and 17% (p = .313 and p = .601, Dunnett's post hoc test) in animals treated with l-DOPA/LY-487,379 0.1 and 1 mg/kg, compared to animals treated with l-DOPA/vehicle, respectively. However, we found that this reduction was not significant. On day 8, integrated cumulative ALO AIMs were significantly lower, by 82%, in animals treated with l-DOPA/LY-487,379 0.1 and 1 mg/kg (both p < .001, Dunnett's post hoc test), compared to animals treated with l-DOPA alone. On day 15, integrated cumulative ALO AIMs were milder, by 88% and 77%, in l-DOPA/LY-487,379 0.1 and 1 mg/kg groups, when compared to the l-DOPA/vehicle treatment (both p < .001, Dunnett's post hoc test). On day 22, integrated cumulative ALO AIMs were 87% and 83% milder in animals administered l-DOPA/LY-487,379 0.1 and 1 mg/kg (both p < .001, Dunnett's post hoc test), compared to animals treated with l-DOPA/vehicle. Following an acute l-DOPA challenge (Figure 5b), rats administered LY-487,379 during the priming period exhibited less severe integrated cumulative ALO AIMs (Kruskal–Wallis statistic [H] = 12.81, p < .01), by 89% and 84% with doses of 0.1 and 1 mg/kg, compared to animals primed with l-DOPA/vehicle (p < .01 and p < .05, respectively Dunn's post hoc test).
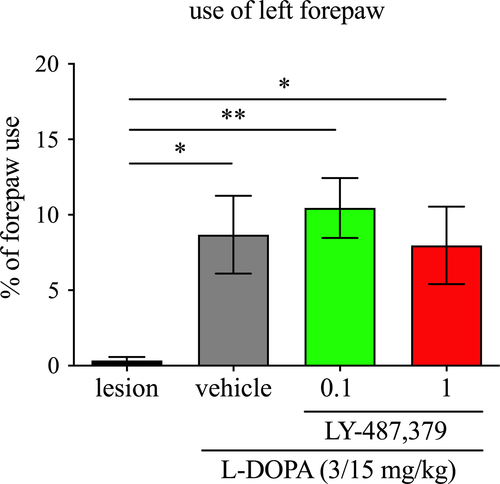
3.5 Effects of LY-487,379 on l-DOPA anti-parkinsonian action
As shown in Figure 6, administration of l-DOPA, with and without LY-487,379, improved the severity of parkinsonism (F(2.463,24.63) = 10.28, p < .001; one-way RM ANOVA). Thus, when 6-OHDA-lesioned rats were administered l-DOPA 3/15 mg/kg, there was a significant increase in the number of rears using the lesioned forepaw, when compared to l-DOPA-untreated 6-OHDA-lesioned animals (p < .05, Tukey's post hoc test). This increase in rears with the lesioned forepaw remained present when LY-487,379 0.1 and 1 mg/kg were combined with l-DOPA (p < .001 and p < .05, respectively).
4 DISCUSSION
In the present study, we have demonstrated that selective mGlu2 positive allosteric modulation significantly alleviates the severity of established l-DOPA-induced dyskinesia and attenuates the development of dyskinesia in the 6-OHDA-lesioned rat, without interfering with the peak anti-parkinsonian action of a low dose of l-DOPA.
It is noteworthy that our experiments were performed exclusively on female rats, because their weight remains stable over time. For this reason, it remains uncertain if an anti-dyskinetic benefit would have been achieved in male rats. It should be pointed out, however, that recent clinical trials performed with amantadine did not encounter different responses to glutamatergic dampening in male and female patients (Hauser et al., 2017; Pahwa et al., 2017).
Here, we have elected to perform the cylinder test to screen our animals for parkinsonism and to assess the effect of LY-487,379 on l-DOPA anti-parkinsonian action. We selected the cylinder test over other approaches, for example, rotational tests, because it enables to keep the animals drug-free, which was critical here, as a de novo experiment, requiring the animals to be drug-naïve prior to commencing l-DOPA, was performed. Indeed, as mentioned above, in the cylinder test, preferential use of the un-lesioned forepaw in ≥70% of the rears, is indicative of ≥88% striatal dopamine depletion (Schallert et al., 2000). Another interest of the cylinder test is that performance at the cylinder test was shown to be improved by l-DOPA (Lundblad et al., 2002), which was critical here to assess the effect of LY-487,379 on the anti-parkinsonian action of l-DOPA.
Unlike the MPTP-lesioned primate, it is virtually impossible, in the 6-OHDA-lesioned rat, to assess the effect of an experimental drug on the anti-parkinsonian action of l-DOPA without performing a second test (Hamadjida, Frouni, Kwan, & Huot, 2019). Here, because we performed such a test at peak behavioural effect of l-DOPA, we can only claim that LY-487,379 did not interfere with the peak anti-parkinsonian effect of l-DOPA and must refrain from speculating on possible effects of LY-487,379 pertaining to the duration of l-DOPA's action. Studies in the MPTP-lesioned primate are warranted to determine the effect of LY-487,379 and other mGlu2 PAMs on the intensity and duration of the reversal of parkinsonian disability conferred by l-DOPA.
Group II metabotropic receptors are localised pre-synaptically on cortico-striatal and subthalamo-nigral terminals (Phillips et al., 2000), where they regulate glutamatergic transmission in these basal ganglia structures (Conn, Battaglia, Marino, & Nicoletti, 2005), and possibly act to mediate an anti-dyskinetic effect when stimulated. It is unclear if a dopamine-related mechanism contributes to the anti-dyskinetic action of mGlu2 activation, as pharmacological studies have reported conflicting results on the effects of mGlu2/3 ligands on dopamine levels or release in the striatum, ranging from activation of mGlu2/3 suppressing dopamine release (Baker, Xi, Shen, Swanson, & Kalivas, 2002) to enhancing dopamine release (Cartmell, Salhoff, Perry, Monn, & Schoepp, 2000).
Few studies have investigated the therapeutic potential of mGlu2 activation as an anti-dyskinetic target. A study found that chronic treatment with the mGlu2/3 agonist LY-379,268 had no significant effect on l-DOPA-induced dyskinesia molecular changes and did not reduce dyskinesia severity in the 6-OHDA-lesioned rat (Rylander et al., 2009). The lack of anti-dyskinetic effect reported with LY-379,268 may be explained by the lack of selectivity for the mGlu2 subunit (EC50 of 2.69 nM) over the mGlu3 one (4.48 nM; Monn et al., 1999). Given the similar EC50 for both receptors, the doses of LY-379,268 administered (1 and 10 mg/kg) may have been sufficient to activate both mGlu2 and mGlu3 receptors. Stimulation of mGlu3 receptors was recently shown to amplify the downstream signalling produced metabotropic glutamate 5 (mGlu5) activation (Di Menna et al., 2018). Because mGlu5 blockade is an investigational anti-dyskinetic strategy (Johnston, Fox, McIldowie, Piggott, & Brotchie, 2010; Tison et al., 2016), we speculate that this agonist effect of LY-379,268 at mGlu3 receptors might have interfered with the anti-dyskinetic benefit conferred by mGlu2 activation, hence the lack of anti-dyskinetic benefit observed by Rylander et al. (2009).
In contrast to the report by Rylander et al. (2009), we recently demonstrated that the mGlu2/3 orthosteric agonist LY-354,740 alleviated dyskinesia in the parkinsonian rat (Frouni et al., 2019). However, LY-354,740 harbours more selectivity than LY-379,268 for mGlu2 over mGlu3 receptors (Monn et al., 1997; Schoepp et al., 1997), which could be why only low doses reduced dyskinesia, with loss of the anti-dyskinetic benefit upon administration of higher doses.
Whereas an interaction with mGlu3 receptors might underlie the loss of anti-dyskinetic effect that we observed with high doses of LY-354,740, this explanation does not stand with LY-487,379, which reportedly does not interfere with targets other than mGlu2 receptors (Johnson et al., 2003). It is therefore possible that the maximal anti-dyskinetic benefit of mGlu2 activation is achieved with lower doses of orthosteric agonists or PAMs. Whether lower doses of LY-487,379 would have led to a greater reduction of dyskinesia remains undetermined. It is noteworthy that we did not encounter such a U-shaped dose–response curve when LY-354,740 was administered to MPTP-lesioned marmosets (Frouni et al., 2019), suggesting that neuro-chemical differences between rodent and primate might also play a role in these differences of anti-dyskinetic dose–response between species.
In addition its therapeutic benefit on l-DOPA-induced dyskinesia, LY-487,379 has also demonstrated efficacy in pre-clinical models of anxiety, psychosis and cognition (Galici, Echemendia, Rodriguez, & Conn, 2005; Nikiforuk et al., 2010), suggesting that mGlu2 positive allosteric modulation could also improve these features, which are also encountered in advanced PD (Hely, Morris, Reid, & Trafficante, 2005) and their effects could therefore extend beyond dyskinesia.
In summary, the highly selective mGlu2 PAM LY-487,379 effectively alleviated the expression, and attenuated the development of, l-DOPA-induced dyskinesia in the 6-OHDA-lesioned rat. These findings suggest that selective mGlu2 activation shows potential as a promising therapeutic strategy to treat dyskinesia.
ACKNOWLEDGEMENTS
PH has research support from Parkinson Canada, Fonds de Recherche Québec–Santé, the Weston Brain Institute, the Michael J Fox Foundation for Parkinson's Research, the Natural Sciences and Engineering Research Council of Canada and Healthy Brains for Healthy Lives.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists. PH has received payments from Valeo Pharma.
AUTHOR CONTRIBUTIONS
Conception: C Rouillard and P Huot; Organisation: A Hamadjida and P Huot; Execution: A Hamadjida, L Sid-Otmane, C Kwan, I Frouni, V Nafade, D Bédard, D Gagnon, and M-J Wallman; Writing of the first draft: A Hamadjida; Review and critique: A Hamadjida, C Kwan, I Frouni, D Gagnon, M-J Wallman, C Rouillard, A Parent, M Parent and P Huot.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available upon written request to the corresponding author.




