Visuomotor signals for reaching movements in the rostro-dorsal sector of the monkey thalamic reticular nucleus
Abstract
The thalamic reticular nucleus (TRN) collects inputs from the cerebral cortex and thalamus and, in turn, sends inhibitory outputs to the thalamic relay nuclei. This unique connectivity suggests that the TRN plays a pivotal role in regulating information flow through the thalamus. Here, we analyzed the roles of TRN neurons in visually guided reaching movements. We first used retrograde transneuronal labeling with rabies virus, and showed that the rostro-dorsal sector of the TRN (TRNrd) projected disynaptically to the ventral premotor cortex (PMv). In other experiments, we recorded neurons from the TRNrd or PMv while monkeys performed a visuomotor task. We found that neurons in the TRNrd and PMv showed visual-, set-, and movement-related activity modulation. These results indicate that the TRNrd, as well as the PMv, is involved in the reception of visual signals and in the preparation and execution of reaching movements. The fraction of neurons that were non-selective for the location of visual signals or the direction of reaching movements was greater in the TRNrd than in the PMv. Furthermore, the fraction of neurons whose activity increased from the baseline was greater in the TRNrd than in the PMv. The timing of activity modulation of visual-related and movement-related neurons was similar in TRNrd and PMv neurons. Overall, our data suggest that TRNrd neurons provide motor thalamic nuclei with inhibitory inputs that are predominantly devoid of spatial selectivity, and that these signals modulate how these nuclei engage in both sensory processing and motor output during visually guided reaching behavior.
Introduction
The motor areas in the cerebral cortex have reciprocal connections with the motor thalamus. Situated around the thalamic relay nuclei, including the motor thalamus, is the thalamic reticular nucleus (TRN), a thin, sheet-like cluster of GABAergic neurons that circumscribes thalamic nuclei laterally (Houser et al., 1980; Guillery & Sherman, 2002; Jones, 2007). The TRN receives collateral inputs from the thalamic neurons projecting to the cerebral cortex and cortical neurons projecting to the thalamus (Chow, 1952; Schell & Strick, 1984; Cicirata et al., 1990; Gonzalo-Ruiz & Lieberman, 1995a,b; Pinault & Deschenes, 1998; Rouiller et al., 1998; Crabtree & Isaac, 2002). Additionally, the TRN receives widespread inputs from the basal ganglia and cholinergic and noradrenergic nuclei (Carman et al., 1964; Jones, 1975; Morrison & Foote, 1986; Pare et al., 1990; Hazrati & Parent, 1991; McCormick, 1992; Gandia et al., 1993; Asanuma, 1994; Steriade, 2005). In turn, TRN neurons send GABAergic, inhibitory outputs to the thalamic relay nuclei. This anatomical organization suggests that the TRN plays an important role in motor control.
The functional role(s) of the TRN in movement remain(s) unknown because TRN neurons have rarely been studied in terms of motor control. In cats, TRN neurons in the somatosensory sector are active during walking, and the activation phase is dependent on the receptive fields (RFs) (Marlinski et al., 2012; Marlinski & Beloozerova, 2014). In monkeys, neurons in the dorsal portion of the TRN respond to somatosensory stimulation (Pollin & Rokyta, 1982). However, neuronal activity has not fully been examined in task-performing monkeys, except in a series of studies showing that neuronal activity in the visual sector of the TRN is modulated dynamically by visual attention (McAlonan et al., 2006, 2008).
To investigate the contribution of the TRN to motor control, we sought to characterize TRN neurons in monkeys while they were performing a visually guided reaching movement. We integrated neuroanatomical and electrophysiological approaches. In the neuroanatomical approach, we used retrograde transneuronal labeling with rabies virus to identify a TRN sector containing neurons that projected across synapses to the ventral premotor cortex (PMv) (Kelly & Strick, 2000; Saga et al., 2011; Takahara et al., 2012). The PMv has been shown to play a central role in visually guided reaching movement (Kurata & Hoffman, 1994; Kurata & Hoshi, 2002; Hoshi & Tanji, 2006; Yamagata et al., 2009). We found that the rostro-dorsal sector of the TRN (TRNrd) contained neurons disynaptically projecting to PMv. In the physiological approach, we analyzed neurons in this portion of the TRN in monkeys while they performed a visually guided target-reaching task. Furthermore, we compared the response properties of TRNrd neurons with those of PMv neurons in other monkeys performing the reaching task. Our results indicate that TRN neurons exhibit a range of responses, such as visual-, set-, and movement-related activity. This suggests that the TRN sends inhibitory signals to the thalamic relay nuclei to modulate activities at multiple time points in the generation of reaching movements.
Materials and methods
Animals and experimental conditions
Six monkeys (Macaca fuscata; five males, 5.0–8.5 kg; one female, 5.0 kg) were used in this study. Four monkeys (Monkeys 1–4) were used for TRN neuron recordings (Monkeys 1 and 2; Saga et al., 2013) and PMv neuron recordings (Monkeys 3 and 4; Yamagata et al., 2009). The other two monkeys (Monkeys 5 and 6) were used for investigating anatomical connectivity of TRN with PMv. Our prior report included results obtained with Monkeys 5 and 6, which showed the origins of multisynaptic projections from the basal ganglia to the PMv (Ishida et al., 2016).
All experiments were conducted in accordance with the National Institutes of Health guidelines and the guidelines of the Tokyo Metropolitan Institute of Medical Science, Tamagawa University, and the Primate Research Institute at Kyoto University. All animal care and experimental procedures were approved by the Animal Care and Use Committee of Tokyo Metropolitan Institute of Medical Science, Tamagawa University, and the Primate Research Institute at Kyoto University.
Electrophysiological study
During the experimental sessions for neuronal recording from task-performing monkeys (Monkeys 1–4), each monkey sat in a primate chair with its head and left arm restrained. A button was installed at waist level in front of the chair, where the monkey could readily press and release it with the right hand. A 19-inch color video monitor equipped with a touch-sensitive screen was placed in front of the monkey (30 cm from the eyes). Eye movement was monitored at 120 Hz using an infrared eye-tracking system (resolution, 0.25° visual angle; RHS-M; Applied Science Laboratories, Spokane, USA). The TEMPONET system (Reflective Computing, Olympia, WA, USA) was used to control the behavioral task, to regulate the opening and closing of a solenoid valve serially installed in the reward delivery system, and to collect data, at 1000 Hz. A liquid reward (apple juice) was delivered via a stainless-steel tube placed in front of the monkey's mouth.
Surgery and electrophysiological recordings
Aseptic surgery was performed under pentobarbital sodium anesthesia (20–25 mg/kg, intravenously) following the induction of anesthesia using ketamine hydrochloride (10 mg/kg, intramuscularly) and atropine sulfate. Antibiotics and analgesics were used to prevent postsurgical infection and pain respectively. Polycarbonate and titanium screws were implanted in the skull, and two plastic pipes were firmly attached using acrylic resin. Part of the skull over the left frontal lobe was removed, and a recording chamber was implanted to permit access to the TRN, thalamic relay nuclei, and the globus pallidus in two monkeys (Monkeys 1 and 2), and the PMv, dorsal premotor cortex (PMd), and primary motor cortex (M1) in two other monkeys (Monkeys 3 and 4).
Neuronal activity was recorded using a glass-insulated tungsten microelectrode or glass-insulated Elgiloy-alloy microelectrodes (0.5–2.0 MΩ at 1 kHz) inserted into the brain through a 23-gauge guide tube that penetrated the dura mater. A hydraulic microdrive (MO-972 or MO-81-S, Narishige, Tokyo Japan) was used to move the electrode using a micrometer step. Single-unit potentials were amplified using a multichannel processor and sorted using a multi-spike detector (MCP and ASD, Alpha Omega Engineering, Nazareth, Israel). Recording sites were verified by examining magnetic resonance images (1.5 T Sonata; Siemens, Erlangen, Germany).
To identify the PMv, we checked the neuronal responses to somatosensory and visual stimuli, and applied intracortical microstimulation (ICMS) through the tip of an inserted electrode (11–44 pulses of 200 μs width at 333 Hz; current, <50 μA; see detail in Yamagata et al., 2009). As described previously, we identified the caudal part, or area F4, and the rostral part, or area F5, of the PMv (Matelli et al., 1985).
Muscle activity was recorded with stainless-steel wire electrodes (single stranded, 0.0762 mm in diameter) inserted percutaneously. The electromyographic activity was amplified, A-D converted, and stored. We monitored activities in the following muscles during task performance: trapezius, supraspinatus, infraspinatus, deltoid, biceps, triceps, flexor carpi radialis, and flexor carpi ulnaris, as well as the neck and paravertebral muscles.
Behavioral tasks
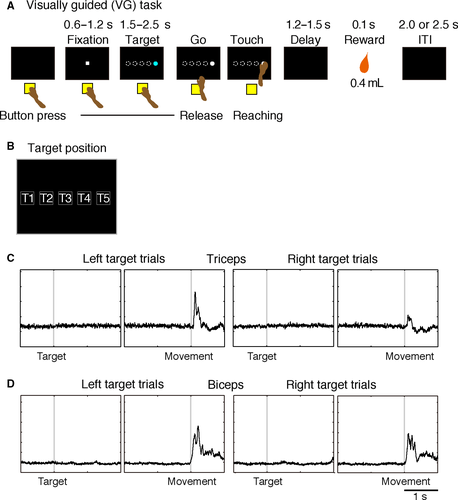
The monkeys were trained to perform three reaching-movement tasks described in our previous reports (Yamagata et al., 2009; Saga et al., 2013). Two monkeys (Monkeys 1 and 2) learned goal-directed tasks, that is, spatial goal, object goal, and a visually guided task (see Saga et al., 2013 for details), and two other monkeys (Monkeys 3 and 4) learned a symbolic cue task and a visually guided task (Yamagata et al., 2009). In the present study, we focused on the visually guided reaching task (VG task, Fig. 1A). For Monkeys 1 and 2 (TRN recording), after an inter-trial interval of 2000–2500 ms, each trial began with the presentation of a fixation point (white square, visual angle of 1.4°) in the center of the screen (corresponding to T3 in Fig. 1B). When the monkey pressed the hold button with its right hand and gazed at the fixation point for 600–1200 ms, a blue-colored target in the shape of a circle or triangle was presented at one of five possible positions on the screen (Fig. 1B; visual angle of 7 × 7° for each target, 11° between the centers of adjacent target positions). For Monkeys 3 and 4 (PMv recording), the target was colored gray, and the shape was square. When the color changed from blue or gray to white (the GO signal) after a delay of 1500–2500 ms, the monkey made a reaching movement toward the target. The difference in VG tasks between recording from the PMv and from the TRN was that the target disappeared after presentation of the target (presentation period: 800 ms) in PMv recording sessions. The target appeared again at the same position after a 1200-ms delay period during PMv recording sessions (Fig. 1D in Yamagata et al., 2009). When the monkey touched the target, it disappeared immediately, and a drop of apple juice (0.4 mL) was delivered after 1200 ms or 1500 ms in TRN recordings and after 500 ms in PMv recordings. If the monkey released the button prematurely, that is, before the appearance of the GO signal, the target disappeared immediately, and an inter-trial interval with no reward began.
Data analyses
To examine visual-, set-, and movement-related activity without confounding by saccade-related activity, we attempted initially to find neurons related to saccadic eye movement. A saccadic eye movement was detected when the velocity of the eye movement was ≥40 °/s and the gaze shift was ≥3°. Then, we compared neuronal activity during the 100-ms period before saccade onset with that during the 100-ms period after onset; activity from −3000 to 0 ms relative to target onset was analyzed separately for saccades in the four directions [upper right, upper left, lower right, and lower left, paired t-test with Bonferroni correction, α = 0.0025; (Nakayama et al., 2016)]. Neurons showing saccade-related activity were excluded from the neuronal database. Next, we analyzed neuronal activity during three task periods of the VG task: the post-target period (50–250 ms after target presentation) to detect visual-related activity; the pre-GO period (200 ms preceding presentation of the GO signal) to detect set-related activity; and the pre-touch period (200 ms preceding touching the target) to detect movement-related activity. We compared the activity during the control period (200 ms before the onset of the visual signal) with that during each of the post-target, pre-GO, and pre-touch periods; this analysis was conducted separately for the left and right target positions (paired t-test with Bonferroni correction; α = 0.01, corrected). If the activity during the post-target, pre-GO, or pre-touch period was significantly different from that during the control period in either right or left-target trials, the neuron was defined as having visual-related, set-related, or movement-related activity respectively.
To examine the spatial selectivity of neuronal activity, visual-related, set-related, and movement-related activities were each analyzed using one-way anova (α = 0.05) with the main factor being target position (left: position T1 or T2 vs. right: position T4 or T5; Fig. 1B). We categorized the target position into two groups following our previous report (Yamagata et al., 2009). Based on this analysis, each response was classified into position-selective (POSITION < 0.05) or non-selective activity (POSITION ≥ 0.05).
Neuroanatomical study
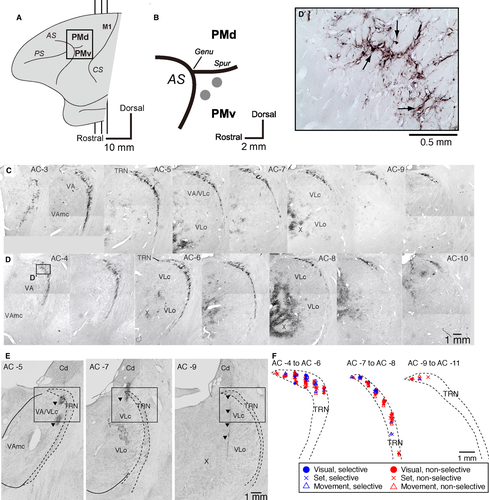
To identify the TRN subdivision interconnected with the PMv, we used retrograde transneuronal labeling with rabies virus in two monkeys (Monkeys 5 and 6: males, 6.9 and 6.0 kg). The rabies virus (CVS-11 strain, 1.0 × 108 focus-forming units/mL) was from the Centers for Disease Control and Prevention (Atlanta, GA, USA) and was provided by Dr. S. Inoue (The National Institute of Infectious Diseases, Tokyo, Japan). For viral injections, we used the method described in our previous reports (Saga et al., 2011; Takahara et al., 2012). A viral suspension was injected slowly through a 10-μL Hamilton microsyringe into the rostral and dorsal portions of the PMv (Fig. 2A and B). Along each injection track, viral deposits were placed at two different depths: 3 and 2 mm below the cortical surface. At each depth, 0.5 μL of the viral suspension was deposited. Then, 3 days after the injections, the two monkeys (Monkeys 5 and 6) were anesthetized deeply with an overdose of sodium pentobarbital (50 mg/kg) and sacrificed by perfusion fixation with a mixture of 10% formalin and 15% saturated picric acid in 0.1 m phosphate buffer (pH 7.4) (Saga et al., 2011; Takahara et al., 2012). This survival time permitted reaching the second stage of retrograde trans-synaptic transport of the virus, resulting in labeling of second-order neurons that projected to the injection sites (PMv) disynaptically (Saga et al., 2011). Then, serial 50- or 60-μm-thick coronal sections were cut on a freezing microtome, and every sixth section was processed for immunohistochemical staining for rabies virus using a standard avidin–biotin–peroxidase complex method (see Saga et al., 2011). Following immersion in 1% skimmed milk, the sections were incubated overnight with rabbit anti-rabies virus antibody (from Dr. S. Inoue) in 0.1 m phosphate-buffered saline (pH 7.4) containing 0.1% Triton X-100 and 1% normal goat serum. The sections were then placed in the same fresh incubation medium containing biotinylated goat anti-rabbit IgG antibody (1 : 200; Vector Laboratories, Burlingame, CA, USA), followed by the avidin–biotin–peroxidase complex kit (ABC Elite; Vector Laboratories). To visualize the antigen, the sections were reacted in 0.05 m Tris–HCl buffer (pH 7.6) containing 0.04% diaminobenzidine, 0.04% nickel chloride, and 0.002% hydrogen peroxide. The sections were mounted on gelatin-coated glass slides and examined by light microscopy (Eclipse 80i, Nikon, Tokyo, Japan).
Histological analysis of a monkey used in the electrophysiological study
After completing the electrophysiological recordings, perfusion fixation was conducted as described above in one monkey (Monkey 1). The perfused brain was removed from the skull, post-fixed in the same fresh fixative overnight at 4 °C, and placed in 0.1 m phosphate buffer (pH 7.4) containing 30% sucrose. Serial 60-μm-thick coronal sections were then cut on a freezing microtome. The sections for verifying recording sites were mounted on gelatin-coated glass slides, stained with 1% cresyl violet, and then examined by light microscopy (Eclipse 80i, Nikon).
Results
TRN subdivision connected with PMv across synapses
The TRN does not send direct projections to the cerebral cortex. This thalamic nucleus is considered to project to cortical areas via the thalamic nuclei. In the cortex, we focused on the PMv, which has been shown to play a central role in reaching movements. To identify the TRN sector projecting to the PMv, we injected rabies virus into the PMv (Fig. 2A and B). By 3 days after the rabies injections, second-order neuron labeling had occurred. Some TRN neurons were labeled with the virus. Dense clusters of labeled neurons were found in the rostro-dorsal sector of the TRN (TRNrd; Fig. 2C, D and D’), and also mainly in the ventral thalamic nuclei. In the thalamic nuclei, dense labeled neurons (the first or second-order ones) were observed in the nucleus X (Fig. 2 C and D), and less densely in the ventroanterior nucleus (VA), the oral division of the ventrolateral nucleus (VLo), the caudal division of the ventrolateral nucleus (VLc), as well as in the mediodorsal nucleus (MD). The TRN neurons were considered to project to the PMv via these thalamic relay nuclei. The results indicate that the TRNrd is part of the multisynaptic network centered on the PMv, and that this portion of the TRN sector may be involved specifically in reaching movements.
Task performance and muscle activity
The monkeys performed the VG task at a success rate of >98%, executing reaching movements promptly after the onset of the GO signal. The reaction time (RT) and movement time (MT) were <600 ms in all monkeys (Table 1). Figure 1C and D show the activity of the triceps and biceps muscles in Monkey 1. As in these examples, the muscles in the right forelimb had movement-related activity changes only during reaching movements. Thus, the monkeys made a movement only during the task performance, while in the remaining periods they sat quietly.
| Target position | ||
|---|---|---|
| Left | Right | |
| Monkey 1 | ||
| Reaction time | 366 ± 104 | 353 ± 99 |
| Movement time | 348 ± 118 | 349 ± 143 |
| Monkey 2 | ||
| Reaction time | 351 ± 112 | 335 ± 97 |
| Movement time | 402 ± 156 | 379 ± 139 |
| Monkey 3 | ||
| Reaction time | 442 ± 55 | 507 ± 68 |
| Movement time | 263 ± 39 | 207 ± 25 |
| Monkey 4 | ||
| Reaction time | 318 ± 20 | 315 ± 34 |
| Movement time | 315 ± 20 | 297 ± 36 |
- Times are expressed as means ± standard deviations (ms).
Neuronal recordings from the TRNrd
We examined neuronal activity of the TRN in the left hemisphere, the side opposite the task-performing right arm. Recordings were carried out from the TRNrd (Fig. 2E), which had been identified as projecting to the PMv through thalamic neurons. The electrode was advanced vertically in the dorsoventral direction. Before contacting neurons in the TRNrd, the electrode went through sporadically firing striatal (caudate) cells and passed the white matter (Fig. 2E). In addition to their spatial localization, TRNrd neurons showed high spontaneous discharge rates. During the 500-ms period before the presentation of the fixation point, the discharge rate of the TRNrd neurons was 40 ± 2 spikes/s (n = 106; mean ± SEM). This value was higher than that of PMv neurons (n = 443; 10 ± 1 spikes/s, mean ± SEM, P < 0.001, two-tailed t-test). The higher discharge rates of the TRNrd neurons are consistent with those in the visual sector of the TRN (McAlonan et al., 2008). Subsequently, at the transition from the TRNrd to the thalamic relay nuclei and white matter, the neuronal discharge rate dropped, and the cellular activity disappeared respectively. In one monkey, the recording sites were verified histologically with reference to markings made by passing anodal direct currents (20–40 μA for 20 s) through the recording electrodes (Nomoto et al., 2010) (Fig. 2E, left and middle panels) and the gliosis along the electrode track (Fig. 2E, right panel). The recording sites were confirmed in accordance with those estimated based on the magnetic resonance images. Although we recorded neuronal activity from the thalamic relay nuclei, we did not sample enough data from the nucleus X that is the main relay nucleus to the PMv. Therefore, we did not analyze neurons in the thalamic relay nuclei.
We examined the impact of saccadic eye movement on activity in each neuron (see 2). Of the 106 TRNrd neurons, none showed significant activity modulation in relation to saccadic eye movement (paired t-test with Bonferroni correction, P > 0.05, corrected). In the PMv, of the 498 neurons, 55 (11%) showed saccade-related activity (paired t-test with Bonferroni correction, P < 0.05, corrected). We excluded those PMv neurons from subsequent analyses. Thus, in this study, we analyzed 106 TRNrd neurons and 443 PMv neurons that had no apparent direct relationship with saccadic eye movement.
Visual-, set-, and movement-related neurons in the TRNrd
Figure 3 shows four TRNrd neurons during the VG task. Generally, TRNrd neurons showed visual-related activity in response to the onset of visual signals (Fig. 3A, B and D), set-related activity during preparation for reaching movement (Fig. 3B and C), and movement-related activity during execution of the movement (Fig. 3A–D).
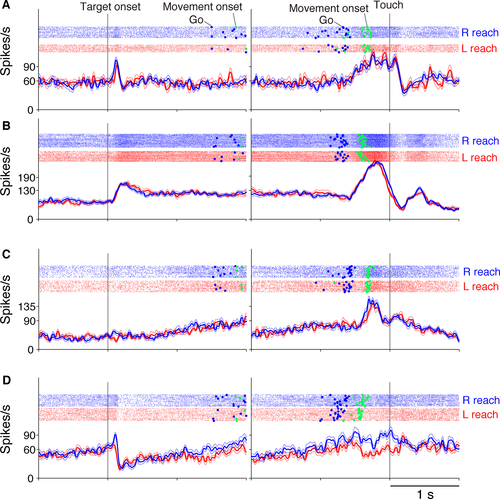
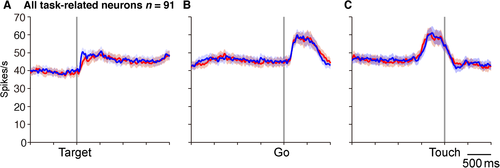
Among the 106 TRNrd neurons, 91 (86%) exhibited activity modulation in at least one of the three task phases. To gain insight into overall activity modulation, population activity in the 91 task-related neurons was assessed separately for the left- and right-side trials (Fig. 4). This indicated that neuronal activity was modulated in response to visual signal onset, during the preparation, and execution of reaching movements. The overall activity was increased and was similar to the right- and left-target trials.
By analyzing the activity of each neuron, we found that 43 (41%), 45 (42%), and 64 (60%) neurons displayed visual-, set-, and movement-related activity respectively (paired t-test with Bonferroni correction, α = 0.05, corrected). Of these, 50 (55%) neurons exhibited activity modulation in two or three task phases. Thirteen neurons (14%) showed visual- and set-related activity, 13 (14%) demonstrated visual- and movement-related activity and 13 (14%) showed set- and movement-related activity. Additionally, 11 (12%) neurons displayed visual-, set-, and movement-related activity. We then compared the baseline activities of single phase and multiple phase neurons, and found that their baseline activities were not different (single phase neurons, 44.6 ± 3.0 spike/s, n = 41; multiphasic neurons, 38.6 ± 3.0 spike/s, n = 50; P = 0.1728, two-tailed t-test).
From the PMv, we sampled 443 neurons having no eye-movement effect. Of these, 375 neurons showed modulation in the VG task: 164 (44%), 292 (78%), and 300 (80%) neurons showed visual-, set-, and movement-related activity respectively. In the following sections, we compare the properties of these activities between the TRNrd and the PMv.
Visual-related neurons in the TRNrd and the PMv
We first analyzed the properties of the 43 visual-related TRNrd neurons (Fig. 1A). The two neurons shown in Figs 3A and B exhibited excitatory phasic responses after the appearance of the visual targets. The activity was similar to the right and left target-positions (P ≥ 0.05, two-sample t-test). The neuron in Fig. 3D showed visual-related phasic responses, and the activity was greater in the right than in the left-target position (P < 0.05, two-sample t-test). Activity in 35 (81%) neurons did not differ between the right and left-target positions, whereas in eight neurons, it did. In the PMv, 75 (46%) of visual-related neurons were not selective for the target position (Fig. 5D), whereas the remaining 89 neurons were. Thus, TRNrd neurons were more frequently non-selective for the target position than PMv neurons were (Table 2, P < 0.0001, Fisher's exact test).
| Spatially non-selective neurons | Neurons with excitatory responses | |
|---|---|---|
| Visual-related neurons | TRN (81%) ≫ PMv (46%) | TRN (91%) ≫ PMv (73%) |
| Set-related neurons | TRN (93%) ≫ PMv (60%) | TRN (76%) ≫ PMv (55%) |
| Movement-related neurons | TRN (69%) > PMv (59%) | TRN (84%) ≫ PMv (59%) |
- ≫, statistically significant (P < 0.05); >, statistically non-significant although the fraction is greater (P ≥ 0.05).
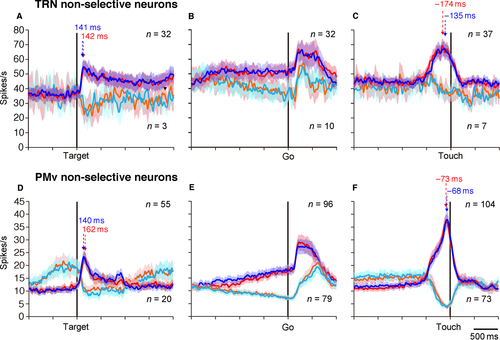
We classified the spatially non-selective activity into activity-increase and activity-decrease types with respect to baseline activity (the 200-ms period before target appearance). In the TRNrd, 91% (32/35) of neurons were classified as the activity-increase type. In the PMv, 73% (55/75) of neurons were the activity-increase type. Thus, the fraction of neurons with activity increase was greater in the TRNrd than the PMv (P = 0.04243, Fisher's exact test). Figure 5A shows the population activity of TRNrd neurons with activity increase (n = 32, blue for right target and red for left target) and with activity decrease (n = 3, cyan for right target and orange for left target). Figure 5D shows the population activity of PMv neurons with activity increase (n = 55) and with activity decrease (n = 20). At the population level, the latencies of spatially non-selective TRNrd neurons with activity increase were 104 ms for the right target and 111 ms for the left target. This timing did not differ when evaluated at the single-neuron level (Table 3, P = 0.6569, two-tailed paired t-test). The latencies of spatially non-selective PMv neurons with activity increase were 105 ms for the right position and 94 ms for the left position at the population level (Table 3, P = 0.9508, two-tailed t-test, evaluated at the single-neuron level). The timing did not differ between the TRNrd and the PMv (P = 0.2686 for right target, P = 0.4069 for left target; two-tailed t-test, evaluated at the single-neuron level). The population activities of TRNrd neurons with activity increases reached peaks at 141 ms for the right target and 142 ms for the left target. The population activities of PMv neurons with activity increases reached peaks at 140 ms for the right target and 162 ms for the left target (Fig. 5A and D). The peak timing was slightly earlier in PMv than in TRNrd for the right target (P = 0.0215, evaluated at the single-neuron level, two-tailed t-test), whereas the two did not differ for the left target (P = 0.1263, two-tailed t-test). These results revealed that the spatially non-selective activity increase in TRNrd and PMv neurons began and developed with similar timing, although the timing for PMv neurons tended to be earlier.
| TRN | PMv | |
|---|---|---|
| Spatially non-selective | Mean ± SEM | |
| Right target | 104 (77 ± 12) | 94 (60 ± 8) |
| Left target | 111 (70 ± 9) | 105 (61 ± 6) |
| Spatially selective | ||
| Preferred target | 99 (75 ± 32) | 86 (72 ± 12) |
| Non-preferred target | 192 (106 ± 57) | 72 (69 ± 8) |
- Numbers indicate the timings of population activity. Numbers in parentheses indicate means ± standard error of means (ms) of single neurons.
Next, the activity decrease in the spatially non-selective neurons was analyzed (Fig. 5A and D). This suggests that the activity decrease occurred via distinct neural mechanisms in the TRNrd and PMv. The PMv neurons showed a build-up activity preceding the appearance of the visual signals, and this activity was suppressed in response to the appearance of the visual targets. In contrast, the activity decrease of the TRNrd neurons was due to reduced baseline activity at higher discharge rates (~40 Hz).
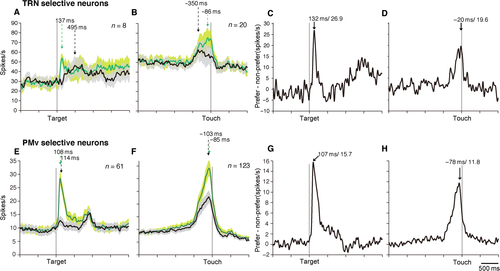
Spatially selective visual-related neurons were also analyzed (n = 8 for TRNrd, n = 89 for PMv). Out of eight neurons, six (75%) exhibited multiphasic response (set-related activity, n = 2; movement-related activity, n = 2; set- and movement-related activity, n = 2). The population activity of spatially selective visual-related neurons in the TRNrd and PMv revealed that phasic activity was observed only when the target appeared in the preferred positions (Fig. 6A and E). The latency of TRNrd neurons’ response to the preferred position was 99 ms. The peak timing of spatially selective visually related neurons did not differ from that of spatially non-selective neurons (P = 0.6136, two-tailed t-test, evaluated at the single-neuron level). The latency of the PMv neuron population for the preferred position was 86 ms, which did not differ from that of TRNrd neurons (P = 0.9417, two-sample t-test, evaluated at the single-neuron level). The population activity in response to the preferred target reached peaks at 137 ms in the TRNrd and 108 ms in the PMv (Fig. 6A and E, P = 0.6807, two-tailed t-test). In terms of spatial selectivity, the TRNrd reached peak selectivity at 132 ms after the target onset, whereas the PMv did so at 107 ms after target onset (Fig. 6C and G). The peak timing of the TRNrd and PMv did not differ (P = 0.1086, two-tailed t-test, evaluated at the single-neuron level). These results showed that spatial selectivity began at similar times in the TRNrd and PMv, whereas the development of selectivity tended to be earlier in the PMv than in the TRNrd.
Set-related neurons in the TRNrd and PMv
Set-related activity during preparation for a reaching movement was also observed. Figure 3C shows a TRNrd neuron with set-related activity. Activity in this neuron built up in preparation for the reaching movement. Figure 3B shows another TRNrd neuron with set-related activity. This neuron showed a sustained activity increase during the preparation period. The set-related activity of these neurons was similar for the right- and left-reach targets. In the TRNrd, 42 of the 45 (93%) set-related neurons were spatially non-selective for the direction of reaching movement. The remaining three neurons were spatially selective and showed multiphasic responses (visual-related activity, n = 1; movement-related activity, n = 1; visual- and movement-related activity, n = 1). In the PMv, 175 of the 292 (60%) neurons were of the spatially non-selective type. Thus, the proportion of spatially non-selective neurons was greater in the TRNrd than in the PMv (P < 0.0001, Fisher's exact test). In the TRNrd, a majority (n = 32; 76%) of the spatially non-selective neurons showed an activity increase, whereas the remaining 10 (24%) showed an activity decrease. In the PMv, non-selective neurons showed decreased (n = 79, 45%) and increased activity (n = 96, 55%; Fig. 5E). The fraction of neurons with activity increase was greater in the TRNrd than in the PMv (Table 2, P = 0.0003, Fisher's exact test). These results showed that during preparation for movement, direction selectivity was lower in the TRNrd than in the PMv and that the TRNrd neurons were characterized by an activity increase.
Movement-related neurons in TRNrd and PMv
The TRNrd neurons in Figs 3A–C showed a robust increase in movement-related activity. Activity in these neurons was similar in the right- and left-target positions (P = 0.4923, two-sample t-test). In the TRNrd, of the 64 movement-related neurons, 20 (31%) were spatially selective for the target position (Fig. 3B and C, P = 0.0037, binomial test). Out of these, 11 (55%) neurons showed multiphasic responses (visual-related activity, n = 5; set-related activity, n = 3; visual- and set-related activity, n = 3). In the PMv, 123 of 300 (41%) neurons were selective for the target position. In contrast to the visual- and set-related neurons, the fraction of spatially selective neurons did not differ between the TRNrd and the PMv (P = 0.1606, Fisher's exact test).
Of the 44 spatially non-selective TRNrd neurons, 37 (84%) showed an activity increase, and the remaining 7 (16%) displayed an activity decrease. In the PMv, 104 spatially non-selective neurons (59%) exhibited an activity increase, and 73 (41%) showed an activity decrease. The fraction of those showing an increase in the TRNrd was significantly greater than that in the PMv (Table 2, P = 0.0015, Fisher's exact test). Thus, the spatially non-selective movement-related TRNrd neurons, like non-selective visual- and set-related neurons, were characterized by activity increases. Figure 5C (blue and red) shows the population activity of the movement-related TRNrd neurons with activity increases, and Fig. 5C (cyan and orange) shows the population activity of those with activity decreases. The lead times of population activity of the TRNrd neurons with activity increases were −496 ms and −514 ms (25% of peak) relative to touching the right and left targets, respectively, and those of the PMv neurons were −457 ms (right target) and −488 ms (left target, Table 4) respectively. The lead times of activity in the TRNrd and PMv neurons at the single-neuron level did not differ for either position (P > 0.05, two-tailed t-test). The activities of TRNrd neurons peaked at −135 ms (67.4 spikes/s) and −174 ms (69.2 spikes/s) relative to touching the right and left targets respectively. The activities of PMv neurons peaked at −73 ms and −68 ms relative to touching the right and left targets respectively (Fig. 5C and F). The peak timing in the TRNrd and PMv (P = 0.3882 for the right target, P = 0.9521 for the left target, two-tailed t-test, evaluated at the single-neuron level) did not differ. Because the overall movement time was <400 ms (Table 1), these results showed that the movement-related activity of spatially non-selective TRNrd neurons, as well as that of the PMv neurons, began earlier than the initiation of movement. They also revealed that the timing of activity modulation of TRNrd neurons was similar to that of PMv neurons.
| Target type | Movement onset | Touch | ||||
|---|---|---|---|---|---|---|
| Lead time (ms) | Timing of activity peak (ms) | Peak firing rate (spikes/s) | Lead time (ms) | Timing of activity peak (ms) | Peak firing rate (spikes/s) | |
| TRN | ||||||
| Right | −121 (−54 ± 67) | 66 | 67.8 | −496 (−407 ± 22) | −135 | 67.4 |
| Left | −165 (−154 ± 49) | 219 | 68.1 | −514 (−430 ± 23) | −174 | 69.2 |
| PMv | ||||||
| Right | −197 (−73 ± 19) | 154 | 38.9 | −457 (−358 ± 12) | −73 | 38.9 |
| Left | −196 (−45 ± 19) | 143 | 38.8 | −488 (−383 ± 11) | −68 | 38.1 |
- Numbers indicate the timings of population activity (ms) and the peak firing rate (spikes/s). Numbers in parentheses indicate means ± standard error of means (ms) of single neurons.
Next, we focused on the direction-selective neurons. The movement-related activity of the TRNrd neuron shown in Fig. 3D was greater for the right than for the left target. With respect to touching the target, activity modulation of the population activity began at −444 ms in the TRNrd and −455 ms in the PMv in the case of the preferred target location (Table 5). At the single-neuron level, PMv neurons started discharging earlier than TRNrd neurons did (two-tailed t-test, P = 0.0001; Table 5). The population activity in the TRNrd reached a peak at −86 ms relative to the target touch in the preferred location, whereas the peak in the case of the PMv was at −85 ms. The greatest activity difference between the preferred and the non-preferred locations was observed at −20 ms (19.6 spikes/s) in the TRNrd and at −78 ms (11.8 spikes/s) in the PMv relative to target touch (Fig. 6D and H). The timing of the greatest differences did not differ between the TRNrd and the PMv (P = 0.9982, two-tailed t-test, evaluated at the single-neuron level). These results revealed that the direction selectivity of movement-related neurons began later in the TRNrd than in the PMv, although the subsequent development of the direction selectivity was similar in the two regions.
| Target type | Movement onset | Touch | ||||
|---|---|---|---|---|---|---|
| Lead time (ms) | Timing of activity peak (ms) | Peak firing rate (spikes/s) | Lead time (ms) | Timing of activity peak (ms) | Peak firing rate (spikes/s) | |
| TRN | ||||||
| Preferred target | −119 (−289 ± 80) | 227 | 76.4 | −444 (−424 ± 17) | −86 | 75.3 |
| Non-preferred target | −133 (−858 ± 55) | −59 | 63.7 | −490 (−425 ± 23) | −350 | 62.8 |
| PMv | ||||||
| Preferred target | −218 (−291 ± 36) | 118 | 30.1 | −455 (−313 ± 16) | −85 | 32.1 |
| Non-preferred target | −232 (−354 ± 40) | 140 | 21.7 | −499 (−393 ± 18) | −103 | 21.4 |
- Numbers indicate the timings of population activity (ms) and the peak firing rate (spikes/s). Numbers in parentheses indicate means ± standard error of means (ms) of single neurons.
Locations of the visual-, set-, and movement-related neurons within the TRNrd
The neurons recorded were located within the TRNrd (Fig. 2E, F), corresponding to the portion in which neurons project to the PMv in a disynaptic manner (Fig. 2C and D). Neurons with visual- (●), set- (×), and movement-related activity (Δ) were distributed widely in the sampled TRN areas (Fig. 2F).
Discussion
We analyzed the roles of TRN neurons in visually guided reaching movements. First, we used retrograde transneuronal labeling with rabies virus, demonstrating that the TRNrd projects disynaptically to the PMv, which plays a central role in reaching movements. Next, we recorded the activities of neurons in the TRNrd and PMv while monkeys performed a visually guided reaching task. The TRNrd and PMv neurons showed visual-, set-, and movement-related activity modulation, indicating that the TRNrd and PMv are involved in the reception of visual signals and in the preparation for and execution of movement. The fraction of neurons that were non-selective for the location of visual signals and the direction of reaching movement was greater in the TRN than in the PMv. Furthermore, the fraction of neurons with activity increases was greater in the TRN than in the PMv. The timing of activity modulations of visual- and movement-related neurons was similar in the TRNrd and PMv neurons. These results indicated that TRN neurons whose activity is modulated in parallel with PMv neurons, send spatially non-selective, inhibitory signals to the thalamic relay nuclei while receiving visual signals and preparing for and executing movements.
The TRN sector projecting disynaptically to the PMv
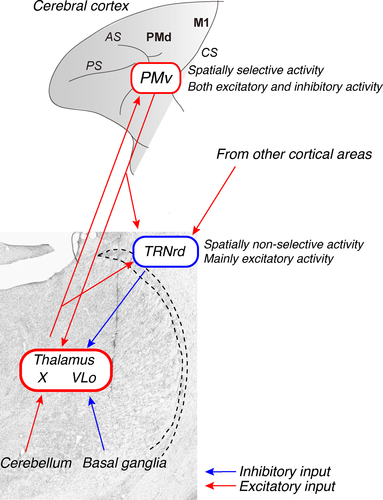
Using the trans-synaptic transport of rabies virus, we identified a sector of the TRN that projected to the PMv in a disynaptic manner. The rabies virus injected into the PMv is considered to be transferred retrogradely to the TRN, primarily via neurons in the thalamic relay nuclei, such as the X and VLo, which send direct projections to the PMv (Fig. 2C and D) (Rouiller et al., 1999). Neurons in these nuclei receive major inputs from the cerebellum (to the X) and basal ganglia (to the VLo) (Schell & Strick, 1984; Sakai et al., 1996). Altogether, the present results suggest that the TRN is involved in modulating the information flow from the cerebellum and basal ganglia to the cerebral cortex via thalamus (Fig. 7). The neurons labeled in the TRN were found rostrocaudally, in the rostral to middle part, and dorsoventrally, in the dorsal to middle part of the TRN. The TRN sector (the TRNrd) defined in this study corresponds largely to the region that receives inputs from the cortical motor areas (Chow, 1952; Schell & Strick, 1984; Rouiller et al., 1998). Additionally, the TRNrd seems to overlap with the region that receives inputs from the prefrontal cortex and amygdala (Zikopoulos & Barbas, 2006, 2012). This suggests that the TRNrd receives not only motor signals from cortical motor areas, such as the PMv, but also cognitive/emotional signals from the prefrontal cortex (Boussaoud & Wise, 1993) and the amygdala (Nishijo et al., 1988; Adolphs et al., 1994).
Although we identified the TRN sector (TRNrd) that sends disynaptic output to the PMv and characterized neurons in the TRNrd and PMv, we did not identify the direct synaptic connectivity between the PMv and the TRNrd. Thus, we only compared the functional roles of the PMv vs. TRNrd neurons at an areal level. To get a deeper insight into the functional interactions between them, it is necessary to employ alternative approaches. In this context, Halassa et al. (2014) employed an optogenetic approach to identify TRN neurons receiving inputs from other thalamic nuclei. By recording neuronal activity from the TRN and other thalamic nuclei, they have shown that their interactions dynamically change according to the sleep-arousal states and the TRN neuron types, that is, sensory-projecting TRN neurons and limbic-projecting TRN neurons. Wimmer et al. (2015) also applied an optogentic approach to identify inputs to TRN and thalamic neurons from the prefrontal cortex and also to intercept inputs to TRN neurons from the prefrontal cortex and the visual cortex. They have demonstrated that TRN neurons receiving inputs from the prefrontal cortex dynamically modulate the activity depending on whether the animals attend to the visual or auditory stimuli. This reveals that the prefrontal cortex exerts top-down control on biased attention via the TRN. In addition, in the future it would be of great interest to identify inputs to single TRNrd neurons from multiple cortical areas (i.e. convergent inputs) by electrical or optical stimulation since Zikopoulos & Barbas (2006) have reported that a single TRN sector receives converging inputs from diverse cortical areas.
Visual-related neurons in TRNrd are characterized by spatially non-selective activity and activity increase
We found that 47% of task-related neurons in the motor TRNrd showed visual-related activity. The fraction of spatially non-selective visual-related neurons was greater in the TRNrd (81%) than in the PMv (46%). In these non-selective neurons, the fraction of neurons with activity increase was greater in the TRNrd (91%) than in the PMv (73%). Moreover, the timing of activity increases in the TRNrd and PMv were fast and similar (~100 ms after target onset). These results showed that the visual-related TRNrd neurons sent inhibitory signals to neurons in the thalamic relay nuclei each time motor targets appeared.
In terms of sensory responses, Shosaku (1985) reported that the somatosensory sector of the rat TRN displayed less directional selectivity in vibrissa-responding neurons and that the receptive field was larger in the TRN than in the somatosensory thalamic nuclei (Pinault, 2004). This less-precise representation of sensory signals is consistent with our findings. In the visual sector of the monkey TRN, McAlonan et al. (2008) showed that the activity of TRN neurons decreased when the monkeys attended to a visual stimulus presented in the receptive field and ignored another visual stimulus presented elsewhere (in visual modality attention), while the activity of neurons in the lateral geniculate nucleus (LGN) increased. These findings indicate that the visual sector of the TRN is involved in the attentional gating of visual signals (Crick, 1984). In cross-modal attention involving visual and auditory signals, neurons in the visual TRN showed enhanced activity when monkeys attended to visual signals rather than to auditory signals (McAlonan et al., 2006). Furthermore, functional deficits in TRN neurons induced by manipulating gene expression in the TRN resulted in abnormalities in attention (Ahrens et al., 2015). These findings indicate that the TRN is involved in the attentional processing of sensory signals, by increasing and decreasing inhibitory output signals to the thalamic relay nuclei. Together with the results of the prior studies, the results of this study showing that visual-related TRNrd neurons send spatially non-selective, inhibitory signals to the thalamic relay nuclei suggest that these signals may play an important role as an attentional gateway by conveying stronger representations and eliminating weaker ones.
Set- and movement-related neurons in TRNrd tend to show spatially non-selective activity and activity increase
TRNrd neurons showed set- and movement-related activity, as described previously in cortical motor areas, including the PMv, and the motor thalamus (Tanji & Kurata, 1982; Weinrich & Wise, 1982; Godschalk et al., 1985; Kurata & Wise, 1988; Alexander & Crutcher, 1990a,b; Boussaoud & Wise, 1993; di Pellegrino & Wise, 1993; Crammond & Kalaska, 1994; Shen & Alexander, 1997; Cisek et al., 2003; Hoshi et al., 2005; Hoshi & Tanji, 2006). Previous studies have stressed that neurons in the cortical motor areas and the motor thalamus encode spatial or directional aspects of movement, which is considered important for selecting and specifying actions (Jones, 1975; Weinrich & Wise, 1982; Wise, 1985; Crutcher & Alexander, 1990; Kalaska & Crammond, 1992; Boussaoud & Wise, 1993; Kurata, 1993, 2005; Johnson et al., 1996; Kalaska et al., 1997; Shen & Alexander, 1997; Crammond & Kalaska, 2000; Wyder et al., 2003; Cisek & Kalaska, 2005; Nakayama et al., 2008; Kunimatsu & Tanaka, 2010). In the present study, we found that the fraction of spatially non-selective, set-related neurons was greater in the TRNrd (93% of set-related neurons) than in the PMv (60%). Of these, 84% of TRNrd neurons showed an activity increase, whereas a smaller number of PMv neurons (59%) did so. In the movement-related neurons, the fraction of spatially non-selective neurons in the TRNrd (69%) did not differ from that in the PMv (59%). However, among them, the fraction of neurons with activity increase was greater in the TRNrd (84%) than in the PMv (59%), as with the visual-related and set-related neurons. These results indicate that along with the visual-related neurons, the set-related and movement-related neurons in the TRNrd send spatially non-selective, inhibitory signals to the thalamic relay nuclei. These signals may be useful in sculpting set-related activity, eliminating unchosen motor signals, setting a threshold for set-related activity to gain effect, and suppressing a movement until a timely initiation.
A subset of movement-related neurons in TRNrd show spatially selective activity increase
In contrast with the visual- and set-related neurons, the fraction of spatially selective movement-related neurons in the TRNrd (31%) did not differ from that in the PMv (41%), and the timing of activity modulation was similar. In cats, movement-related activity of neurons in the motor sector of the TRN has been shown during locomotion (Marlinski et al., 2012; Marlinski & Beloozerova, 2014). The TRN activity was modulated depending on the required locomotion type (i.e. simple vs. ladder locomotion). Thus, such movement-related activity is considered to actively encode the direction of a reaching movement and the type of locomotion. Our results suggest that the TRNrd may play an important role in specifying the movement direction or spatial information when monkeys make reaching movements.
Theoretical considerations
The TRN receives inputs from the thalamic nuclei and neurons in layers VI and V of the cortical areas (Guillery & Harting, 2003; Zikopoulos & Barbas, 2006, 2012; Sherman & Guillery, 2011). Thus, the TRN receives feedback modulation from layer VI neurons and a feedforward driver from layer V neurons (Zikopoulos & Barbas, 2006). We found that the timing of visual- and movement-related neurons was similar in the TRNrd and PMv. This may suggest that the TRNrd receives feedforward driver inputs concerning visual and motor responses from cortical areas. Of the movement-related activity, 31% showed direction selectivity. Such a directional movement signal may also be carried by the feedforward driver signal from the cortical motor areas, including the PMv. The feedback modulation inputs may also contribute to generating spatially non-selective activity or form the background or baseline activity of TRN neurons. It would be of interest to determine the functional roles played by these two types of cortical input. With respect to the interaction between the PMv and the TRNrd, there are two additional possibilities. One possibility is that non-spatially selective PMv neurons preferentially project to the TRN, and the other is that PMv neurons with distinct spatial positions send converging inputs to single TRN neurons. These different mechanisms may underlie the non-spatially selective activity of TRNrd neurons.
Another important anatomical issue concerns local circuits. It has been proposed that TRN neurons have intrinsic connections with other TRN neurons, which may constitute a neural basis of lateral inhibition within the nucleus (Cox et al., 1996). We found that high-baseline discharges were suppressed at several time points, such as after visual cue onset, during preparation for movement, and during execution of movement. These findings suggest that the activity decrease, as well as any activity increase, may contribute to information processing by the TRN; furthermore, local circuits within the TRN, as well as interneurons in the thalamic relay nuclei, may also be involved in these processes. In relation to the lateral inhibition within the TRN, a recent study in a rodent has shown that the TRN intrinsic connection is functional only during a short period after birth (Hou et al., 2016). This finding is in line with a prior report showing that the lateral inhibition between TRN neurons is not so substantial (Pinault et al., 1997). This important issue should further be investigated in the TRN of nonhuman primate. Moreover, an intrinsic connection has been shown to mediate the interaction between first- and higher order thalamic relay nuclei (Pinault, 2004; Lam & Sherman, 2007) and between nuclei representing different sensory modalities (Crabtree et al., 1998; Crabtree, 1999; Crabtree & Isaac, 2002). Thus, it is possible that the TRNrd mediates the interaction between sensory and motor signals. In fact, we found that single TRNrd neurons exhibited visual- and movement-related activity. Another possibility is that the TRNrd mediates the interaction between the primary motor cortex and higher order motor areas such as the PMv. Multiple cortical motor areas are known to project to the rostro-dorsal sector of the TRN, corresponding to the TRNrd examined in the present study (Rouiller et al., 1998). Furthermore, inputs from the prefrontal cortex and inferotemporal cortex converge in the TRN (Zikopoulos & Barbas, 2006). These findings indicate that the TRN may contribute to the integration of multiple systems, such as the PMv and the primary motor cortex, by collecting inputs from multiple cortical areas and by specific interaction mechanisms formed with thalamic relay nuclei.
TRN neurons use GABA as their neurotransmitter, indicating that they exert a suppressive action on the thalamic relay neurons. Previous studies showed that modulating neuronal activity of the TRN by microinjection of GABA-related substances resulted in changes in the size of the receptive fields of neurons in the thalamic relay nuclei (Sillito & Kemp, 1983; Hicks et al., 1986; Lee et al., 1994a,b; Cotillon-Williams et al., 2008). In a similar manner, the TRN may be involved in sharpening the direction selectivity of set- and movement-related activity in neurons in the motor thalamic relay nuclei. One possibility is that the spatially non-selective activity functions as a filter and sets a threshold for passing stronger activity and for blocking weaker activity. Another possibility is that general suppression enhances the contrast of direction selectivity. Further, Pinault & Deschenes (1998) showed anatomical evidence that TRN and thalamic neurons form open loop connections in the rat thalamus. Thus, a lateral inhibition mechanism may play an important role in the information processing in the thalamic relay nuclei.
A further possible function of the TRN concerns efference copies from cortical areas. Sherman & Guillery (2011) showed that the TRN was involved in blocking efference copy signals from the cerebral cortex that were irrelevant for computations in the thalamic relay nuclei. The similar timing of activity modulation between the TRNrd and the PMv indicate that TRNrd can inhibit signals from the PMv at the level of the thalamic relay nuclei. Only the part of the motor-related activity issued by the PMv neurons that is needed in the thalamic relay nuclei may be transferred to them, while other signals may be suppressed.
In this study, we identified several features of TRN function. Further work based on these theoretical perspectives would enhance our understanding of the role(s) of the TRN in information processing through thalamo-cortical networks that are essential for almost all brain functions beyond sensory and motor aspects (Jones, 2007).
Conflict of interest
None of the authors has any competing financial interests.
Acknowledgements
The authors thank T. Ogata and T. Kuroda for technical assistance. This work was supported by AMED-CREST, AMED (to E. H. and M. T.), Japan Society for the Promotion of Science (JSPS) Postdoctoral Fellow for Research Abroad (to Y.S.), and Global COE Program of Tamagawa University (to Y.S., Y.N., T.Y., M.H., and E.H.).
Abbreviations
-
- AS
-
- arcuate sulcus
-
- CS
-
- central sulcus
-
- ICMS
-
- intracortical microstimulation
-
- LGN
-
- lateral geniculate nucleus
-
- M1
-
- primary motor cortex
-
- MD
-
- mediodorsal nucleus
-
- MT
-
- movement time
-
- PMd
-
- dorsal premotor cortex
-
- PMv
-
- ventral premotor cortex
-
- PS
-
- principal sulcus
-
- RF
-
- receptive field
-
- RT
-
- reaction time
-
- TRN
-
- thalamic reticular nucleus
-
- VA
-
- ventroanterior nucleus
-
- VG task
-
- visually guided reaching task
-
- VLc
-
- caudal division of ventrolateral nucleus
-
- VLo
-
- oral division of ventrolateral nucleus
Author contributions
Y.S., Y.N., K.I., T.Y., M.H., L.T., M.T., and E.H. designed research; Y.S., Y.N., K.I., and T.Y., performed research; Y.S., and Y.N., analyzed data; Y.S., and E.H. wrote the paper.