Rizatriptan overuse promotes hyperalgesia induced by dural inflammatory stimulation in rats by modulation of the serotonin system
Abstract
Clinical and preclinical studies have implicated serotonin (5-HT) and the 5-HT2A receptor (5-HT2AR) in the pathogenesis of medication-overuse headache (MOH). However, with no appropriate animal model to study this phenomenon it is difficult to differentiate the effects of chronic exposure to analgesics from the consequences of frequent headache attacks during the development of MOH. Therefore, this study used a novel animal model of MOH established by a combination of the overuse of rizatriptan (RIZ) and stimulation with dural inflammatory soup (IS) to investigate whether 5-HT and 5-HT2AR are involved in central plasticity and hyperalgesia. Similar to an IS infusion, IS-RIZ treatment induced nociception-related behaviours in Sprague–Dawley rats and increased Fos expression in the cortex and trigeminal pathway, whereas the RIZ injection alone did not. In addition, overuse of RIZ, administration of an IS stimulus, and a combination of these treatments, decreased the periorbital withdrawal threshold, with IS-RIZ treatment having the most significant effects. Both chronic RIZ exposure and recurring nociception decreased 5-HT expression, whereas IS-RIZ treatment led to decreased expression of 5-HT and upregulation of 5-HT2AR, which was positively correlated with Fos activation. These findings suggest that overuse of RIZ does not directly induce pain via the activation of nociceptive pathways but may increase hyperalgesia by influencing the pain modulation system. Furthermore, decreased 5-HT levels and upregulation of 5-HT2AR may play important roles in this system. Taken together, these findings indicate that medication overuse and frequent headache attacks can promote the neural plasticity associated with MOH.
Introduction
Recently, medication-overuse headache (MOH) has generated increasing interest due to its widespread influence (Evers & Marziniak, 2010). The International Headache Society (IHS) defines MOH as a condition in which patients with headache regularly overuse therapeutic agents for ≥3 months (2013). MOH has a prevalence of 0.6–2.0% in the general population (Evers & Marziniak, 2010; Yu et al., 2012), and drug discontinuation is an important treatment strategy for patients because of the close relationship of medication overuse with this disease (Chiang et al., 2016). Some headache experts regard the association between the acute overuse of medication and the development of MOH as causal, but whether the medication overuse is a consequence of living with chronic headaches or vice versa remains debated (Dodick, 2002; Bahra et al., 2003; Lipton & Bigal, 2003). Furthermore, the mechanisms by which chronic exposure to medication lead to MOH remain unclear because not all patients who overuse medications develop MOH.
Rodent studies have demonstrated that neural plasticity is important in the pathogenesis of MOH (Bongsebandhu-phubhakdi & Srikiatkhachorn, 2012). Of the various neurotransmitters involved in the pain modulation process, serotonin (5-HT) is the most important (Sommer, 2004; Panconesi, 2008). Similarly, clinical studies have shown that individuals with headaches due to medication overuse tend to exhibit low 5-HT levels and upregulation of the 5-HT2A receptor (5-HT2AR) (Srikiatkhachorn & Anthony, 1996a,b), which also indicates that the 5-HT system plays a role in MOH.
Several animal models have been developed to investigate the pathogenesis of MOH (Srikiatkhachorn et al., 2000; De Felice et al., 2010). For example, systemic administration of triptan to rats (De Felice et al., 2010) elicits time-dependent and reversible cutaneous tactile allodynia and increased expression of calcitonin gene-related peptide in trigeminal dural afferents. Chronic paracetamol administration results in an adaptation of the 5-HT system in rats (Srikiatkhachorn et al., 2000), which may provide a basis for transformation of migraines to MOH. However, these models cannot represent the repeated episodic nature of headache attacks in patients with MOH, and overuse of analgesics seems unreasonable in the absence of headache attacks. Moreover, an efficient method to differentiate the effects of overuse of analgesics from the consequences of frequent headache attacks is needed.
Repeated infusions of inflammatory soup (IS), a mixture of proinflammatory mediators, onto the intact dura in awake rats is widely used in animal models of chronic migraine to simulate recurrent activation of the trigeminal nociceptive system, which is assumed to occur in patients with headache (Oshinsky & Gomonchareonsiri, 2007; Melo-Carrillo & Lopez-Avila, 2013). To our knowledge, no published studies have investigated the effects of the combination of medication overuse and migraine in an animal model. Thus, this study focused on generating a novel animal model of MOH by employing the combined effects of overuse of rizatriptan (RIZ) and dural inflammatory stimulation with IS. To elucidate the pathophysiology underlying MOH, this study also investigated the involvement of 5-HT and 5-HT2AR in central plasticity and hyperalgesia using this novel animal model of MOH.
Materials and methods
Ethical concerns
This study included 40 male Sprague–Dawley rats that weighed 190 to 200 g (Research and Technology Service Centre of the Chinese PLA 302 Hospital, Beijing, PR China). All experimental procedures were approved by the Committee on Animal Use for Research and Education of the Laboratory Animals Center at Chinese PLA General Hospital (Beijing, PR China) and were consistent with the ethical guidelines recommended by the International Association for the Study of Pain in conscious animals (Zimmermann, 1983). All efforts were made to minimize animal suffering.
Habituation
All rats were individually housed in a controlled environment with a constant room temperature of 23 °C ± 2 °C and a constant humidity level of 50 ± 10% under standard lighting conditions (12/12-h light/dark cycle with the lights turned on at 07:00). The rats were allowed to habituate for 3 days prior to the surgery and given unrestricted access to food and water. During the habituation period, the researchers held each animal, touched the periorbital region of its head several times with von Frey monofilaments to acclimate it to the testing apparatus, and then measured its baseline sensory thresholds. Subsequently, each rat was placed in the observation room daily for a period of 15 min. After the completion of all procedures, the rats were returned to their individual cages.
Surgical procedure
Following the habituation period, each rat was put under general anaesthesia with 10% chloral hydrate (4 mL/kg intraperitoneally) and then placed into a stereotactic frame. An incision was made on the dorsal surface of the scalp to expose the skull. A cranial window was drilled into the left frontal bone (1.0 mm in diameter, 1.5 mm beyond the transverse sinuses and 1.5 mm left of the superior sagittal sinus) to expose the dura mater adjacent to the superior sagittal sinus with care taken not to damage the dura. A plastic cap with a stainless steel inner cannula (C = 1 mm, 62001; RWD Life Science Co., Ltd., Shenzhen, China) was then manoeuvred into the drilled hole and placed just above the skull without touching the meningeal tissue to ensure that the dura mater was not lacerated. The plastic cap was attached to the skull using small screws and dental cement, and the cannula was sealed with an obturator cap (G = 0 mm, 62101; RWD Life Science Co., Ltd.) that extended just beyond the end of the cannula over the dura, preventing scar tissue from forming over the hole of the inner cannula. Finally, the sectioned skin was sutured leaving only the obturator cap outside of the skin. All rats received prophylactic treatment with antibiotic injections (penicillin, 0.1 million IU/100 g) for 3 days following the surgery. In addition, all rats were allowed to recover for at least 3 days prior to the initiation of the experimental procedure so that their sensory thresholds could return to presurgical baselines.
Experimental procedure
The rats were randomly divided into the following four groups (n = 10 per group): IS plus RIZ benzoate (IS-RIZ), IS, RIZ benzoate (RIZ) and control (Con). Rats in the IS-RIZ group received a subcutaneous injection of RIZ benzoate dissolved in saline (4 mg/kg) and an infusion of IS (10 μL) once daily for 21 days. Rats in the IS, RIZ and Con groups were subjected to the same procedure, but either the RIZ benzoate was replaced with saline, the IS was replaced with saline, or both were replaced with saline respectively. The RIZ or saline injection occurred 5 min prior to either the IS or saline infusion. The IS contained histamine (1 mm), 5-HT (1 mm), bradykinin (1 mm) and prostaglandin E2 (0.1 mm) in normal saline. The IS or saline infusion was administered over 5 min while the rat was freely moving to allow for diffusion into the tissue surrounding the dura. A visual inspection of the skin was performed to ensure that there was no irritation due to leakage of the IS onto the skin.
Tactile sensory tests were conducted every other day prior to the injection of RIZ benzoate or saline, and data obtained prior to the first treatment were recorded as baseline data (d0). Each rat was restrained by hand to prevent it from walking away during the sensory testing, but this restraint was atraumatic because the rats could turn around with some difficulty. Nociceptive thresholds were measured by perpendicularly applying a von Frey monofilament (North Coast Medical Co., Ltd., Gilroy, CA, USA) to the periorbital region of the rats until it buckled slightly and then maintaining that position for 3–6 s or until a positive response was observed. The thresholds were determined using the ‘up-down’ method (Chaplan et al., 1994). When the rat withdrew its face from the von Frey monofilament, a positive response was recorded. Rats that did not respond to the 26-g stimulus, which is the maximum filament strength, were assigned 26 g as their threshold for analysis.
After undergoing the dura infusion and subcutaneous injections, the rats were transported to the observation room in their cages. A high-definition video camera was placed 150 cm in front of the observation cage and positioned so that the camera covered all angles of the cage to obtain a complete image of the rat's behaviour. After the first treatment (d1), each rat was recorded once every 2 days for 15 min throughout the duration of the experiment (21 days). Specific behaviours were assessed to analyse meningeal nociception as described by (Melo-Carrillo & Lopez-Avila, 2013); these included exploratory and resting behaviours and ipsilateral and bilateral facial grooming behaviours. The total time spent engaged in each of these behaviours was manually measured in seconds. All videos were analysed three times by three investigators who were unaware of the group assignments.
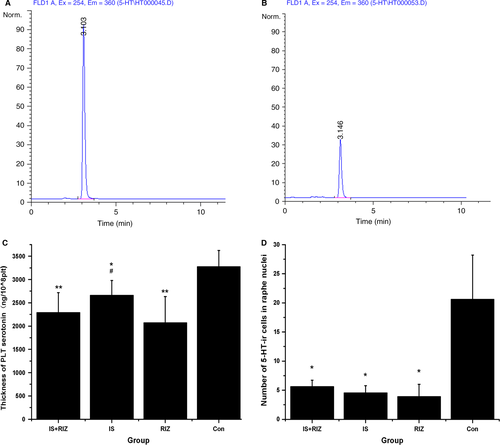
After 21 days, all rats were humanely killed by an overdose of 10% chloral hydrate (15 mL/kg intraperitoneally), and blood and brain tissue samples were immediately obtained. Platelets were obtained from the whole blood as described by Audhya (Srikiatkhachorn et al., 2000) and prepared for the detection of 5-HT using a high-performance liquid chromatography-fluorescence detector (HPLC-FLD). In addition, five rats from each group were transcardially perfused with 200 mL of cold fresh saline and then 400 mL of fresh fixative (0.1 m phosphate buffer containing 4% paraformaldehyde [pH 7.4]). The brain of each rat was removed, post-fixed and stored until the immunohistochemistry assay. The other five rats from each group were perfused through the heart with 200 mL of cold fresh saline, and brain tissue samples were quickly collected on ice. The frontal cortex and brain stem of each rat were isolated and stored at −80 °C until western blot analysis.
Determination of platelet 5-HT levels
Platelet 5-HT levels were analysed using the HPLC-FLD method. An HPLC chromatography system (Agilent, Santa Clara, CA, USA) was used for chromatographic separation, was achieved on a ZORBAX SB-C18 chromatography column (Agilent). The column temperature was maintained at 35 °C, and the flow rate was 1.0 mL/min for all experiments. The mobile phase consisted of a phosphoric buffer solution (pH 6.5, 10 mm phosphoric acid, 0.1 mm KH2PO4-methanol-acetonitrile (85 : 10 : 5, v/v/v). Fluorescence was monitored at excitation and emission wavelengths of 254 and 360 nm respectively. Peaks were identified by comparing their retention time in the sample solution with that of a standard solution. Each sample was analysed in duplicate, and the platelet 5-HT content was expressed as ng per 108 platelets.
Immunohistochemistry assays
The Fos and 5-HT2AR immunohistochemistry assays were performed on brain tissue samples from the frontal cortex, periaqueductal grey (PAG), rostral ventromedial medulla (RVM) and trigeminal nucleus caudalis (TNC), all of which play significant roles in the pain modulation process. Fixed samples were embedded in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA, USA) and cut into 10-μm-thick serial sections on a freezing microtome (CM1850; Leica, Wetzlar, Germany). The cortical areas were chosen by placing the grid on the uppermost aspect of the orbital pole of the frontal cortex; the same procedure was used for all sections to minimize variation.
The sections were collected at intervals in three sets of glass slides coated with poly-l-lysine (ZLI-9005; ZSGB-BIO, Beijing, China). The sections were permeabilized in 0.2% Triton X-100, blocked with 10% goat serum (ZLI-9005; ZSGB-BIO) and incubated for 24 h at 4 °C with either rabbit antiserum against Fos (1 : 100, sc-52; Santa Cruz Biotechnology, Dallas, TX, USA) or rabbit antiserum against 5-HT2AR (1 : 500, PC176; Millipore, Billerica, MA, USA) in primary antibody dilution buffer (ZLI-9028; ZSGB-BIO). The sections were then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (PV-6002; ZSGB-BIO) for 30 min at 37 °C, and peroxidase activity was visualized using a 3,3′-diaminobenzidine kit (ZLI-9033; ZSGB-BIO). The sections were weakly counterstained with haematoxylin, dehydrated and then sealed with mounting medium. Between each step, the sections were thoroughly washed with 0.01 m phosphate-buffered saline.
5-HT immunohistochemistry was performed on tissue samples from the brain stem and raphe nuclei area because this is a major location for central 5-HT neurons. The above-mentioned staining method was used except that a specific antiserum against 5-HT2AR (1 : 500, Ab16007; Abcam, Cambridge, UK) was used as the primary antibody. Immunoreactive cells were counted in squares at 200× magnification using a light microscope (DP73; Olympus, Tokyo, Japan), and the brain regions were identified according to the atlas by Paxinos and Watson (Watso, 2004). Three squares per section per brain region were randomly selected, and three sections for each region were quantitated to determine the number of Fos-immunoreactive (Fos-ir), 5-HT2AR-immunoreactive (5-HT2AR-ir) and 5-HT-immunoreactive (5-HT-ir) neurons in the targeted brain regions. The data from nine sampling areas from each rat were averaged and expressed as the number of neurons per brain region. The identity of each rat was concealed throughout the counting process.
Western blot analysis
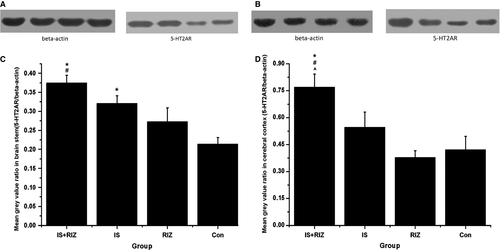
The obtained frontal cortex and brain stem tissue samples were chopped into small pieces and homogenized in ice-cold radioimmunoprecipitation assay buffer (P0013B; Beyotime Biotechnology, Haimen, China) containing 0.1% phenylmethylsulfonyl fluoride (ST506; Beyotime Biotechnology). The homogenates were centrifuged at 12 000 g for 20 min, the dissolved proteins were collected from the supernatant, and protein concentrations were determined using a bicinchoninic acid protein assay kit (CW0014; Beyotime Biotechnology). Identical amounts of protein (40 μg) were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis and then transferred onto a polyvinylidene fluoride membrane (Millipore).
After being blocked with 5% non-fat milk in Tris-buffered saline and Tween 20 (TBST) (Amresco LLC, Solon, OH, USA) for 1 h, the membranes were incubated overnight at 4 °C with respective primary antibodies for anti-5-HT2AR (1 : 500, PC176; Millipore) and beta-actin (inner control, 1 : 1000, AA128; Beyotime Biotechnology). After being washed with TBST three times, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1 : 4000, A0208; Beyotime Biotechnology) for 1 h at room temperature. The antibody-reactive bands were visualized using enhanced chemiluminescence detection reagents (P0018; Beyotime Biotechnology) and a gel imaging system (Beyotime Biotechnology).
Statistical analysis
The spss 19.0 (IBM Corp., Armonk, NY, USA) and Origin 9.1 (OriginLab, Northampton, MA, USA) software packages were used for the statistical analyses and graph generation respectively. Levene's test for homogeneity was conducted, and abnormally distributed data were analysed using the Kruskal–Wallis test to determine differences among the groups. Difference in the periorbital withdrawal threshold among groups were compared using a two-way repeated-measures anova with a Holm–Sidak post hoc comparison after data were examined for non-normality and equal variance. Otherwise, all data were analysed using analysis of variance (anova) and then a least significant differences (LSD) t-test (when the variance was regular) or Dunnett's T3 test (when the variance was irregular) to compare the differences among the groups. Pearson's correlation coefficient test was conducted to determine the relationship between the expressions of Fos and 5-HT2AR. All data are presented as the mean ±standard deviation (SD), and P values of <0.05 were considered to indicate statistical significance.
Results
Nociception-related behaviour elicited by IS-RIZ
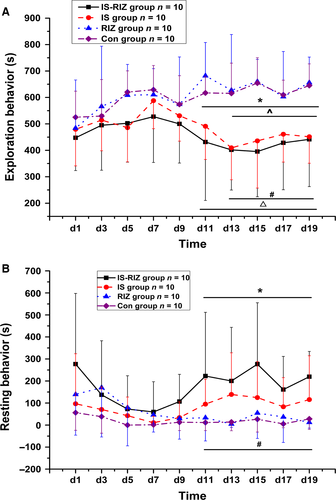
Four types of possible nociception-related behaviour were analysed to confirm whether the rats in this study had ‘headache attacks’. In contrast to previous findings (Melo-Carrillo & Lopez-Avila, 2013), there were no significant differences in ipsilateral or bilateral facial grooming behaviours among the groups (Kruskal–Wallis test, P > 0.05; data not shown). However, the IS-RIZ and IS groups exhibited a significant decrease in exploratory behaviours and a significant increase in resting behaviours compared with the RIZ and Con groups (Kruskal–Wallis test, P < 0.05).
The times spent performing exploratory behaviours (Fig. 1A) were shorter in the IS-RIZ group than in the RIZ and Con groups from day 11 (Kruskal–Wallis test, χ23 = 13.749 (N = 40), P = 0.015) and day 13 (Kruskal–Wallis test, χ23 = 16.178 (N = 40), P = 0.041) of the experiment respectively. In addition, the IS group exhibited fewer exploratory acts than the RIZ and Con groups from and day 11 (Kruskal–Wallis test, χ23 = 13.749 (N = 40), P = 0.012) and day 13 (Kruskal–Wallis test, χ23 = 16.178 (N = 40), P = 0.031) respectively. Meanwhile, the time spent performing resting behaviours (Fig. 1B) was markedly higher in the IS-RIZ group from day 11 than in both the RIZ (Kruskal–Wallis test, χ23 = 13.749 (N = 40), P = 0.018) and Con (Kruskal–Wallis test, χ23 = 13.749 (N = 40), P = 0.013) groups; however, no significant increases were observed in the IS group.
Changes in the periorbital withdrawal threshold
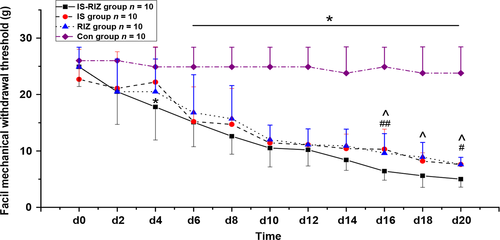
The two-way anova comparing facial allodynia threshold showed a significant interaction between group and time (F0.05, (3,36) = 45.275, P = 0.011; Fig. 2). And post hoc tests showed that the IS (two-way anova, P = 0.002) and RIZ groups (two-way anova, P = 0.010) showed significantly persistent declines in their periorbital mechanical withdrawal thresholds than those observed in the Con group from day 6 (Fig. 2). In particular, the IS-RIZ group began to exhibit lower thresholds relative to the Con group after the application of the third IS stimuli (two-way anova, P = 0.037). Moreover, the IS-RIZ group had lower thresholds than the IS (two-way anova, P = 0.009) and RIZ (two-way anova, P = 0.028) groups from day 16 to the end of the experiment, and these differences increased with time. These results indicate that the IS-RIZ group was in a state of relatively greater hyperalgesia.
Effects of IS-RIZ on Fos expression in headache-related brain regions
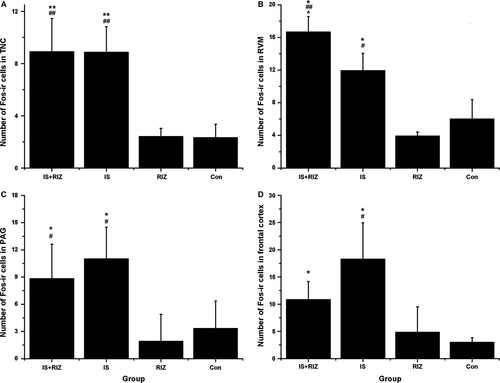
Fos, which is the protein product of the activated c-Fos proto-oncogene, is often used as a marker of neuronal activity (Harris, 1998). Consequently, Fos expression was examined in this study to investigate the activation of nociceptive pathways by the IS infusions. There was an obvious increase in the number of Fos-ir neurons in the RIZ-IS and IS groups compared with the Con group in the TNC (anova, F0.05, (3,16) = 19.191, P < 0.001), RVM (anova, F0.05, (3,16) = 49.328, P < 0.001), PAG (anova, F0.05, (3,16) = 7.675, P = 0.003) and frontal cortex (anova, F0.05, (3,16) = 10.859, P < 0.001) (Fig. 3). In contrast, there were no significant differences between the RIZ and Con groups in any of these brain regions (anova, P = 1.000, 0.458, 0.537 and 0.983 in the TNC, RVM, PAG and frontal cortex respectively).
Effects of IS-RIZ on peripheral and central 5-HT expression
Platelets are widely used as a model of the expression of neuronal serotonergic cells (Audhya et al., 2012). In this study, the 5-HT content of platelets was detected using the HPLC-FLD method to evaluate the effects of IS infusion and RIZ administration (Fig. 4A and B). The IS, RIZ and IS-RIZ groups had lower platelet 5-HT levels than did the Con group (anova, F0.05, (3,36) = 15.256, P < 0.001) (Fig. 4C), and the RIZ group had lower platelet 5-HT levels than did the IS group (LSD t-test, P = 0.004). Similarly, the central 5-HT system, which is primarily located in the raphe nuclei, exhibited an obvious decrease in the number of 5-HT-ir neurons in the IS-RIZ, IS and RIZ groups compared with the Con group (anova, F0.05, (3,16) = 19.889, P < 0.001) (Fig. 4D). Taken together, these data demonstrate that both the repeated administration of noxious stimuli and the overuse of triptan induced decreases in 5-HT levels.
Effects of IS-RIZ on 5-HT2AR expression
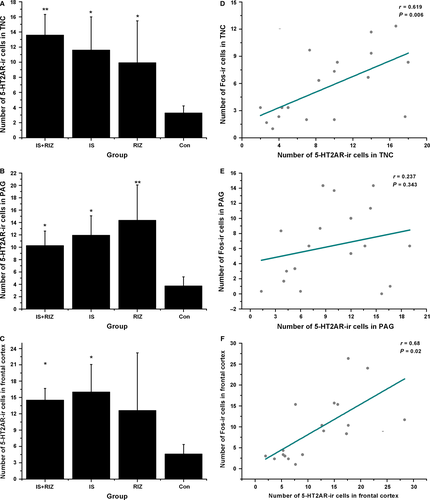
Both the IS-RIZ and IS groups expressed more 5-HT2AR-ir neurons than did the Con group in the TNC (anova, F0.05, (3,16) = 4.176, P = 0.025), PAG (anova, F0.05, (3,16) = 8.675, P = 0.001) and frontal cortex (anova, F0.05, (3,16) = 6.985, P = 0.003) (Fig. 5A–C). In addition, an upregulation of 5-HT2AR was observed in the RIZ group in the TNC and PAG (Fig. 5A and B). This study also assessed 5-HT2AR protein levels using Western blot analyses (Fig. 6). In the brain stem (Fig. 6C), both the IS-RIZ (LSD t-test, P = 0.001) and IS (LSD t-test, P = 0.010) groups expressed more 5-HT2AR receptor proteins than did the Con group, and there was a significant difference between the IS-RIZ and RIZ groups (LSD t-test, P = 0.013). On the other hand, the IS-RIZ group exhibited a greater degree of 5-HT2AR upregulation than did the other three groups in the frontal cortex (anova, F0.05, (3,12) = 6.284, P = 0.008) (Fig. 6D).
Correlation between expression of Fos and 5-HT2AR
In this study, the number of Fos-ir neurons was positively correlated with the number of 5-HT2AR-ir neurons in the TNC (r18 = 0.619, P = 0.006) (Fig. 5D) and frontal cortex (r18 = 0.68, P = 0.020) (Fig. 5F), which suggests that Fos expression was associated with 5-HT2AR expression levels. No significant results were found in the PAG (r18 = 0.237, P = 0.343) (Fig. 5E).
Discussion
This study aimed to develop an animal model of MOH using a combination of dural inflammation-induced headaches and the overuse of RIZ. This model was then used to differentiate the effects of chronic analgesic intake and the consequences of frequent headache attacks. In terms of behaviour, the IS-RIZ group exhibited an increase in resting behaviours and a decrease in exploratory behaviours, which is correlated with previous clinical studies showing that migraineurs have a tendency to reduce their routine physical activity and prefer to rest during headache attacks (Melo-Carrillo & Lopez-Avila, 2013). On the other hand, the IS group showed a reduction in routine physical activity but not an increase in resting behaviour. These findings suggest that the IS-RIZ group suffered more severe headache attacks than the IS group. No nociception-related behaviours were observed in the RIZ group, which indicates that RIZ overuse does not cause headache attacks but that it might aggravate the nociception-related behaviours induced by the infusion of IS.
Various types of noxious stimulation, including thermal, mechanical and chemical stimuli, induce the expression of Fos in the brain and spinal cord (Han et al., 2003). A previous study from our group (Wang et al., 2011) demonstrated that Fos-ir neurons have comparatively more dense distributions in the TNC, RVM and PAG than in other brain regions in an animal model of migraine, which is why these brain regions were assessed for Fos-ir neurons in this study. The frontal cortex was also assessed in this study because of its possible role in headache modulation (Bongsebandhu-phubhakdi & Srikiatkhachorn, 2012). The expression of Fos in these pain-modulating brain regions significantly increased in rats that received IS infusions both with and without RIZ injections, whereas the administration of RIZ itself did not increase Fos expression. These results imply that overuse of RIZ cannot induce pain directly via the activation of the nociceptive pathway but that it may cause hyperalgesia by influencing this pain-modulating system.
This interpretation would explain why the overuse of medication is neither necessary nor sufficient to induce chronic daily headaches in and of itself (Lipton & Bigal, 2003). If patients do not have a history of headaches, then they do not develop MOH even when receiving triptans or other acute pharmacological headache treatments for non-headache indications. Conversely, patients with a history of episodic headaches frequently progress to chronic daily headaches following the overuse of headache treatment drugs (Bahra et al., 2003). In this study, the IS-RIZ group showed high expression of Fos, which demonstrated the activation of the nociceptive pathway and ultimately resulted in nociception-related behaviours.
Although the precise mechanisms underlying MOH remain unclear, the development of central sensitization might be a key factor (De Felice et al., 2011). Some clinical evidence has shown that patients with migraine complicated by MOH show a higher prevalence of allodynia than do patients with episodic migraine (Bigal & Lipton, 2008), which indicates that MOH represents a state of central sensitization. In this study, the repeated administration of IS induced hyperalgesia, which is in agreement with previous reports (Oshinsky & Gomonchareonsiri, 2007; Stucky et al., 2011). The present findings also demonstrated that the frequent administration of RIZ led to a significant decrease in periorbital withdrawal thresholds and exacerbated the decrease induced by IS infusion because the IS-RIZ group manifested a state of hyperalgesia more quickly (after just the third treatment) and more severely. Thus, it is possible that an overdose of RIZ may serve as an aggravating factor for hyperalgesia rather than as a therapy. These results are similar to those demonstrating hyperalgesia in patients with MOH and suggest that RIZ-induced hyperalgesia may account for the exacerbation of central sensitization, at least to some extent.
5-HT has also been implicated in the development of MOH (Srikiatkhachorn et al., 2000; Ayzenberg et al., 2008), and the impact of IS-RIZ on the 5-HT system was therefore investigated in this study. Consistent with other research findings (Srikiatkhachorn et al., 2000), the overuse of analgesics decreased 5-HT levels in this study. The IS group exhibited a decline in 5-HT levels, and their average platelet 5-HT level was higher than that of the RIZ group. However, there was no significant difference in central 5-HT-ir neurons between these two groups, which implies that medication overuse may have a greater influence on the peripheral 5-HT system than frequent headache attacks. In addition, it is possible that the decreased 5-HT levels in the IS-RIZ group may have been due to the combined effects of RIZ and recurring nociception.
It is well documented that the modulation of pain mediated by the noradrenergic and serotonergic pathways is important for the descending inhibitory control of spinal nociceptive activity (Bannister et al., 2009). However, more recent studies have found that 5-HT is important in the mediation of descending facilitation from the brain stem (Wei et al., 2010), particularly the role of 5-HT2AR in regulating the excitatory drive (Rahman et al., 2011). Moreover, accumulating evidence has demonstrated the facilitating effects of 5-HT2AR in the pain perception process. For instance, an animal study found that chronic exposure to paracetamol results in an enhancement of cortical excitability and facilitates trigeminal nociception (Supornsilpchai et al., 2010), in which 5-HT2AR may be implicated. Similarly, other studies have shown that 5-HT2AR can act centrally to facilitate nociception (Van Steenwinckel et al., 2008; Kupers et al., 2009).
The present observation that IS-RIZ treatment induced the upregulation of 5-HT2AR, which was positively correlated with Fos expression in the TNC and frontal cortex, supports the role of this receptor in central sensitization. However, the results of the correlation test were not sufficiently robust to verify a causal relationship between the upregulation of 5-HT2AR and the high expression levels of Fos. Thus, future studies that involve the application of agonists and/or antagonists of 5-HT2AR are needed. The present data show that IS-RIZ, IS and RIZ all caused central upregulation of 5-HT2AR, whereas IS-RIZ generally had the most significant effects and RIZ had the least significant effects. These results indicate that both medication overuse and repeated inflammatory stimuli can both promote neural adaption processes but that chronic headache may be more influential to some extent.
Taken together, the present findings suggest that the pathogenesis of MOH may develop in the following manner. First, both the recurring headache attacks and the overuse of analgesics can induce a depletion of 5-HT and lead to an upregulation of 5-HT2AR, although chronic headaches might play a more important role. Second, the upregulation of 5-HT2AR may augment central sensitization, which would in turn enhance pain perception. Finally, based on this central sensitization, a pre-existing headache would ultimately develop into a new type of headache; namely, MOH.
In summary, this study describes the development of a novel animal model of MOH that combines the overuse of RIZ and the application of dural inflammatory stimulation. Experiments using this model demonstrated the role of the 5-HT system, particularly 5-HT2AR, in the development of hyperalgesia. This novel model has several advantages over previous animal models simply by employing the overuse of analgesics. On the one hand, IS-RIZ treatment induced nociception-related behaviours and hyperalgesia in rats, which resembled the clinical features of MOH; on the other hand, the present findings concern the effects of RIZ overuse as well as the effects of recurring nociception. Nonetheless, further investigation is necessary because this study did not assess the response to medication withdrawal, include migraine therapeutic agents other than RIZ, or evaluate the role of 5-HT2AR using agonists and/or antagonists of this receptor.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgements
This work was supported by the National Science Foundation of China (grants 81171058 and 81471147) and National Key Technology Support Program (grants 2013BAI04B04). The authors thank Hubei OULY Pharmaceutical Co., LTD (Hubei, China) for the gift of rizatriptan.
Abbreviations
-
- 5-HT
-
- serotonin
-
- 5-HT2AR
-
- 5-HT2A receptor
-
- IS
-
- inflammatory soup
-
- MOH
-
- medication-overuse headache
-
- PAG
-
- periaqueductal grey
-
- RIZ
-
- rizatriptan
-
- RVM
-
- rostral ventralmedial medulla
-
- TNC
-
- trigeminal nucleus caudalis