The complex relationship between the light-entrainable and methamphetamine-sensitive circadian oscillators: evidence from behavioral studies of Period-mutant mice
Abstract
The methamphetamine-sensitive circadian oscillator (MASCO) is an enigmatic circadian clock whose output is observed during continuous consumption of low-dose methamphetamine. The MASCO rhythm persists when the light-entrainable pacemaker in the suprachiasmatic nucleus (SCN) is lesioned, but the anatomical location of MASCO is unknown. We recently found that the period of the MASCO rhythm is unusually short (21 h) in mice with disruption of all three paralogs of the canonical clock gene, Period. In this study, we investigated the contribution of each Period paralog to timekeeping in MASCO. We measured wheel-running activity rhythms in intact and SCN-lesioned Per1-, 2- and 3-mutant mice administered methamphetamine, and found that none of the mice displayed a short (21-h) period, demonstrating that no single Period gene is responsible for the short-period MASCO rhythm of Per1−/−/Per2−/−/Per3−/− mice. We also found that the periods of activity rhythms in constant darkness were lengthened by methamphetamine treatment in intact wild-type, Per1−/− and Per3−/− mice but not Per2−/− mice, and Per2−/− mice had two distinct activity rhythms upon release to constant light. These data suggest that the SCN and MASCO are not coupled in Per2−/− mice. The MASCO rhythm in Per1−/−/Per2−/− mice in constant darkness alternated between a short (22-h) and a long (27-h) period. This pattern could result from two coupled oscillators that are not synchronised to each other, or from a single oscillator displaying birhythmicity. Finally, we propose a working model of the in vivo relationship between MASCO and the SCN that poses testable hypotheses for future studies.
Introduction
Circadian rhythms are self-sustained oscillations in physiology and behavior with endogenous periods of ~ 24 h that can be entrained to environmental cues such as the light–dark cycle and food availability (Takahashi et al., 2001). The mammalian circadian system is organised hierarchically, such that the master light-entrainable circadian clock in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus coordinates the phases of oscillators in peripheral tissues (Yamazaki et al., 2000; Yoo et al., 2004). Behavioral experiments have revealed the existence of two enigmatic oscillators, the methamphetamine-sensitive circadian oscillator (MASCO) and the food-entrainable oscillator (FEO), whose overt circadian activity rhythms are revealed by methamphetamine and food restriction (followed by food deprivation), respectively (Honma et al., 1986; Mistlberger, 1994; Tataroglu et al., 2006). Both MASCO- and FEO-controlled activity rhythms persist after SCN lesion, but the anatomical loci of these oscillators are unknown (Honma et al., 1987; Mistlberger, 1994; Davidson, 2009). Indeed, it is possible that they are the same circadian oscillator. Like the SCN, the MASCO and FEO can coordinate the phases of peripheral oscillators (Pezuk et al., 2010), suggesting that they function at the top of the hierarchy of the mammalian circadian system.
As the location of MASCO is unknown, recent studies have focused on the molecular timekeeping mechanism of this oscillator. The primary conclusion from these studies was that the MASCO is a ‘non-canonical’ circadian oscillator because free-running MASCO rhythms persist when the canonical circadian genes are non-functional (in Cry1−/−/Cry2−/−, Bmal1−/− and Per1−/−/Per2−/− mice) even though those mutations render SCN-driven locomotor activity arrhythmic (Honma et al., 2008; Mohawk et al., 2009). In contrast, we recently found that the MASCO rhythm is unusually short (~21 h) in mice with disruption of all three paralogs of the canonical clock gene Period (Pendergast et al., 2012). These data demonstrate that the Period genes are involved in the timekeeping mechanism in MASCO, but they are not necessary to sustain the oscillation. In the current study, we investigated the contribution of each of the three Period paralogs to timekeeping in MASCO. Our data demonstrate that no single Period gene is responsible for the short-period MASCO rhythm of Per1−/−/Per2−/−/Per3−/− mice. However, during the course of our experiments we identified several intriguing properties of MASCO and its relationship with the SCN. From the data described herein, we propose a working model of the in vivo relationship between the MASCO and SCN.
Materials and methods
Animals
We obtained mPer1−/−, mPer2−/− and mPer3−/− mice (Shearman et al., 2000; Bae et al., 2001) (provided by Dr David Weaver, University of Massachusetts Medical School; congenic with the 129/sv genetic background) and backcrossed them with wild-type C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) for at least 15 generations (Pendergast et al., 2009, 2010a; C57BL/6J Per1−/−, Per2−/− and Per3−/− mice are available from The Jackson Laboratory, stock numbers 10 491, 10 492 and 10 493, respectively). Wild-type and Per-mutant mice were generated from intercrossing Per-mutant mice. Adult male and female mice (6–39 weeks old) were used for all experiments. Genotyping for the Per genes was performed as previously described (Shearman et al., 2000; Bae et al., 2001). The mice were bred and group-housed in the Vanderbilt University animal facility in a 12-h light–12-h dark cycle (light intensity ~350 lux) and provided food and water ad libitum. All experiments were carried out in accordance with the National Institutes of Health Guidelines regarding the care and use of animals for experimental procedures and were approved by the Institutional Animal Care and Use Committee at Vanderbilt University (M/08/096).
Recording of circadian behavior and methamphetamine treatment
Animals were singly housed in cages (length × height × width: 33 × 17 × 14 cm) with unlimited access to a running wheel (diameter 11 cm), food and water. The cages were placed in light-tight ventilated boxes in light–dark conditions (light intensity, 200–300 lux), in constant darkness (DD) or in constant light (LL; light intensity, 200–300 lux), as indicated for each experiment. Mice were provided with 0.005% methamphetamine hydrochloride (Sigma) in their drinking (tap) water as indicated. Cages were changed every 3 weeks. Wheel-running activity (recorded every minute by computer) was monitored and analysed using ClockLab (Actimetrics, Wilmette, IL, USA). Activity data were double-plotted in actograms in 5-min bins using the normalised format in ClockLab.
SCN lesions
All surgery was performed under anesthesia by intraperitoneal administration of ketamine (100 mg/kg; Putney Inc., Portland, ME, USA) and xylazine (10 mg/kg; Lloyd Laboratories, Inc., Shenandoah, IA, USA). Buprenex (0.05 mg/kg; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA) was administered subcutaneously immediately after surgery. Mice were placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA) and bilateral lesions were made by passing a 1.1-mA direct current through a teflon-coated tungsten electrode (Unique Medical, Osaka, Japan, or A-M systems, catalog number 575500, Sequim, WA, USA) for 20 s. The tip of the electrode (0.5-mm exposed tip) was positioned 0.4 mm anterior, ± 0.25 mm lateral and 6.0 mm ventral to bregma. After surgery, mice that drank excessive amounts of water (more than ~50 mL/day) were excluded from the study. Mice that did not exhibit circadian rhythms of wheel-running in DD (for at least 10 days) were subsequently administered methamphetamine.
Period analyses
Periods were determined by χ2 periodogram with alpha set to 0.001 (periods ranging from 10 to 50 h) of days 12–22 of methamphetamine treatment in intact and SCN-lesioned mice in DD (Clocklab). Changes in phases and/or periods of methamphetamine-induced rhythms occurred frequently, and the days used for χ2 periodogram analyses were altered in these instances. A minimum of 5 days was used for periodogram analyses of all mice. Methamphetamine-induced activity rhythms are characterised by period instability (especially when the SCN is disabled), so the first period that was observed after day 12 of methamphetamine treatment is reported in Table 1. Data are presented as the mean ± SD period (Table 1). However, if a mouse expressed alternating short (< 24 h) and long (> 24 h) periods, the individual values are reported in Table 2 (in this case the periods are not reported in Table 1). Mice were subsequently released into LL and periods were determined by χ2 periodogram of days 12-22 in LL (except where changes in the phases and/or periods occurred), with the exception of Per2−/− and Per1−/−/Per2−/− mice. Upon release into LL, Per2−/− mice simultaneously exhibited two distinct rhythmic components so χ2 periodogram analysis was performed on days 1–35 in LL, and periodograms for each mouse are shown in the Supporting Information. After release into LL, some Per1−/−/Per2−/− mice sequentially expressed short and long periods so the days analysed were altered to detect both periods (reported in Table 2). Actograms for all individual mice are shown in the Supporting Information figures and the days used for χ2 periodogram analysis are indicated for each mouse on these actograms. Locomotor activity was defined as arrhythmic if no significant period was detected by χ2 periodogram analysis.
| Genotype | DD | SCN-lesioned | LL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No MA | n | +MA | n | No MA | +MA | n | No MA | n | +MA | n | |
| Wild-type | 23.63 ± 0.16 | 28* | 24.23 ± 0.22 | 4 | AR | 29.25, 30.58 | 2 of 3† | 25.32 ± 0.32 | 9‡ | 29.25 ± 1.23 | 3 |
| Per1 −/− | 23.49 ± 0.16 | 8§ | 24.71 ± 0.24 | 6 | AR | 29.67 ± 6.67 | 3 of 4† | 27.25 ± 0.79 | 10‡ | 34.96 ± 5.98 | 4 |
| Per2 −/− | 23.48 ± 0.20 | 12¶ | 23.56 ± 0.14 | 4 | AR | 27.75 ± 2.71 | 3 | 23.40 ± 0.29 | 5‡ | 23.14 ± 0.09 | 4 of 5 |
| 30.03 ± 1.82 | 5 of 5** | ||||||||||
| Per3 −/− | 23.51 ± 0.16 | 10¶ | 24.26 ± 0.20 | 8 | AR | 26.28 ± 1.35 | 5 | 25.00 ± 0.07 | 4‡ | 30.70 ± 2.55 | 5 |
| Per1 −/− /Per2 −/− | AR | 23.08 | 1 of 6†† | ND | ND | AR | 7 | 17.58, 17.17, 35.33 | 3 of 5‡‡ | ||
| Per1 −/− /Per2 −/− /Per3 +/− | AR | 21.68 ± 1.51 | 9 | ND | ND | ND | ND | ||||
| Per1 −/− /Per2 −/− /Per3 −/− | AR | 21.81 ± 0.64 | 7 of 8§§ | ND | ND | 18.91 ± 2.33 | 3 of 4¶¶ | 15.21 ± 0.64 | 4 of 5¶¶ | ||
| Per1 +/+ /Per2 −/− /Per3 −/− | 23.12 ± 0.40 | 3 | 23.28 ± 0.27 | 3 | ND | ND | ND | ND | |||
| Per1 +/− /Per2 −/− /Per3 −/− | 21.58, 22.25 | 21.85 ± 0.50 | 6 | ND | ND | ND | ND | ||||
| Per1 −/− /Per2 +/− /Per3 +/+ | 22.92, 22.94 | 24.42, 24.25 | ND | ND | ND | ND | |||||
| Per1 −/− /Per2 +/− /Per3 −/− | 22.63, 22.25 | 23.67, 23.00 | ND | ND | ND | ND | |||||
- Where indicated, mice were provided with 0.005% methamphetamine (MA) in their drinking water in DD or LL. SCN-lesioned mice were examined in DD. Periods were determined by χ2 periodogram with alpha set to 0.001 (periods ranging from 10 to 50 h). Arrhythmic (AR) mice had no significant period (in the circadian range). Data are the mean (h) ± SD. The number of mice (n) in each condition is reported. If n = 2 then the two periods are listed. ND, not determined. *Wild-type data from Pendergast et al. (2009, 2010a). †One mouse sequentially exhibited a short and a long period (not reported in this table). See Table 2 for individual period values. ‡Wild-type and Per-mutant mice in LL (no MA) data from Pendergast et al. (2010b). §Per1−/− data from Pendergast et al. (2009). ¶Per2−/− and Per3−/− data from Pendergast et al. (2010a). **Four of five Per2−/− mice simultaneously exhibited a short (23.1 h) and long (30 h) period upon release into LL (see Results). Periodograms are shown for each mouse in Fig. S10. ††Five of six mice displayed alternating short and long periods (not reported in this table) See Table 2 for individual periods for each mouse. ‡‡The mean is not reported as mice displayed either short (n = 2) or long (n = 1) periods. In addition, two of five mice displayed alternating short and long periods (not reported in this table). See Table 2 for individual periods for each mouse. §§One of eight mice displayed alternating short and long periods (not reported in this table). See Table 2 for individual periods for this mouse. Results from six of the eight mice have been previously reported (Pendergast et al., 2012). ¶¶One mouse had no significant period in LL.
| Genotype | Mouse number | Lighting condition | Figures | Period (h) | |
|---|---|---|---|---|---|
| Short (< 24 h) | Long (> 24 h) | ||||
| Per1 −/− /Per2 −/− | 5479 | DD | 3A, S12A | 19.92 | 27.33 |
| Per1 −/− /Per2 −/− | 5217 | DD | 3B, S12B | 21.00 | 27.58 |
| LL | 4B, S15B | 17.00 | 32.50 | ||
| Per1 −/− /Per2 −/− | 5493 | DD | 3C, S12C | 18.58 | 26.67 |
| Per1 −/− /Per2 −/− | 5250 | DD | 3D, S12D | 21.75 | 24.33 |
| LL | 4A, S15A | 17.92 | 30.08 | ||
| Wild-type, SCN-lesioned | DD | 1B, S1E | 23.00 | 27.50 | |
| Per1−/−, SCN-lesioned | DD | S2I | 20.08 | 25.08 | |
| Per1 −/− /Per2 −/− Per3 −/− | DD | S13H | 21.16 | 25.50 | |
- Mice were provided with 0.005% methamphetamine (MA) in their drinking water in DD or LL. Periods were determined by χ2 periodogram with alpha set to 0.001 (periods ranging from 10 to 50 h). For each mouse, 5–10 days were analysed for the dominant short and long periods. The days used for χ2 periodogram analysis are shown for each mouse in the Supporting Information. The data reported here are not included in Table 1.
Statistical analyses
The pooled variance method was used to define the 95% confidence interval (t-distribution) of the difference between untreated and methamphetamine-treated intact wild-type, Per1−/−, Per2−/− and Per3−/− mice, respectively. The periods of individual methamphetamine-treated wild-type and Per-mutant mice (intact and SCN-lesioned) were compared to the 95% confidence interval (t-distribution) of the period of Per1−/−/Per2−/−/Per3−/− mice. In addition, the minimum period value in each group (methamphetamine-treated, intact or SCN-lesioned, wild-type and Per-mutant mice) was used to determine the number of SDs between the period of that group and the period of methamphetamine-treated Per1−/−/Per2−/−/Per3−/− mice.
Results
Methamphetamine-induced locomotor activity rhythms in Period-mutant mice in DD
As Per1−/−/Per2−/−/Per3−/− mice had a MASCO period of ~21 h (Pendergast et al., 2012) we examined MASCO rhythms in single Per-mutant mice to determine whether they also had short MASCO periods. We first administered methamphetamine (0.005% in drinking water) to wild-type (n = 4; Fig. 1A; Fig. S1A–D), Per1−/− (n = 6; Fig. 1C; Fig. S2A–F), Per2−/− (n = 4; Fig. 1E; Fig. S3A–D) and Per3−/− (n = 8; Fig. 1G; Fig. S4A–H) mice in DD. During methamphetamine administration, the period of the wheel-running activity rhythm was longer than 24 h in wild-type, Per1−/− and Per3−/− mice (Table 1; these mice have periods of ~23.6 h without methamphetamine treatment (Pendergast et al., 2009, 2010a). In contrast, the period of the wheel-running rhythm was 23.5 h with and without methamphetamine administration in Per2−/− mice (Table 1; also Pendergast et al., 2010a). The confidence intervals of the differences in periods between untreated and methamphetamine-treated wild-type [0.37, 0.84], Per1−/− [0.97, 1.47] and Per3−/− [0.56, 0.94] mice were greater than (and did not overlap with) those of Per2−/− mice [−0.12, 0.28], demonstrating that intact Per2−/− mice did not respond to methamphetamine treatment in DD. In addition, none of the methamphetamine-treated single Per-mutant mice exhibited a rhythm with a period that was within the 95% confidence interval [21.22, 22.40] of the period of Per1−/−/Per2−/−/Per3−/− mice and all mice were at least 2 SD away from the Per1−/−/Per2−/−/Per3−/− mean period (Table S1; period values are shown for each mouse in figures in Supporting Information). These data demonstrate that no single Per gene confers a short (21-h) period in intact mice.
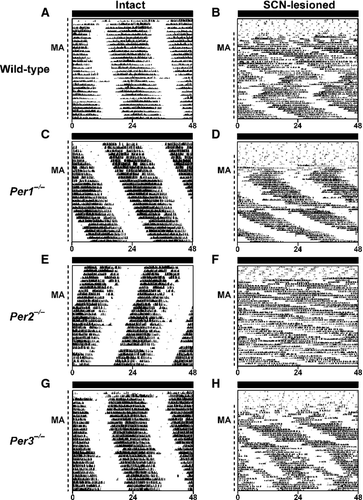
Because the period of the circadian activity rhythm was 23.5 h with and without methamphetamine treatment in Per2−/− mice, we also assessed the periods of other genotypes of mice that lacked either one or both copies of functional Per2. The periods of the wheel-running rhythms were < 24 h with and without methamphetamine treatment in other mice lacking functional PER2 (Per1+/−/Per2−/−/Per3−/−, Fig. S5; Per1+/+/Per2−/−/Per3−/−, Fig. S6, Table 1). In mice with at least a single copy of functional Per2, the periods of the wheel-running rhythms were < 23 h without methamphetamine administration, but ≥ 23 h with methamphetamine treatment (Per1−/−/Per2+/−/Per3+/+, Fig. S7A and B; Per1−/−/Per2+/−/Per3−/−, Fig. S7C and D; Table 1).
The locomotor activity rhythm in wild-type rodents during short-term (typically < 30 days in mice) methamphetamine treatment represents the integrated outputs of the SCN and MASCO. To eliminate the influence of the SCN on MASCO, we lesioned the SCN in wild-type (n = 3; Fig. 1B; Fig. S1E–G), Per1−/− (n = 4; Fig. 1D; Fig. S2G–J), Per2−/− (n = 3; Fig. 1F; Fig. S3E–G) and Per3−/− (n = 5; Fig. 1H; Fig. S4I–M) mice and investigated the methamphetamine-induced activity rhythm. The MASCO periods in wild-type, Per1−/−, Per2−/− and Per3−/− mice were > 25 h (Table 1). None of the SCN-lesioned single Per-mutant mice had a rhythm with a period that was within the 95% confidence interval [21.22, 22.40] of the period of Per1−/−/Per2−/−/Per3−/− mice and all mice were at least 3 SD away from the Per1−/−/Per2−/−/Per3−/− mean period (Table S1; period values are shown for each mouse in Supporting Information figures). Therefore, none of the SCN-lesioned single Per-mutant mice had a short (~21 h) MASCO period. Interestingly, one wild-type (Fig. S1E) and one Per1−/− mouse (Fig. S2I) administered methamphetamine after SCN lesion exhibited a short (< 24 h) and a long (> 24 h) period (Table 2).
Methamphetamine-induced locomotor activity rhythms in Period-mutant mice in LL
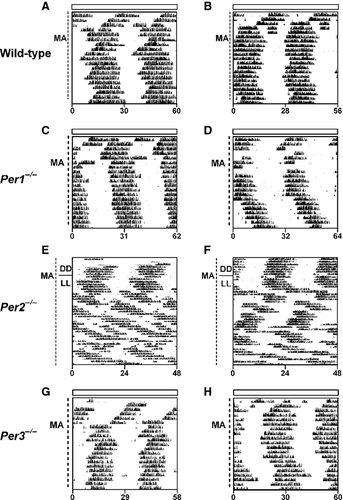
Constant light lengthens the period of the SCN-controlled activity rhythm and lowers the amplitude of the SCN oscillation in wild-type mice (Daan & Pittendrigh, 1976; Ohta et al., 2005). Compared to wild-type mice in LL, the period of the SCN-controlled activity rhythm is longer (by ~2 h) in C57BL/6J Per1−/− mice, while Per3−/− mice do not differ from wild types (Pendergast et al., 2010b). The period of the activity rhythm in C57BL/6J Per2−/− mice is 23.5 h in both DD and LL (Pendergast et al., 2010b). We investigated the effect of LL on the methamphetamine-induced locomotor activity rhythms in wild-type (Fig. 2A and B; Fig. S8), Per1−/− (Fig. 2C and D; Fig. S9), Per2−/− (Fig. 2E and F; Fig. S10) and Per3−/− (Fig. 2G and H; Fig. S11) mice. In LL, the periods of the methamphetamine-induced wheel-running rhythms were 29 h in wild-type and Per3−/− mice but 35 h in Per1−/− mice (Table 1). Upon release of Per2−/− mice from DD to LL, two activity rhythms were simultaneously observed (Fig. S10A–C). One activity rhythm had a short (23-h) period (similar to the period of the SCN-controlled activity rhythm in Per2−/− mice without methamphetamine) and the other rhythm had a 30-h period (similar to the MASCO period in SCN-lesioned Per2−/− mice in DD). In some Per2−/− mice, both components persisted for the length of the recording in LL (Fig. 2E) while in other mice the short-period component became less prominent or disappeared (Fig. 2F; Fig. S10C) and the long-period rhythm persisted. When Per2−/− mice were maintained in 18L : 6D prior to release into LL, the short (23 h) period was absent or disappeared quickly (Fig. S10D and E), suggesting that there may be aftereffects of entrainment to the light–dark cycle.
Two distinct MASCO rhythm periods in Per1−/−/Per2−/− mice in DD and LL
As Per1−/−/Per2−/−/Per3−/− mice had a MASCO period of ~21 h, but none of the intact and SCN-lesioned single Per-mutant mice had a period of 21 h, we next examined methamphetamine-induced locomotor activity in Per1−/−/Per2−/− mice in DD (Fig. 3; Fig. S12). Before methamphetamine treatment, Per1−/−/Per2−/− mice did not exhibit circadian rhythms of wheel-running activity, demonstrating that the SCN rhythm was abrogated in DD (Fig. 3). After methamphetamine administration (1–20 days), Per1−/−/Per2−/− mice exhibited circadian rhythms of wheel-running activity. While the period of the MASCO rhythm was predominantly short, with a period of ~21 h, similar to Per1−/−/Per2−/−/Per3−/− mice (Fig. S13; Pendergast et al., 2012), the Per1−/−/Per2−/− MASCO rhythm alternated between short (~21 h) and long (~27 h) periods of methamphetamine-induced locomotor activity (Table 2). The change in the period of the methamphetamine-induced activity rhythm always coincided with an environmental disturbance, such as changing the methamphetamine water, opening the light-tight box or changing the cage (environmental disturbances are shown on actograms in Fig. S12).
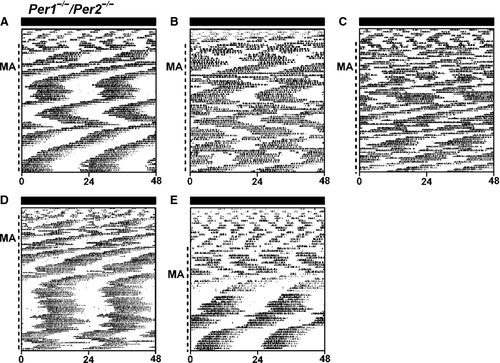
In contrast, mice with an additional single copy of Per3 (Per1−/−/Per2−/−/Per3+/−; Fig. S14) expressed mostly short (22 h) periods of methamphetamine-induced activity, similar to Per1−/−/Per2−/−/Per3−/− mice (only one mouse switched from a short to along period; Fig. S13H, Table 2).
When placed in LL, some Per1−/−/Per2−/− mice exhibited short (~17 h) and then long (~35 h) periods of methamphetamine-induced activity rhythms, as if the short-period component observed in DD was further shortened and the long-period component was further lengthened in LL (Fig. 4A and B; Fig. S15; Table 2). Some Per1−/−/Per2−/− mice had only one short (~17 h) or one long (~35 h) period activity rhythm in LL (Fig. 4C–E, Fig.S15; Table 1). Wheel-running activity was arrhythmic in Per1−/−/Per2−/− mice maintained in LL without methamphetamine (Fig. S16).

The methamphetamine-induced activity rhythm in Per1−/−/Per2−/−/Per3−/− mice was short in LL
We previously demonstrated that the period of the MASCO rhythm in Per1−/−/Per2−/−/Per3−/− mice is short (~21 h) in DD (Fig. S13; Pendergast et al., 2012). In LL, the period of the MASCO rhythm was 14-16 h in Per1−/−/Per2−/−/Per3−/− mice (in four of five mice: Fig. 5 and Fig. S17; one mouse did not exhibit a detectable rhythm in LL; Table 1). Similar to previous studies of circadian-mutant mice (Spoelstra et al., 2002; Abraham et al., 2006), rhythms of wheel-running activity were sometimes conferred to Per1−/−/Per2−/−/Per3−/− mice by LL even without methamphetamine treatment (Fig. S18).

Discussion
MASCO and the SCN are distinct circadian oscillators
Methamphetamine-sensitive circadian oscillator is a circadian oscillator whose output is observed in rodents during continuous consumption of low-dose methamphetamine (Honma et al., 1986; Tataroglu et al., 2006). Previous studies have demonstrated that MASCO is distinct from the SCN because (i) the methamphetamine-induced activity rhythm persists in SCN-lesioned rodents (Honma et al., 1987; Tataroglu et al., 2006), and (ii) during long-term (> 30 days) methamphetamine administration (in the light–dark cycle and in DD), MASCO de-couples from the SCN and two activity rhythms are observed, one corresponding to SCN-controlled activity and the other to MASCO-controlled activity (Honma et al., 1986; Masubuchi et al., 2000; Cuesta et al., 2011; Pendergast et al., 2012). During short-term methamphetamine administration (typically < 30 days), rodents with intact SCN express a single, free-running wheel-running activity rhythm with a period that is intermediate to the short period of the SCN rhythm and the long period of the MASCO rhythm, suggesting that the SCN and MASCO are coupled to each other (Honma et al., 1991; Masubuchi et al., 2000; Tataroglu et al., 2006). Thus, when methamphetamine is present, locomotor activity is the integrated output of the SCN and MASCO (and other oscillators) and the system is plastic over time.
No single Period gene confers a short-period MASCO rhythm
Previously we found that the MASCO rhythm in Per1−/−/Per2−/−/Per3−/− mice in DD (the SCN is disabled) is unusually short (21.5 h; Pendergast et al., 2012). In contrast, wild-type and other circadian-mutant mice have MASCO periods > 24 h (Tataroglu et al., 2006; Mohawk et al., 2009). To determine the contribution of each Per gene to timekeeping in MASCO, we analysed the MASCO rhythms in single Per-mutant mice. We found that the periods of the methamphetamine-induced activity rhythms in Per1−/−, Per2−/− and Per3−/− mice were 24 h. None of the methamphetamine-treated single Per-mutant mice had periods approximating 21 h. As it was possible that the 24-h MASCO periods in single Per-mutant mice could result from coupling of MASCO to the SCN, we lesioned the SCN and investigated the methamphetamine-induced rhythms. We found that the periods of MASCO in SCN-lesioned Per1−/−, Per2−/− and Per3−/− mice were 26–30 h (with the exception of one SCN-lesioned wild-type and one SCN-lesioned Per1−/− mouse that displayed two sequential periods, as discussed below). SCN-lesioned wild-type mice also have MASCO periods > 24 h (this study and Tataroglu et al., 2006). These data demonstrate that no single Period gene is responsible for the short-period MASCO rhythm of Per1−/−/Per2−/−/Per3−/− mice.
Two stable MASCO periods are expressed in Per1−/−/Per2−/− mice
As we found that no single Per gene is responsible for the short-period MASCO rhythm in Per1−/−/Per2−/−/Per3−/− mice, we next examined the MASCO rhythm in Per1−/−/Per2−/− mice in DD (the SCN is disabled). We found that the MASCO rhythm alternated between a short (~22 h) and a long (~27 h) period. The Per1−/−/Per2−/− MASCO phenotype could result from two oscillators that are not synchronised to each other (because they have different periods and because coupling is weak as compared to wild types), resulting in relative coordination between the two. In this case, the activity rhythm would alternate, even in the absence of external stimulation. An alternative explanation for the methamphetamine-induced alternating and stable short and long periods is that the Per1−/−/Per2−/− MASCO displays birhythmicity, which is the existence of two stable regimes of limit-cycle oscillations (Decroly & Goldbeter, 1982; Goldbeter, 1996; Leloup & Goldbeter, 1999). In birhythmicity, two limit cycles are simultaneously stable, so switches of the periods between two distinct values can be observed. In addition, perturbations cause the switch between the two equally stable limit cycles (it does not occur in a constant environment). In accordance with the tenets of birhythmicity, the Per1−/−/Per2−/− MASCO rhythm exhibited two fixed period values and all of the switches between the short and long period were concurrent with an environmental disturbance (most often changing the methamphetamine drinking water, but also with opening the light-tight box and changing the cage). Mohawk and colleagues found that Per1−/−/Per2−/− mice exhibit a long (~27 h) MASCO period in DD, consistent with the long period that we observed in our experiments (Mohawk et al., 2009). It is possible that there were fewer environmental disturbances in the Mohawk et al. (2009) experiment, thus eliminating the switches between short and long periods in Per1−/−/Per2−/− mice. It is also possible that differences in the genetic background (i.e. generations of backcrossing to the C57BL/6J strain) of the Per1−/−/Per2−/− mice used in this study (N15 C57BL/6J) and the mice used by Mohawk and colleagues (mixed genetic background) contributed to differences in MASCO. Computer simulations of these putative scenarios are necessary to further explore the nature of the Per1−/−/Per2−/− MASCO.
Interestingly, when Per1−/−/Per2−/− mice were placed in LL, some mice sequentially expressed two periods of methamphetamine-induced activity, a short ~17-h period and then a long ~34-h period. In these mice, the effect of LL on the methamphetamine-induced activity rhythm may be to shorten the short period and lengthen the long period. The short-period MASCO rhythm in Per1−/−/Per2−/−/Per3−/− mice was also short (~15 h) in LL.
Alternating short and long MASCO periods were also occasionally observed in other mice with disabled SCN (SCN-lesioned wild-type and Per1−/− mice and one Per1−/−/Per2−/−/Per3−/− mouse). These data, in addition to the observation that phase shifts in the MASCO rhythm occur frequently in SCN-lesioned and Per1−/−/Per2−/−/Per3−/− mice, suggest that instability may be a characteristic of MASCO. MASCO may be an inherently weak oscillator, whose instability is masked by coupling to the SCN but revealed when the SCN is disabled or its timekeeping mechanism is compromised as in Per1−/−/Per2−/− mice.
Coupling between MASCO and the SCN is altered in Per2−/− mice
In this study, we also sought to understand the relationship between the SCN and MASCO by analysing wheel-running activity in intact (not SCN-lesioned) wild-type and Period-mutant mice in DD and LL. In DD, without methamphetamine, C57BL/6J wild-type and Per-mutant mice expressed similar periods of wheel-running activity (23.5 h; Pendergast et al., 2009, 2010a). Upon administration of methamphetamine, the period of the activity rhythm was 24 h in wild-type, Per1−/− and Per3−/− mice, suggesting that the SCN and MASCO are coupled (at least during short-term, i.e. less < 30 days, methamphetamine treatment). In contrast, the period of the wheel-running activity rhythm in Per2−/− mice was 23.5 h with and without methamphetamine consumption. This may occur if the Per2−/− MASCO is insensitive to methamphetamine. However, this is an unlikely explanation because the long-period MASCO rhythm (similar MASCO period as SCN-lesioned wild-types) was clearly expressed in Per2−/− mice when the SCN was lesioned. Thus, it is more likely that the SCN and MASCO are not coupled in Per2−/− mice. Because we only observed one circadian activity rhythm component in SCN-intact Per2−/− mice, we propose that MASCO output is masked or inhibited in DD. Furthermore, our data suggest that a single copy of Per2 is sufficient to restore coupling between the SCN and MASCO.
Rather than expressing a single integrated methamphetamine-induced wheel-running activity rhythm in LL, Per2−/− mice initially had two free-running components, one corresponding to the period of the SCN (which is 23.5 h in LL in Per2−/− mice; Pendergast et al., 2010b) and the other to the MASCO period. This is additional evidence that the SCN and MASCO are not coupled in Per2−/− mice. However, unlike in DD, MASCO output is not masked in LL (and thus we observe both the SCN- and MASCO-controlled activity rhythms). With extended exposure to LL, the short-period component became less visible and disappeared in some mice; this may correspond to the gradual effect of LL to desynchronise SCN neurons (Ohta et al., 2005). It is possible that, as the amplitude of the SCN oscillator decreases, MASCO gradually suppresses SCN output.
Similar to our results in Per2−/− mice, Tataroglu et al. (2006) reported that methamphetamine treatment did not affect the period the locomotor activity rhythm in intact C3H mice in DD. In contrast, the MASCO period in SCN-lesioned C3H mice was ~24.5 h, while the MASCO period in SCN-lesioned Per2−/− mice was ~27 h, and C3H mice express only one activity component during methamphetamine treatment in LL. Therefore, unlike Per2−/− mice, coupling between the SCN and MASCO is probably intact in C3H mice, but the period of MASCO may be shortened in C3H mice compared to C57BL/6J mice.
Working model of the in vivo relationship between MASCO and the SCN
Based on our experiments, we propose a working model of the SCN–MASCO integrated circuit in wild-type mice (Fig. 6). In our model, the SCN and MASCO are mutually coupled circadian oscillators that also inhibit each other's output, but the strengths of these interactions are modulated by environmental factors. The effector, which is an unidentified neural circuit or locus, integrates the output from the MASCO and SCN and drives wheel-running activity. Wheel-running activity itself may be critical for coupling between MASCO and the SCN as it was previously demonstrated that the period of the locomotor activity rhythm was not lengthened in rats administered methamphetamine without a running wheel (Honma et al., 1991). Future studies should examine the role of wheel-running activity in MASCO–SCN coupling.
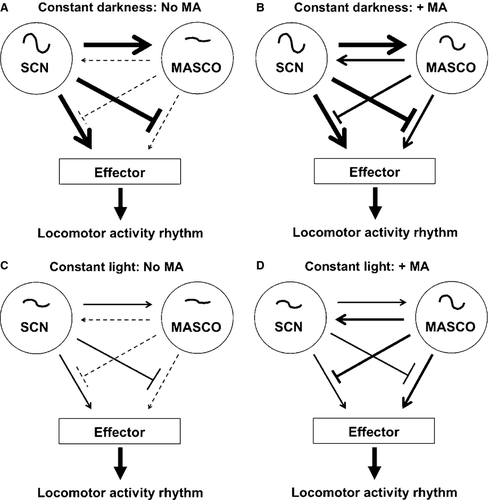
In DD, the SCN is a robust circadian oscillator that is coupled to MASCO and also suppresses its output (Fig. 6A). We propose that, in the absence of methamphetamine, MASCO is a weak oscillator so it has little influence on the SCN and it does not appreciably suppress the output of the SCN. Thus, in DD without methamphetamine, the locomotor activity rhythm is largely (or completely) controlled by the SCN. Upon addition of methamphetamine, MASCO becomes a robust circadian oscillator, thus increasing its coupling to the SCN and its suppression of SCN output (Fig. 6B). The resulting locomotor activity rhythm is an integration of both SCN and MASCO outputs. During long-term methamphetamine administration (> 30 days), the SCN and MASCO become decoupled and two activity rhythms are expressed. In our model, we assume that methamphetamine does not affect the period of the SCN; we thus conceptualise that the SCN and MASCO become decoupled rather than exhibit relative coordination. However, our interpretation is limited as it is possible that methamphetamine affects the period of the SCN. Unfortunately, the effect of methamphetamine on the period of the SCN cannot be determined as this would require methamphetamine treatment of mice with MASCO lesions and we do not know the anatomical locus of the MASCO.
In LL, without methamphetamine, both the SCN and MASCO are weak oscillators (Fig. 6C). Upon addition of methamphetamine, MASCO becomes a robust oscillator, increasing its coupling to the SCN and its suppression of SCN output (we assume that LL does not affect the amplitude of MASCO; Fig. 6D). As we assume that DD and LL do not differentially affect MASCO, we propose that the period-lengthening of the methamphetamine-induced activity rhythm in LL is largely attributed to the effects of light on the SCN. This is consistent with our data showing that the magnitude of lengthening of the methamphetamine-induced rhythm in Per1−/− mice is greater than the lengthening in wild-type and Per3−/− mice in LL (which mirrors the respective effects of LL on the SCN-controlled activity rhythms in these mice; Pendergast et al., 2010b). However, we cannot rule out the possibility that light acts directly on MASCO.
Per2−/− mice are unique in that their SCN and MASCO are probably not coupled (even during short-term methamphetamine administration). Because we found that Per2−/− mice exhibit one component (corresponding to the period of the SCN) in DD but two components in LL, we propose that the SCN suppresses the output of MASCO in DD but not in LL. We attribute the strength of the suppression to the robustness of the SCN oscillator. In DD, the Per2−/− SCN is a robust oscillator that is not coupled to MASCO, but suppresses MASCO output. However, in LL the Per2−/− SCN is a weak oscillator that is not coupled to MASCO and does not suppress MASCO output, so two components are observed upon release into LL. The function of PER2 in SCN–MASCO coupling is unknown and should be investigated in future studies.
This study demonstrates the complexity of the multi-oscillator circadian system and highlights the fact that the output measured from the animal represents the integration of multiple oscillators. Computational modeling is critical for further understanding the complex relationships between these oscillators.
Acknowledgements
This research was supported by a National Science Foundation grant IOS-1146908 to S.Y. K.D.N. was supported by resources of the Tennessee Valley Healthcare System and National Institutes of Health grants, DK085712 and the Diabetes Research and Training Center DK020593. The Vanderbilt University Department of Biostatistics Basic Science Clinic provided guidance regarding statistical analyses of data. We thank Dr Gisele A. Oda and Dr Gen Kurosawa for insightful discussion of the data. We also thank Dr Wataru Nakamura for technical advice in performing SCN lesion surgery. The authors declare no competing financial or non-financial interests.
Abbreviations
-
- DD
-
- constant darkness
-
- LL
-
- constant light
-
- MASCO
-
- methamphetamine-sensitive circadian oscillator
-
- Per
-
- Period
-
- SCN
-
- suprachiasmatic nucleus




