Role of Cytokines in Predicting Early Major Bleeding in Patients With Acute Pulmonary Embolism
Funding: This work was supported by Eugenio Rodriguez Pascual Foundation grant (December 2021).
ABSTRACT
Introduction
Anticoagulant therapy is critical for venous thromboembolism (VTE) management, though bleeding remains a major concern, ranging from mild to fatal events. This study aimed to assess the predictive value of cytokines for major bleeding in patients with acute pulmonary embolism (PE).
Methods
In this prospective, observational study, patients aged ≥ 18 years with acute PE were enrolled from April 2021 to September 2022 and followed for 30 days. Exclusion criteria included asymptomatic PE, VTE without PE, and chronic anticoagulation. Major bleeding was defined as bleeding that required ≥ 2 transfused units of red blood cells, occurred in critical areas, or was fatal. Blood samples were collected at diagnosis to measure IL-6, IL-1beta, IL-8, IL-10, and TNF-alpha. Statistical analyses used bivariate and multivariate logistic regression (p < 0.05).
Results
Out of 191 patients (mean age 68.6 years, 52.9% male), 8.4% died, and 4.2% experienced major bleeding within 30 days. IL-8 > 40 pg/mL and TNF-alpha > 8.5 pg/mL were linked to major bleeding. IL-8 > 40 pg/mL independently predicted early major bleeding (adjusted OR 9.40; 95% CI 1.38–63.69). Cox regression showed HRs of 12.60 for IL-8 and 5.61 for TNF-alpha.
Conclusion
High IL-8 levels at diagnosis were predictive of major bleeding in acute PE patients. Further studies are required to confirm these findings.
Abbreviations
-
- BMI
-
- body mass index
-
- HR
-
- hazard ratio
-
- IL
-
- Interleukin
-
- IQR
-
- interquartile ranges
-
- OR
-
- odds ratio
-
- PE
-
- pulmonary embolism
-
- ROC
-
- Receiver operating characteristic
-
- SD
-
- standard deviations
-
- TNF
-
- tumor necrosis factor
-
- VTE
-
- venous thromboembolism
1 Introduction
Anticoagulant treatment is the cornerstone of venous thromboembolism (VTE) management, but bleeding remains a significant complication, ranging from mild episodes to potentially fatal events. The risk of major bleeding within the first 3–6 months following a VTE is approximately 1%–7% for non-cancer-associated VTE and 7%–10% for cancer-associated VTE [1]. However, in patients undergoing extended anticoagulant treatment, the estimated 5-year bleeding risk varies significantly: from 2.3% in patients receiving reduced doses of rivaroxaban or apixaban to nearly 10% in patients treated with vitamin K antagonists, compared to an estimated 5-year bleeding risk of 2% in patients without anticoagulant treatment [2-4].
This study aimed to evaluate the predictive capacity of different cytokines for major bleeding events in a cohort of patients with acute pulmonary embolism (PE).
2 Methods
This prospective, observational, single-center study included consecutive patients with acute PE between April 2021 and September 2022, with a 30-day follow-up period. Inclusion criteria included being 18 years or older and providing informed consent. Exclusion criteria included the presence of incidental or asymptomatic PE, VTE without PE, and chronic anticoagulant therapy (at any dose or indication). Major bleeding was defined in this study as bleeding that required transfusion of ≥ 2 units of red blood cells, occurred in a critical location, or resulted in death.
Whole blood samples were collected at diagnosis and frozen at −20°C. Interleukin (IL) 6 was measured using the ROCHE Diagnostics cobas e411 analyzer via electrochemiluminescence immunoassay. For IL-1beta, IL-8, IL-10, and tumor necrosis factor (TNF) alpha, the SIEMENS IMMULITE 1000 analyzer was used. The normal ranges were as follows: IL-1beta (0.0–5.0 pg/mL), IL-6 (0.0–4.3 pg/mL), IL-8 (0–62 pg/mL), IL-10 (0.0–9.1 pg/mL), and TNF-alpha (0.0–8.1 pg/mL).
The sample size was calculated based on an incidence of 10%, with a 95% confidence interval, an alpha error of 0.05, and accounting for a 10% potential loss, resulting in a minimum required sample size of 155 patients. Categorical data were reported as proportions, while continuous data as means with standard deviations (SD) or medians with interquartile ranges (IQR). The Student's t-test or ANOVA was used for normally distributed variables, while the Mann–Whitney U or Kruskal–Wallis tests were applied for non-normally distributed variables. Receiver operating characteristic (ROC) curve analysis was employed to assess the predictive capacity of cytokines, with the optimal cut-off determined by Youden's index. Crude odds ratios (OR) were calculated using bivariate logistic regression, and multivariate logistic regression was used to assess the independent association (adjusted OR). Time-to-event analyses were conducted using Cox regression for hazard ratios (HR) and the Kaplan–Meier estimator. All tests were two-sided, and the level of statistical significance was set at 0.05. Statistical analysis was performed using STATA software (v14.2).
This study was approved by the Hospital General Universitario Gregorio Marañón ethical committee (approval #PE-9/2021) and written informed consent was obtained from all patients.
3 Results
A total of 191 patients were included, with a mean age of 68.6 years (±17), and 52.9% (99 patients) were male. Of the cohort, 42.9% (82 patients) had unprovoked PE, 18.3% (35 patients) had cancer-associated thrombosis, and 8.4% (16 patients) died. Among the deaths, 20% were attributed to PE, 20% to cancer, 20% to respiratory failure, 3% to infections, and the remainder to other causes. A total of 9.9% (19 patients) presented clinically relevant bleeding or major bleeding. Major bleeding within the first 30 days occurred in 4.2% (8 patients), with a median time to onset of 4.5 days (IQR: 3–5) and a bleeding case-fatality of 25% (2 patients). The locations of major bleeding included the gastrointestinal tract (3 patients), retroperitoneum (2 patients), urinary tract (2 patients), and intracerebral region (1 patient). Table 1 summarizes the clinical and laboratory findings of the cohort.
| Variable | Total (n = 191) | No bleeding, group (n = 172) | Bleeding, group (n = 19) | p |
|---|---|---|---|---|
| Age, years (mean, SD) | 68.6 (17) | 68.3 (18) | 71 (13) | 0.52 |
| Sex male, n (%) | 101 (52.9) | 94 (54.7) | 7 (36.8) | 0.15 |
| BMI > 30, n (%) | 71 (37.2) | 61 (35.5) | 10 (62.5) | < 0.01 |
| Hypertension, n (%) | 95 (59.7) | 87 (50.6) | 8 (42.1) | 0.48 |
| Diabetes, n (%) | 25 (13.1) | 23 (13.4) | 2 (10.5) | 0.72 |
| Heart failure, n (%) | 14 (7.3) | 11 (6.4) | 3 (15.8) | 0.14 |
| Chronic ischemic cardiopathy, n (%) | 11 (5.8) | 10 (5.8) | 1 (5.3) | 0.92 |
| Previous stroke, n (%) | 19 (9.9) | 19 (11.0) | 0 (0.0) | 0.22 |
| Concomitant infection, n (%) | 64 (33.5) | 54 (31.8) | 10 (52.6) | 0.08 |
| Cancer, n (%) | 35 (18.3) | 27 (15.7) | 8 (42.1) | < 0.01 |
| SBP < 90 mmHg, n (%) | 11 (5.8) | 10 (5.8) | 1 (5.3) | 0.92 |
| sPESI (high-risk), n (%) | 124 (64.9) | 109 (63.4) | 15 (78.9) | 0.17 |
| RV disfunction, n (%) | 91 (47.6) | 81 (47.1) | 10 (52.6) | 0.20 |
| Laboratory findings | ||||
| Hemoglobin, g/dL (median, P25-P75) | 13.4 (11.9–14.7) | 13.4 (11.9–14.9) | 12.6 (10.9–14.5) | 0.03 |
| Leukocytes, ×1000 μL−1 (median, P25-P75) | 9.2 (7.7–11.7) | 9.2 (7.5–11.4) | 12.6 (9.2–15.3) | < 0.01 |
| Platelets, ×1000 μL−1 (median, P25-P75) | 212 (168–268) | 212 (168–265) | 210 (152–299) | 0.88 |
| CRP, mg/L (median, P25-P75) | 31.0 (12.0–66.0) | 31.1 (12.4–67.5) | 24.0 (8.5–52.0) | 0.60 |
| CRP > 50 mg/L, n (%) | 64 (33.5) | 59 (35.1) | 5 (26.3) | 0.44 |
| D-dimer, ng/mL (median, P25-P75) | 2566 (1336–6137) | 2427 (1300–5312) | 3307 (2233–8065) | 0.04 |
| IL-1beta (median, P25–P75) | 2.5 (2.4–2.6) | 2.5 (2.4–2.6) | 2.5 (2.4–5.3) | 0.79 |
| IL-6 (median, P25-P75) | 31.6 (16.6–61.6) | 29.1 (15.7–61.6) | 45.4 (31.1–78.5) | 0.19 |
| IL-8 (median, P25-P75) | 14.0 (7.8–30.6) | 13.8 (7.6–26.6) | 40.2 (13.4–124.0) | < 0.01 |
| IL-10 (median, P25-P75) | 2.5 (2.4–5.0) | 2.5 (2.4–2.6) | 2.5 (2.4–6.0) | 0.68 |
| TNF-alpha (median, P25-P75) | 6.7 (4.4–9.2) | 6.5 (4.3–9.1) | 8.8 (5.9–14.5) | 0.02 |
| Outcomes | ||||
| Mortality, n (%) | 19 (9.7) | — | — | — |
| Major bleeding, n (%) | 8 (4.2) | — | — | — |
- Abbreviations: BMI: body mass index; CRP: C-reactive protein; IL: interleukin; PCT: procalcitonin; RV: right ventricle; SBP: systolic blood pressure; sPESI: simplified Pulmonary Embolism Severity Index; TAPSE: tricuspid annular plane systolic excursion; TNF: tumoral necrosis factor.
Median cytokine levels and interquartile ranges (IQR) for patients with major bleeding (8 patients) versus those without (183 patients) were as follows: IL-1beta 2.5 pg/mL (2.4–9.7) vs. 2.5 pg/mL (2.4–2.6), p = 0.09; IL-6 45.8 pg/mL (24.3–80.1) vs. 29.7 pg/mL (15.7–58.8), p = 0.37; IL-8 55.5 pg/mL (26.9–101.5) vs. 13.6 pg/mL (7.6–24.9), p < 0.01; IL-10 2.5 pg/mL (2.4–2.6) vs. 2.5 pg/mL (2.4–5.0), p = 0.46; and TNF-alpha 8.9 pg/mL (8.5–15.2) vs. 6.5 pg/mL (4.4–9.2), p = 0.02. The ROC curve analysis showed a C-statistic of 0.62 (95% CI: 0.42–0.81), 0.77 (95% CI: 0.59–0.95), and 0.76 (95% CI: 0.62–0.91) with an optimal cut-off > 5.2 pg/mL, > 40.0 pg/mL, and > 8.5 pg/mL for IL-1beta, IL-8, and TNF-alpha, respectively (Figure 1A,B). The Kaplan–Meier curve for IL-8 > 40 pg/mL demonstrated a significant difference in survival (p = 0.001), while the curve for TNF-alpha > 8.5 pg/mL also showed a significant difference (p = 0.02) (Figure 1C,D, respectively). The optimal cut-off for IL-6 and IL-10 could not be determined due to their poor C-statistic (< 0.60), indicating insufficient predictive accuracy for these biomarkers.
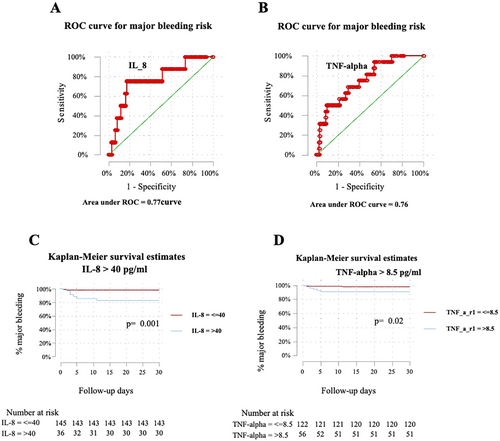
Bivariate logistic regression analysis was conducted to assess potential cofounders for variables potentially associated with major bleeding, revealing a potential association for the prediction of major bleeding with a crude OR of 1.03 (95% CI: 0.88–1.07; p = 0.22) for IL-1beta > 5.2 pg/mL, 14.30 (95% CI: 2.74–74.65, p < 0.01) for IL-8 > 40.0 pg/mL, and 5.88 (95% CI: 1.10–31.47; p = 0.04) for TNF-alpha > 8.5 pg/mL. Furthermore, the multivariate model included only those variables that showed statistically significant association (p < 0.05) in bivariate analysis. Multivariate analysis identified IL-8 levels > 40 pg/mL as the only independent predictor of early major bleeding (adjusted OR 9.40; 95% CI: 1.38–63.69; p = 0.02), adjusted for obesity, presence of cancer, hemoglobin < 12 g/dL, TNF-alpha > 8.5 pg/mL, C-reactive protein, and other variables (Table 2). Additionally, Cox regression analysis showed an HR of 12.60 (95% CI: 2.51–63.21; p < 0.01) for IL-8 > 40 pg/mL and 5.61 (95% CI: 1.10–28.76; p = 0.04) for TNF-alpha > 8.5 pg/mL.
| Bivariate logistic regression | ||||
|---|---|---|---|---|
| Variables | Odds ratio (crude) | 95% confidence interval | p | |
| Age (years) | 1.03 | 0.99 | 1.06 | 0.14 |
| Sex (male) | 1.72 | 0.47 | 6.31 | 0.41 |
| Body mass index > 30 | 6.59 | 1.28 | 33.82 | 0.02 |
| Cancer | 5.16 | 1.41 | 18.96 | 0.01 |
| Concomitant infection | 2.26 | 0.47 | 11.0 | 0.31 |
| C-reactive protein > 50 mg/L | 1.31 | 0.36 | 4.84 | 0.68 |
| Hemoglobin < 12 g/dL | 4.6 | 1.24 | 17 | 0.02 |
| Platelets < 100 × 1000 μL−1 | 3.43 | 0.37 | 31.73 | 0.28 |
| D-dimer > 1000 ng/mL | 1.36 | 0.16 | 11.27 | 0.78 |
| sPESI (high risk) | 2.11 | 0.43 | 10.27 | 0.35 |
| Interleukin 1beta > 5.2 pg/mL | 1.03 | 0.88 | 1.07 | 0.22 |
| Interleukin 6 > 50.0 pg/mL | 2.09 | 0.50 | 8.68 | 0.31 |
| Interleukin 8 > 40.0 pg/mL | 14.30 | 2.74 | 74.65 | < 0.01 |
| Interleukin 10 > 10.0 pg/mL | 0.94 | 0.11 | 8.03 | 0.95 |
| TNF-alpha > 8.5 pg/mL | 5.88 | 1.10 | 31.47 | 0.04 |
| Multivariate logistic regression* | ||||
|---|---|---|---|---|
| Variables | Odds ratio (adjusted) | 95% confidence interval | p | |
| BMI > 30 | 6.59 | 1.28 | 33.82 | 0.02 |
| Cancer | 5.16 | 1.41 | 18.96 | 0.01 |
| Hemoglobin < 12 g/dL | 4.6 | 1.24 | 17 | 0.02 |
| IL-8 > 40.0 pg/mL | 14.30 | 2.74 | 74.65 | < 0.01 |
- Abbreviations: RV: right ventricle; SBP: systolic blood pressure; sPESI: simplified Pulmonary Embolism Severity Index; TNF: tumoral necrosis factor.
- * Multivariate logistic regression model was built with variables with a p < 0.05 in the bivariate analysis.
4 Discussion
Various scores are used to evaluate bleeding risk in patients undergoing anticoagulant therapy, including those designed for patients with VTE, such as the RIETE, VTE-BLEED, and VTE-PREDICT scores. These scores predominantly rely on clinical variables, including age, sex, arterial hypertension, cancer, body mass index (BMI), history of bleeding, chronic kidney disease, anemia, and thrombocytopenia [4-6]. Mathonier et al. [5], assessed the predictive capacity of different bleeding scores (HAS-BLED, RIETE, ORBIT, HAEMORR2HAGES, ATRIA, and VTE-BLEED) in a multicenter French cohort of 2754 PE patients, where 2.9% patients experienced major bleeding. All prognostic scores demonstrated a C-statistic of less than 0.7, indicating an overall limited predictive capacity. Previously, our group evaluated the predictive role of soluble P-selectin for major bleeding in patients with acute symptomatic PE, yielding poor results (c-statistic 0.49; 95% CI: 0.24–0.68) [7]. In contrast, the presence of elevated C-reactive protein (CRP) levels > 50 mg/L, a well-established inflammatory biomarker, at diagnosis appeared to be associated with increased bleeding risk in patients with acute VTE (c-statistic 0.65; 95% CI: 0.54–0.75), suggesting that inflammatory biomarkers may play a role in bleeding risk assessment [8].
Cytokines are a broad group of chemical messengers involved in various immunological processes, and their role in immune thrombosis remains unclear. To date, most research on cytokines in VTE has focused on their potential association with predicting new thromboembolic events [9-11]. However, very few studies have examined whether the inflammatory state associated with VTE might also increase bleeding risk, potentially as a cause or consequence of endothelial damage [8]. Recently, Dong et al. [12], analyzed patients with acute promyelocytic leukemia and found that among various cytokines, both IL-8 and TNF-alpha showed a significant cumulative correlation with intracerebral hemorrhage, potentially facilitating leukocyte migration during the inflammatory response, which could explain a mechanism for bleeding risk.
The evaluation of the inflammatory state through interleukin measurements has not yet been thoroughly studied specifically. The results of this study reveal a significant association between IL-8 levels > 40 pg/mL at diagnosis and the prediction of major bleeding within 30 days (adjusted OR 9.40; 95% CI 1.38–63.69) (Figure 1), independently of age, sex, obesity, presence of cancer, anemia, concomitant infection, and CRP levels > 50 mg/L, among other pro-inflammatory cytokines.
This study has several limitations. First, it is a single-center study with a relatively small number of events due to the limited sample size, restricting the ability to perform more complex analyses for robust results. Second, a healthy control group was not included to assess the cytokine distribution in our local population. Third, cytokine levels were measured only once at diagnosis, leaving the dynamics and evolution of these levels over time unknown. Fourth, the bleeding risk was not evaluated according to the type of anticoagulation therapy received during the acute and extended phases.
5 Conclusion
High levels of IL-8 were predictive of major bleeding events within 30 days in patients with acute symptomatic PE. Further studies are required to confirm these findings.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: Crhistian-Mario Oblitas, Francisco Galeano-Valle and Pablo Demelo-Rodríguez. Data collection: Crhistian-Mario Oblitas, Marta-Olimpia Lago-Rodríguez and Marina López-Rubio. Analysis and interpretation of results: Crhistian-Mario Oblitas, Mercedes García-Gámiz, and Angielys Zamora-Trillo. Draft manuscript preparation: Crhistian-Mario Oblitas, Francisco Galeano-Valle, and Pablo Demelo-Rodríguez. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of Interest
Dr. Galeano-Valle has received speaker's honoraria from the following pharmaceutical companies: ROVI, Techdow, Pfizer, Bristol-Myers, and Daichii-Sankyo. Dr. Demelo-Rodríguez has received speaker's honoraria from the following pharmaceutical companies: ROVI, Bayer, Techdow, Menarini, Leo Pharma, Pfizer, Bristol-Myers, Sanofi, and Daichii-Sankyo. In addition, he has engaged in advisory consultancy work for Techdow, Leo Pharma, and Pfizer. Dr. Crhistian-Mario Oblitas has received speaker's honoraria from the pharmaceutical company ROVI. The other authors have no conflicts of interest.
Open Research
Data Availability Statement
Data available on request due to privacy/ethical restrictions.




