Venetoclax Combined With FLAG-IDA in Refractory or Relapsed Acute Myeloid Leukemia
Funding: The authors received no specific funding for this work.
Kai Wille and Marvin Dumke contributed equally to this work.
ABSTRACT
Introduction
The prognosis of patients with refractory or relapsed AML (R/R-AML) is very limited. To (re)achieve complete remission, there has recently been increasing evidence that the combination of venetoclax (VEN) with chemotherapy is associated with improved outcomes.
Patients and Methods
Our retrospective, single-center study of 53 R/R-AML patients with a median follow-up time of 11.0 months compared standard salvage chemotherapy (FLAG-Ida or HAM in n = 35 patients) with a combination of venetoclax (VEN) and FLAG-Ida (FLAVIDA in n = 18 patients) concerning safety and efficacy.
Results
Regarding the primary endpoints, there was a statistically significant increased event free survival (EFS) in the FLAVIDA group compared to patients with standard chemotherapy based on the univariate log-rank-test and in the multivariate Cox regression analysis (HR 0.22 [95% CI 0.05, 0.97]). There were no differences between the two groups in terms of patients developing febrile neutropenia CTCAE III° and IV° or a delay in hematological recovery. In addition, a clear trend towards an improved overall response rate (78% vs. 51%) was demonstrated in the FLAVIDA group.
Conclusions
The FLAVIDA regimen represents a promising treatment alternative for R/R AML patients with a high response rate and significantly improved EFS compared to standard chemotherapy.
1 Introduction
Although the prognosis of patients with acute myeloid leukemia (AML) has improved since the 1970s, 40% of all AML patients develop a relapse or do not respond to induction chemotherapy with anthracycline and cytarabine [1-3]. To date, younger patients with relapsed or refractory AML (R/R-AML) who are eligible for allogeneic hematopoietic cell transplantation (alloHCT) are mainly treated with multi-agent chemotherapy regimens such as FLAG-Ida [4] (fludarabine, cytarabine, and idarubicin) or high-dose cytarabine in combination with mitoxantrone (HAM) [4, 19] in order to achieve complete remission (CR) prior to alloHCT. However, the CR rates of these therapies are 30%–40% and the median EFS and overall survival (OS) are only 6–12 months [5-8].
The BCL-2 inhibitor venetoclax (VEN) in combination with 5-azacitidine [9], decitabine [10], or low-dose cytarabine [11] has been approved by the EMA for the palliative first-line treatment of elderly and/or unfit AML patients and has significantly increased remission and OS rates. Since 2021, there has been increasing evidence suggesting that younger AML patients with R/R-AML can also benefit from VEN in combination with standard chemotherapeutic agents [12, 13]. In a prospective phase Ib/II-study by DiNardo et al. [14], in which the combination of VEN with FLAG-Ida (so called “FLAVIDA” regimen) was investigated, 29 of the 86 included AML patients were diagnosed with R/R AML (and were investigated in the phase II part of the study). The composite complete response rate (CRc) for these patients was 67%, and 46% of patients were switched to alloHCT. After a median follow-up of 12 months, the median EFS and median OS were not reached, and the corresponding 12-month EFS and OS were 77% and 94%, respectively. In another retrospective study by Wolach et al. [15] with 25 patients diagnosed mainly with R/R-AML, the FLAVIDA-protocol was also associated with a high CRc rate of 72%. The incidence of relapse-free and OS at 12 months was 67% and 50%, respectively. However, a comparison of FLAVIDA to “standard chemotherapy” regimes such as FLAG-Ida or HAM was not carried out in either study.
In 2020, Stelljes et al. [16] published the prospective “ETAL3” study, which challenged the strategy of achieving a CR in R/R-AML patients prior to alloHCT. In this analysis, 276 R/R-AML patients were randomized to receive salvage chemotherapy prior to alloHCT or were switched primarily to alloHCT within 4–6 weeks after the diagnosis of R/R-AML (without salvage therapy). The remission rates and OS did not differ between the two groups. Of note, the group of patients with salvage chemotherapy prior alloHCT (n = 137) were treated with HAM.
Shahswar et al. [17] published a retrospective cohort study of 128 R/R-AML patients treated with FLAVIDA (n = 37) or FLAG-Ida (n = 81) in 2024. The overall response rate (ORR) was significantly higher in the FLAVIDA group (p = 0.001), while the EFS and OS did not differ. A comparison with R/R-AML patients who received HAM was not performed. However, the authors concluded that the FLAVIDA regimen is an effective treatment option for R/R-AML patients, particularly as a bridge to alloHCT which produces high response rates.
The aim of our retrospective, single-center study of 53 patients with relapsed or refractory AML was therefore to obtain further “real-world” data on the putative superior efficacy of FLAVIDA in order to achieve a CR compared to standard chemotherapy regimens (excluding venetoclax).
2 Patients and Methods
2.1 Inclusion Criteria
The clinical data of all R/R-AML patients aged 18 years or older who regularly presented to our institution were collected from January 1, 2008 to September 30, 2023 (the time of the last data cut-off). Refractory AML was defined according to the European Leukemia NET (ELN) 2010 recommendations [4] as no CR or no CR with incomplete recovery (CRi) at the time of response (after initial treatment). Relapsed disease was defined as recurrence of bone marrow blasts ≥5% or recurrence of blasts in the blood, or development of extramedullary disease [4]. Secondary AML was defined as AML with myelodysplasia-related changes and/or therapy-related AML [4]. The 2010 ELN recommendations [4] were used to ensure that missing mutation analyses do not result in AML misclassifications. The study was approved by the ethics committee of our institution. All patients had given written informed consent to off-label use of venetoclax, genetic analysis, and use of clinical data according to the Declaration of Helsinki and institutional guidelines. The follow-up period began with the start of chemotherapy for R/R-AML and with the last visit to our center or the death of the patient. Data were collected retrospectively from medical records. If necessary, further information was requested from the patients and/or the treating physicians. Patients diagnosed with acute promyelocytic leukemia were excluded.
2.2 Treatment Administration
Fifty-three R/R-AML patients who met the inclusion criteria were identified. All patients received at least one salvage chemotherapy for R/R-AML between 2008 and 2023. In 18 of the 53 patients (34%), VEN was combined with fludarabine, cytarabine, and idarubicin (FLAVIDA) [14-17] between 2020 and 2023. The FLAVIDA regime consisted of one cycle of intravenous (IV) fludarabine (20–30 mg/m2) and cytarabine (1000–2000 mg/m2) on days (D) 1–5, idarubicine (IV; 8–10 mg/m2) on D1-3, and filgrastim (5 μg/kg subcutaneous) in combination with VEN. Filgrastim was administered in all patients receiving FLAVIDA daily until the white blood count (WBC) >500/μL. The VEN dose was administered orally without dose escalation at a dose of 100 mg instead of 400 mg once daily (days 1–7), as co-medication with a CYP3A4 inhibitor for antifungal prophylaxis, primarily posaconazole, was prescribed [17, 18].
The 35 (66%) control patients were selected from our institution's internal database and treated with fludarabine, cytarabine and idarubicine (FLAG-Ida) (n = 12), or HAM (n = 23) between 2008 and 2020. The FLAG-Ida regime consisted of intravenous (IV) fludarabine (20–30 mg/m2) and cytarabine (1000–2000 mg/m2) on D1-5, idarubicine (IV; 8–10 mg/m2) on D1-3 and filgrastim (5 mcg/kg subcutaneous) [4, 6, 7]. The HAM regime comprised IV cytarabine (1000–3000 mg/m2, twice daily) on D1-3 and mitoxantrone (IV; 10 mg/m2) D3-5 [8, 19]. Filgrastim was administered according to the physician's choice in 7/35 (20%) patients with FLAG-Ida or HAM.
2.3 Cytogenetic and Molecular Analysis
Molecular and cytogenetic analysis was performed centrally by G- and R-banding analysis and next-generation sequencing (NGS) from peripheral blood or bone marrow [19]. Molecular analysis was performed on each patient at the start of primary therapy. All patients were assigned a risk classification according to the ELN 2010 guidelines [4]. Data on MRD were not available for most patients and were therefore not analyzed.
2.4 Safety and Efficacy Assessment
Safety and efficacy analyses were performed for all patients who received at least one cycle of salvage chemotherapy. Consistent with previous studies [17, 18], the primary objectives of this study included the safety and tolerability of a seven-day VEN regimen with FLAG-Ida and 6-month event free survival (EFS; time from the start of treatment to the occurrence of disease progression, relapse, or death, whichever occurred first). Secondary objectives included the assessment of ORR and of 6-month OS (time from the start of treatment to death). ORR was defined according to the ELN 2010 criteria [4] and included CR, complete remission with incomplete blood recovery (CRi) (composite complete remission CRc = CR + CRi), and morphologically leukemia-free status (MLFS, defined as less than 5% blasts in an aspirate sample without hematological recovery; absence of extramedullary disease). Patients with “resistant disease” (RD) according to ELN 2010 [4] were evaluable for the response but did not fulfill the criteria for CR, Cri, or PR. Only patients were included surviving ≥7 days following completion of initial treatment, with evidence of persistent leukemia by blood and/or bone marrow examination. Bone marrow evaluation was done at day 28 ±2 days after the start of salvage chemotherapy. Treatment response was evaluated earlier than that if laboratory markers showed either complete hematologic recovery or recurrent appearance of blasts as a sign of treatment failure.
2.5 Statistical Methods
The median and range were specified for continuous variables. Differences in proportions or absolute frequencies were estimated using the Chi-square test, the Kruskal–Wallis test, the Mood's median test the Fisher's exact test, the Odds ratio, and the two-sided log-rank test. The Fligner-Killeen test was used to compare the variances between the two groups. The Cox regression model was used to account for the effects of multiple variables on ORR/EFS/OS and was significant, that is, the covariates were adequate to explain the dependent variable (p < 0.001).
The significance level was used for all analyses.
Statistical analyses were performed using statistical software environment R, version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Patient Characteristics
Eighteen patients with R/R-AML received FLAVIDA and 35 were treated with standard chemotherapy (FLAG-Ida: n = 12 [34%] or HAM: n = 23 [66%]). The median follow-up time for the FLAVIDA patients was 8.5 months (range 0–33.0) and 19.0 months (range 0–160.0) for the patients with standard chemotherapy.
The demographics data, baseline situation, and treatment characteristics did not differ statistically between the two groups (Table 1). Specifically, the median overall age at the start of salvage therapy was 56 years, and most patients were diagnosed with de novo AML (72% and 83%, respectively). Two patients who received standard chemotherapy relapsed after the previous alloHCT. There was a numerical difference in distribution between refractory (61% and 46%) and relapsed (39% and 54%) AML, which was not statistically significant. The distribution of AML subtypes and the number of previous treatment lines were also similar in the two study groups. In terms of molecular mutations, NPM1 and FLT3-ITD were the most frequent, while the complex karyotype was equally distributed, resulting in a similarity of ELN risk groups [4]. After salvage chemotherapy, 72% and 74% of patients underwent alloHCT. With regard to blood counts at baseline, there was no significant difference between median white blood cell counts (FLAVIDA: 2.1 × 109/L; standard chemotherapy: 2.7 × 109/L), hemoglobin (8.5 and 9.4 g/dL) and platelet counts (56 × 109/L and 39 × 109/L), respectively.
| Baseline characteristics | FLAVIDA (n = 18) | Standard chemotherapy (n = 35) | p |
|---|---|---|---|
| Male/female, n (%) | 7/11 (39/61) | 21/14 (60/40) | 0.24 |
| Age at start of salvage therapy a , years (range) | 53 (24–70) | 57 (27–69) | 0.66 |
| Type of AML, n (%) | 0.48 | ||
| De novo | 13 (72) | 29 (83) | |
| Secondary | 5 (28) | 6 (17) | |
| ELN 2010 b risk group, n (%) | 0.68 | ||
| Favourable | 4 (22) | 8 (23) | |
| Intermediate 1 | 6 (33) | 20 (57) | |
| Intermediate 2 | 3 (17) | 1 (3) | |
| Adverse | 5 (28) | 6 (17) | |
| Disease status at time of salvage chemotherapy a , n (%) | 0.44 | ||
| Refractory | 11 (61) | 16 (46) | |
| Relapse | 7 (39) | 19 (54) | |
| Refractory after n cycles of chemotherapy | 1.00 | ||
| One cycle, n (%) | 17 (94) | 34 (97) | |
| ≥2 cycles, n (%) | 1 (6) | 1 (3) | |
| Molecular mutations, n (%) | 0.18 | ||
| NPM1 | 3 (17) | 4 (11) | |
| FLT3-ITD | 3 (17) | 5 (14) | |
| IDH1 | 1 (6) | 0 (0) | |
| IDH2 | 3 (17) | 2 (6) | |
| KRAS | 1 (6) | 0 (0) | |
| ASXL1 | 2 (11) | 0 (0) | |
| DNMT3A | 1 (6) | 0 (0) | |
| KMT2A | 1 (6) | 0 (0) | |
| CEBPA | 1 (6) | 3 (9) | |
| CBFB | 1 (6) | 1 (3) | |
| RUNX1 | 0 (0) | 3 (9) | |
| Complex karyotype, n (%) | 4 (22) | 6 (17) | 0.71 |
| Prior alloHCT c before salvage chemotherapy, n (%) | 0 (0) | 2 (6) | 0.54 |
| AlloHCT c after salvage chemotherapy, n (%) | 13 (72) | 26 (74) | 0.73 |
- a Salvage therapy: FLAVIDA (n = 18), FLAG-Ida (n = 12) or HAM (n = 23).
- b European LeukemiaNET 2010 [4].
- c Allogeneic hematopoietic cell transplantation.
3.2 Treatment Response
Response to treatment was evaluated after one cycle of FLAVIDA or standard chemotherapy and is summarized in Table 2. The ORR of 78% (CRc + MLFS in 14 of 18 patients) was higher in the FLAVIDA group, but not statistically different from 51% (18 of 35 patients) in the standard chemotherapy group.
| FLAVIDA (n = 18) | Standard chemotherapy (n = 35) | p | OR (95% CI) | |
|---|---|---|---|---|
| ORR a (CRc + MLFS), n (%) | 14 (78) | 18 (51) | 0.05 | OR 4.16 [1.14–15.17] |
| CRc a (CR + CRi), n (%) | 13 (72) | 16 (46) | 0.07 | n.a. |
| CR a , n (%) | 10 (56) | 15 (43) | 0.38 | |
| CRi a , n (%) | 3 (17) | 1 (3) | 0.07 | |
| MLFS a , n (%) | 1 (6) | 0 (0) | n.a. | |
| Resistant disease b , n (%) | 3 (17) | 14 (40) | 0.54 | OR 0.25 [0.01–5.13] |
- Abbreviation: n.a. = not applicable.
- a ORR (overall response rate) according to ELN 20104 includes CR, complete remission with incomplete blood recovery (CRi) (composite complete remission CRc = CR + CRi), and morphologic leukemia-free state (MLFS, defined as less than 5% blasts in an aspirate sample without hematological recovery).
- b Resistant disease according to ELN 20104: Patients evaluable for response but not meeting the criteria for CR, CRi, MLFS, or PR; only includes patients surviving ≥7 days following completion of initial treatment, with evidence of persistent leukemia by blood and/or bone marrow examination.
On the other hand, a higher proportion of patients who received standard chemotherapy had RD (40% vs. 17%). These differences were not statistically significant, which is probably due to the small sample size.
3.3 Survival
Six-month EFS was 82% in the FLAVIDA group compared to 51% in the standard chemotherapy group. Figure 1 presents the Kaplan–Meier curves of the 6-month EFS along with a log-rank-test, which shows a statistically significant difference based on the univariate log-rank-test for the 6-month censored data between the two treatments.
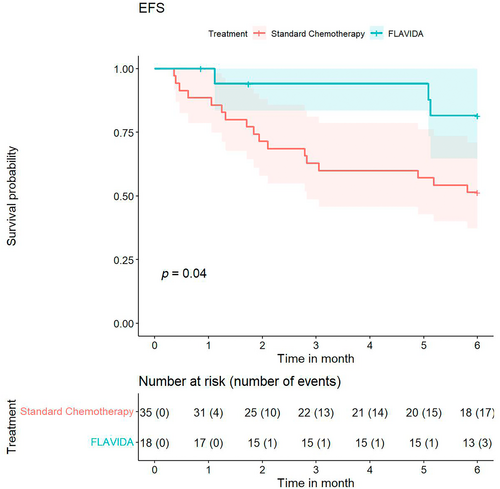
To analyze additional covariates that could influence 6-month EFS, we performed multivariate Cox regression with age at the start of salvage therapy, gender, FLAVIDA administration, ELN 2010 [4] risk group “intermediate 1/2” and “adverse,” overall response, and secondary AML as covariates. The estimates for the covariates are shown in Table 3. According to this analysis, treatment with FLAVIDA compared to standard chemotherapy (HR 0.22 [95% CI 0.05, 0.97]) and no response compared to overall response (CRc, CRi, or MLFS) (HR 14.84 [95% CI 3.91, 56.39]) were significant.
| Variable | HR [95% CI] |
|---|---|
| Multivariate Cox regression on the 6-month EFS | |
| Age at start of salvage therapy | 1.05 [1.00, 1.11] |
| Gender | 1.05 [0.40, 2.78] |
| FLAVIDA | 0.22 [0.05, 0.97] a |
| Secondary AML | 2.51 [0.71, 8.81] |
| ELN 2022 “intermediate” | 0.47 [0.11, 1.92] |
| ELN 2022 “adverse” | 0.17 [0.03, 1.09] |
| Overall response | 14.84 [3.91, 56.39] a |
| Multivariate Cox regression on the 6-month OS | |
| Age at start of salvage therapy | 1.03 [0.96, 1.09] |
| Gender | 1.74 [0.48, 6.27] |
| FLAVIDA | 0.40 [0.09, 1.81] |
| Secondary AML | 5.15 [1.15, 23.12] a |
| ELN 2022 “intermediate” | 0.33 [0.06, 1.74] |
| ELN 2022 “adverse” | 0.29 [0.04, 2.06] |
| Overall response | 26.43 [3.18, 219.46] a |
- Note: The 95% confidence intervals for the estimates are provided in parentheses. FLAVIDA treatment (HR 0.22) and overall response (HR 14.84) were significant regarding the 6-month EFS. Secondary AML (HR 5.15) and overall response (HR 26.43) were significant concerning the 6-month OS.
- a Statistically significant.
In terms of 6-month OS, 82% of patients were alive in the FLAVIDA group and 69% in the standard chemotherapy group (Figure 2). This difference was not statistically different using the two-sided log-rank test for the 6-month censored data between the two treatments.
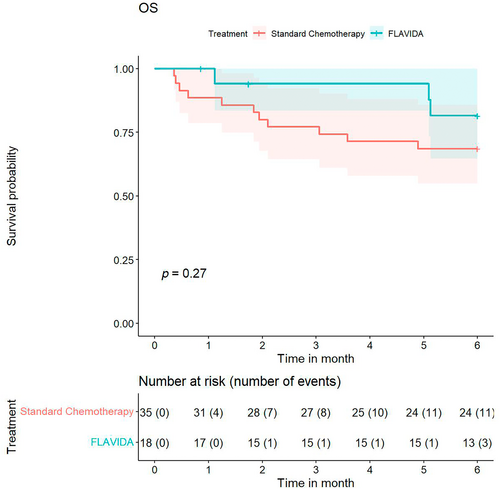
In addition, a multivariate Cox regression analysis was performed with 6-month OS as the dependent variable and with the same independent variables used for the EFS analysis (Table 3). According to this analysis, secondary AML compared to de novo AML (HR 5.15 [95% CI 1.15, 23.12]) and no response compared to overall response (CRc, CRi, or MLFS) (HR 26.43 [95% CI 3.18, 219.46]) were significant.
Regarding the entire follow-up period, no significant differences in terms of EFS and OS were observed in the log-rank test between the two groups. The corresponding Kaplan–Meier curves are presented in the supplement (Figures S1 and S2).
3.4 Safety
Adverse events such as neutropenia, anemia, and thrombocytopenia occurred in all patients in both groups (Table 4). During treatment, febrile neutropenia was the most common adverse event (96%), with only two patients (treated with standard chemotherapy) achieving hematological recovery without developing fever.
| FLAVIDA (n = 18) | Standard chemotherapy (n = 35) | p | |
|---|---|---|---|
| Time to neutrophil recovery >500/μL (ANC05) | |||
| Median days (95% CI) | 24 (19–32) | 27 (19–62) | 0.40 |
| Missing/not recovered, n (%) | 2 (11) | 10 (29) | |
| Time to neutrophil recovery >1000/μL (ANC1) | |||
| Median days (95% CI) | 26 (20–36) | 32 (20–71) | 0.21 |
| Missing/not recovered, n (%) | 2 (11) | 12 (34) | |
| Time to platelet recovery >50/nL (PLT50) | |||
| Median days (95% CI) | 28 (20–75) | 28 (17–62) | 0.59 |
| Missing/not recovered, n (%) | 3 (17) | 10 (29) | |
| Time to platelet recovery >100/nL (PLT100) | |||
| Median days (95% CI) | 31 (21–75) | 30 (19–71) | 0.33 |
| Missing/not recovered, n (%) | 6 (33) | 13 (37) | |
- Abbreviation: n.a. = not applicable.
Of note, patients with a “quick SOFA-Score” (qSOFA = systolic blood pressure < 100 mmHg, respiratory rate ≥ 22/min, altered mental status) ≥1 were considered as a life-threatening disease (CTCAE grade 4), which resulted in admission to either our intermediate care or intensive care unit. Patients who had negative qSOFA and were not treated in our IMC/ICU respectively were classified as CTCAE grade 3.
According to this definition, no statistical difference between the groups was observed with regard to febrile neutropenia ≥grade 3. In detail, 15 out of 18 patients (83%) in the FLAVIDA group and 23 of 35 patients (66%) in the standard chemotherapy group developed febrile neutropenia CTCAE grade 3. Two of 18 patients (11%) with FLAVIDA experienced CTCAE grade 4 febrile neutropenia compared to 5 out of 35 patients (14%) with standard chemotherapy.
The median time to neutrophil recovery at levels of >500/μL was 24 days (range 19–32) in FLAVIDA patients and 27 days (range 19–62) in patients in the standard chemotherapy group. For the recovery of neutrophils >1.000/μL, the difference was 26 (20–36) and 32 (20–71) days, respectively.
Regarding recovery times for platelet counts above 50/nL, a median of 28 days was determined for FLAVIDA (range 20–75) and for standard chemotherapy (range 17–62). For platelet counts above 100/nL, 31 days (range 21–75) after chemotherapy with FLAVIDA were documented compared to 30 days (19–71) after standard chemotherapy.
To compare the mean values of time to ANC and platelet recovery, we used Mood's median and the Kruskal–Wallis test. No statistical difference could be observed for ANC recovery >500/μL (ANC05) and >1000/μL (ANC1). Regarding platelet recovery, the differences in the median time to a platelet count >50/nL and 100/nL were also not significantly different.
Of note, neutrophil and platelet recovery data were missing for several patients in both groups and/or several patients did not recover in both groups (Table 4). In the next step, Kaplan–Meier curves of ANC and platelet along with log-rank-test were performed (the corresponding figures are presents in the supplement; Figures S3–S6). Only ANC recovery >1000/μL differs significantly between the FLAVIDA and standard chemotherapy groups.
4 Discussion
The prognosis of AML patients who do not response to first-line therapy or who relapse after achieving a CR is very limited. To date, (re)achieving CR by administration of salvage chemotherapy followed by alloHCT as consolidation is considered a suitable approach to achieve long-term remission [4, 5].
In recent years, there is increasing evidence, particularly from single-arm studies [14, 15], that a combination of VEN with FLAG-Ida (FLAVIDA) in patients with R/R-AML is associated with a high response rate and an acceptable toxicity profile. In 2024, Shahswar et al. [17] published their retrospective study comparing R/R-AML patients treated with FLAVIDA versus FLAG-Ida. In this analysis, FLAVIDA was associated with an increased ORR and a comparable toxicity profile. However, EFS and OS were similar between the two groups and no comparison was performed with patients treated with HAM. In 2022, Stelljes et al. [16] published data from the prospective “ETAL3” study. In this analysis, 276 R/R-AML patients were randomized to receive salvage chemotherapy prior to alloHCT or were switched primarily to alloHCT within 4–6 weeks after the diagnosis of R/R-AML (without salvage therapy before transplantation). The remission rates and OS did not differ between the two groups, whereas toxicity was significantly increased in the group of patients who received salvage therapy. Of note, all 137 included patients with salvage chemotherapy before alloHCT were treated with HAM.
In our retrospective study, a comparison was made between R/R AML patients treated with FLAVIDA and patients with standard chemotherapy in terms of EFS and (hematological) toxicity, with almost 2/3 of patients in the standard chemotherapy arm receiving HAM. In contrast to previous studies [17, 18], a statistically significant benefit with regard to EFS was demonstrated in the univariate log-rank-test and in the multivariate Cox regression (HR 0.22) with a comparable toxicity profile. Furthermore, there was a clear trend towards improved ORR and even an improved 6-month OS. However, these differences were not statistically significant, possibly due to the smaller number of patients included. Additional limitations included the retrospective, single-center design of the study, the lack of MRD data, and the short follow-up period for the FLAVIDA patients. It is noteworthy that the short follow-up period results from the use of the FLAVIDA regimen in our center from 2020 and beyond, while previously mainly standard chemotherapy regimens were used. Additionally, 15.1% (8/53) of the R/R AML patients in our study were FLT3-ITD mutated. Since the “ADMIRAL”-study [20], Gilteritinib has been EMA approved for the treatment of R/R AML with FLT3 mutation and therefore represents another treatment option in this situation.
5 Conclusions
In summary, our analysis demonstrated a statistically significant improvement in 6-month EFS of R/R AML patients treated with FLAVIDA compared to standard chemotherapy, supporting the data of DiNardo et al. and Shahswar et al. [9, 17, 18]. Importantly, our study also showed that administration of FLAVIDA does not increase the risk of serious complications. In view of the “ETAL3”- [16] and the “ADMIRAL”-study [20], FLAVIDA appears to represent a promising treatment alternative for R/R AML patients (without FLT3 mutation), as more than 4–6 weeks remain until alloHCT.
Author Contributions
Study conception and design: Kai Wille, Marvin Dumke, and Martin Griesshammer. Data collection: Kai Wille, Marvin Dumke, Parvis Sadjadian, Vera Kolatzki, Stephanie Jender-Bartling, and Anette Horstmann. Analysis and interpretation of results: Kai Wille, Marvin Dumke, Nadine Wilsdorf, Artur Schneider, Raphael Meixner, Marina Jiménez-Muñoz, Christiane Fuchs, Hans-Joachim Tischler, and Martin Griesshammer. Draft manuscript preparation: Kai Wille, Marvin Dumke, and Martin Griesshammer. All authors reviewed the results and approved the final version of the manuscript.
Ethics Statement
This study protocol was reviewed and approved Ethics Committee of the Ruhr-Universität Bochum, based in Bad Oeynhausen, approval number 2023-1123.
Conflicts of Interest
Dr. Wille declares funding, advisory board honoraria and other financial support (e.g., travel support) from Amgen, AOP Orphan, Novartis, BMS, AbbVie, Pfizer, Roche, Janssen, Gilead, AstraZeneca, Lilly. Professor Dr. Griesshammer declares funding, advisory board honoraria and other financial support (e.g., travel support) from Amgen, AOP Orphan, Novartis, BMS, AbbVie, Pfizer, Roche, Janssen, Gilead, AstraZeneca, Sierra, Lilly. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are not publicly available due to their containing information that could compromise the privacy of research participants but are available from the corresponding author (K.W.) upon reasonable request.




