Incidence of cardiovascular and bleeding events and reasons for discontinuation in patients with chronic lymphocytic leukemia treated with ibrutinib—A retrospective analysis on consecutive patients from a well-defined region
Abstract
Objective
Ibrutinib treatment is associated with cardiovascular side effects, in particular atrial fibrillation (AF) and hypertension, as well as increased risk of bleeding. Here, we aimed at describing the incidence of these events during long-term follow-up in patients with chronic lymphocytic leukemia treated outside clinical trials as well as identifying clinical factors predictive of developing AF. Additionally, other reasons for treatment withdrawal were analyzed.
Methods
The study was retrospective, data were collected from medical records.
Results
A total of 134 patients were identified. Median follow-up was 32 months (range 3–103) and median duration of ibrutinib treatment was 26 months (range 1–103). Of 110 patients with no prior history of AF, 24.5% were diagnosed during treatment. Newly diagnosed or worsening of pre-existing hypertension occurred in 15.7%. Sixty-six % of the patients experienced bleeding events, of which 7.5% grade 3–4. Treatment discontinuation and dose reduction occurred in 68% and 47% of the patients, respectively, mostly due to toxicity.
Conclusions
The incidence of AF was high and at a median follow-up of 2.5 years, two-thirds of the patients discontinued treatment mostly due to bleeding and infections. Treatment-related toxicity of any grade should be regarded as a concern of prolonged ibrutinib therapy.
1 INTRODUCTION
Ibrutinib, the first-in-class Bruton tyrosine kinase inhibitor (BTKi), has paved the way for the introduction of these drugs in the treatment algorithm of chronic lymphocytic leukemia (CLL). The approval of ibrutinib by the US Food and Drug Administration (FDA) for the treatment of CLL in 2014 has been a major breakthrough for the treatment of this disease. Relapsed/refractory (R/R) patients with high-risk characteristics, such as TP53 aberration and unmutated immunoglobulin heavy chain variable region genes benefit from treatment with ibrutinib as well as treatment-naïve patients with favorable characteristics.1-3 The drug is assumed once daily until disease progression or unacceptable toxicity occurs.
In contrast to the treatment benefits, toxicity including infections, bleeding, diarrhea, arthralgia, hypertension and atrial fibrillation (AF) is a growing concern. Discontinuation due to toxicity has been reported in various rates in clinical trials and real-world studies, usually more frequent in the latter.4-6 With regard to cardiovascular toxicity, most frequently in the form of AF and hypertension as well as bleeding, the incidence varies in clinical trials. Available long-term follow-up data in the real-world setting are, however, limited.
Most of these side effects are thought to be related to the off-target inhibitory activity of ibrutinib on kinases other than BTK. Indeed, ibrutinib can also bind, with various affinities, to other kinases, which have a corresponding cysteine residue as BTK. It is speculated that ibrutinib-induced AF is an off-target effect related to inhibition of the C-terminal Src kinase expressed in the cardiac tissue.7 Bleeding events are thought to result from both off- and on-target effects since inhibition of BTK and TEC in different pathways affects platelet activation.8, 9
CLL is a disease of the elderly and cardiovascular comorbidity is common in this patient population by the time of diagnosis and when treatment is initiated.10 As discussed above, the occurrence of one or a combination of cardiovascular adverse events, especially AF, during ibrutinib treatment is not uncommon and the management of AF requires prophylactic anticoagulation, thus adding to the bleeding risk related to ibrutinib.
Structured risk assessment of thromboembolic events related to AF, in particular ischemic stroke, has improved long-term prognosis for patients with AF by allowing a better selection of the patients eligible for prophylactic anticoagulant treatment. Despite the fact that the two most established risk scores for AF-related thromboembolic stroke, the CHADS₂score (congestive heart failure, hypertension, age ≥ 75 years, diabetes, previous stroke/transient ischemic attack/thromboembolism) and the CHA₂DS₂-VASc score (addition of the risk factors vascular disease, age 65–74 years, female sex) were not primarily validated for cancer patients, emerging data support their usefulness.11, 12 In cancer patients, a multidisciplinary team decision is recommended to evaluate the risk of stroke versus risk of bleeding depending on type of cancer and anti-cancer treatment. This is particularly relevant for patients treated with an agent increasing the risk of bleeding such as ibrutinib.13
The European Society of Cardiology Guidelines on CardioOncology published in 2022 recommend that a cardiovascular risk assessment including measurement of blood pressure and screening for AF is performed before start of treatment with BTKis and at every clinical follow-up. In addition, a transthoracic echocardiography should be performed in patients considered at high risk of cardiotoxicity from BTKis (i.e., male, age ≥ 65 years, diabetes mellitus, QTc ≥480 ms, AF, heart failure, cardiomyopathy or severe valvular heart disease).14, 15
Most of the data available derive from clinical trials which are not always representative for the general patient population due to selection of a more homogenous patient group and standardized and close monitoring.
The aim of this retrospective real-world study was to describe the incidence of cardiovascular and bleeding events in a real-world cohort of consecutive patients with CLL treated with ibrutinib. In addition, we analyzed length of treatment with ibrutinib therapy and reasons for withdrawal.
Furthermore, we aimed at identifying possible risk factors for developing AF during ibrutinib treatment, such as the pre-existence of cardiovascular comorbidity and metabolic disorders, which could be considered in the treatment decision process and/or motivate a more intensive clinical follow-up during ibrutinib treatment in selected patients.
2 METHODS
2.1 Study population
Patients with CLL, age > 18 years, treated with single-agent ibrutinib for at least 1 month during the period January 2014 to September 2021 at the Lymphoma unit, Department of Hematology, Karolinska University Hospital were identified and followed until December 2022. The research project was approved by the Swedish Ethics Authority (www.epm.se) and conducted in accordance with the Declaration of Helsinki.
2.2 Data collection
Clinical data were gathered retrospectively from each patient's individual medical records from the hematology clinic, emergency room, primary care, and other specialized clinics. Patients with other active malignancies in need of non-CLL systemic cancer treatment including chemotherapy, immunotherapy, biological drugs, hormone therapy, or radiotherapy at the time of start of ibrutinib treatment were excluded.
Cumulative Illness Rating Scale (CIRS)16 was used to determine overall multimorbidity burden. Information of dose reductions was included if lasting >1 month.
The occurrence of new or worsening of pre-existing cardiovascular events including AF, coronary artery disease, heart failure, valvular disease, myocardial infarction, and hypertension were reported as they were diagnosed and managed by physicians. Worsening of pre-existing AF and hypertension was defined as increased symptoms with the need of new or increased dosage of medicine and/or need for intervention. Bleeding events were reported and graded according to Common terminology criteria for adverse events version 5.0.
2.3 Statistical analysis
Descriptive analyses present distributions of clinical characteristics as counts and percentages for categorical variables, and median values together with their minimum and maximum values for continuous variables. Time at risk in progression-free survival (PFS) was calculated from the date of treatment start to the date of progression, date of start of new treatment, or date of death, whichever comes first. For event-free patients, time was calculated from the date of treatment start to the date of last clinical visit. In overall survival (OS), time at risk was calculated from the date of treatment start to the date of death, or for patients still alive, to the date of last clinical visit. Survival was graphically presented using the Kaplan–Meier technique.
Distributional differences in baseline characteristics between patients with newly debuted AF and without AF, were assessed using the Chi-squared test—or Fischer exact tests when proper—for categorical variables, and with the Mann–Whitney test for continuous variables.
The time to failure (TTF) is calculated from the date of treatment start to the date of AF, discontinuation due to any cause, that is, toxicity, CLL progression, allogeneic stem cell transplantation, patient's decision or death/other reason, whichever comes first. For event-free patients, that is, with no of the events listed above, TTF was calculated from the date of treatment start to the last clinical visit date. Cumulative incidence functions are used to graphically illustrate the risk of AF over time taking the competing risk of other events into account. The effect covariates on the subhazards were modeled using competing risk regression.17 Results from these models are presented as subhazard ratios, 95% confidence intervals, and Wald p-values.
All statistical analyses were performed using Stata, and StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.
3 RESULTS
3.1 Patient characteristics
A total of 134 patients with CLL treated with ibrutinib were identified. The baseline characteristics are shown in Table 1. The median age at start of ibrutinib treatment was 74 years (range 22–94), 72% were males and 33% had del(17p) and/or TP53 mutations. The median number of prior treatments was 2 (range 0–7) and 33 (25%) patients received ibrutinib as first-line treatment. Sixty-five patients (47%) had a CIRS score ≥6. The most common cardiovascular comorbidities at the time of ibrutinib initiation were hypertension (41%), diabetes mellitus (20%), and AF (18%).
| Total population, n | 134 | |
|---|---|---|
| Gender | ||
| Male, n (%) | 97 | |
| Female, n (%) | 37 | (27.6) |
| Median age, years (range) | 74 | (22–94) |
| CIRS score ≥6, n (%) | 63 | (47.0) |
| del(17p)/TP53 mutation, n (%) | 44 | (32.8) |
| Previous treatment lines, median (range) | 2 | (0–7) |
| Prior treatment with anthracyclines, n (%) | 15 | (11.2) |
| Ibrutinib as first line treatment, n (%) | 33 | (24.6) |
| Cardiovascular comorbidities | ||
| BMI≥30, n (%) | 22 | (16.4) |
| Smoking previous/current, n (%) | 51 | (38.1) |
| Diabetes, n (%) | 27 | (20.1) |
| Hypertension, n (%) | 55 | (41.0) |
| Atrial fibrillation, n (%) | 24 | (17.9) |
| Heart failure, n (%) | 19 | (14.2) |
| Coronary ischemic artery disease, n (%) | 17 | (12.7) |
| Valvular disease, n (%) | 7 | (5.2) |
| Ischemic cerebral infarction/TIA, n (%) | 11 | (8.2) |
| Venous thromboembolism, n (%) | 17 | (12.7) |
| Medications, cardiovascular and anticoagulants | ||
| Beta-blocker, n (%) | 43 | (32.1) |
| ACE inhibitor/ARB, n (%) | 33 | (23.1) |
| Calcium channel blocker | ||
| Vascular selectivity, n (%) | 14 | (10.4) |
| Myocardial selectivity, n (%) | 1 | (0.7) |
| Digoxin, n (%) | 3 | (2.2) |
| Diuretics, n (%) | 25 | (18.7) |
| Antiplatelet, n (%) | 18 | (13.4) |
| DOAC, n (%) | 11 | (8.2) |
| LMWH, n (%) | 15 | (11.2) |
| Warfarin, n (%) | 1 | (0.7) |
- Abbreviations: ARB, angiotensin receptor blocker; ACE, angiotensin converting-enzyme; BMI, body mass index; CIRS, cumulative illness rating score; DOAC, direct oral anticoagulant; LMWH, low molecular weight heparin; TIA, transient ischemic attack.
3.2 Progression-free survival and overall survival
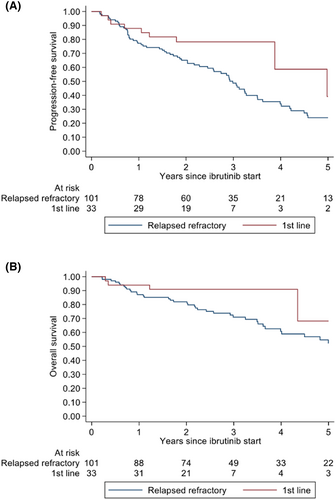
The median follow-up time for the whole cohort was 32 months (range 3–103), for first-line treatment 27 months (range 3–103), and R/R 35 months (range 3–102). At last follow-up, 43 patients (32%) were still on treatment (first-line n = 18, R/R n = 25). Forty-seven patients had died due to progressive disease (n = 16), infection (n = 16), or other (n = 15). Median PFS and OS were not reached when ibrutinib was used as first-line treatment. At 2 years, the PFS rate was 77% and OS rate 91%. For R/R patients, the median PFS was 3 years and median OS 5 years. At 2 years, PFS and OS rate was 65%, respectively, 81% (Figure 1).
3.3 Treatment discontinuation and dose reductions
Median duration of ibrutinib treatment was 26 months (1–103). At the end of the study period, 91 patients (68%) had discontinued treatment permanently. In the whole cohort, the most common reason for discontinuation was toxicity (25%; 33/134), followed by death (20%; 27/134) and progressive disease (19%; 26/134). Of the patients with progressive disease, 6 developed Richter's transformation. Of the 33 patients who discontinued treatment due to toxicity, the most common reason was bleeding events (n = 9, of which 5 grade 3–4) and infection (n = 8). The median time to discontinuation due to toxicity was 10 months (range 1–81). In the majority of the cases (88%), the strategy after discontinuation was watch and wait, while in the remaining cases, treatment was switched to acalabrutinib.
The reason for discontinuation differed between patients with R/R disease and patients receiving ibrutinib as first-line treatment. Discontinuation due to toxicity was 23% (23/101) and 27% (9/33), respectively in the two different patient groups (Figure 2). All reasons for discontinuation are listed in Table 2.

| Relapsed/refractory n = 101 | First line treatment n = 33 | |||
|---|---|---|---|---|
| Discontinuation, total, n (%) | 76 | (75.2) | 15 | (45.5) |
| Toxicity, total, n (%) | 23 | (22.8) | 9 | (27.3) |
| Hypertension, n | 1 | - | ||
| Atrial fibrillation, n | 3 | 1 | ||
| Bleeding, n | 4 | 5 | ||
| Infection, n | 7 | 1 | ||
| Cytopenia, n | 4 | 1 | ||
| Diarrhea, n | 1 | - | ||
| Arthralgia, n | 1 | - | ||
| Elevated liver markers, n | 2 | 1 | ||
| CLL progression, total, n (%) | 22 | (23.7) | 4 | (12.1) |
| Transformation, n | 4 | 2 | ||
| Allogeneic stem cell transplantation, n (%) | 1 | (1.0) | - | |
| Death, total, n (%) | 25 | (24.8) | 2 | (6.1) |
| Infection, n | 11 | 1 | ||
| Secondary malignancy, n | 5 | - | ||
| Sudden cardiac arrest, n | 3 | - | ||
| Heart failure, n | 2 | - | ||
| Respiratory insufficiency, n | 2 | - | ||
| Transformation, n | - | 1 | ||
| Cytopenia unknown, n | 1 | - | ||
| Unknown, n | 1 | - | ||
| Other, total, n (%) | 5 | (5.0) | - | |
| Interaction with other medicine, n | 1 | - | ||
| Patient's choice, n | 4 | - | ||
Reasons for death during ibrutinib treatment were infection (n = 12), secondary malignancy (n = 4), sudden cardiac arrest (n = 3), heart failure (n = 2), Richter's transformation (n = 1), and other (n = 5). Of the three patients who died due to sudden cardiac arrest, one had been treated with ibrutinib for 2.5 years and had multiple cardiovascular comorbidities at the time ibrutinib was initiated, that is, heart failure, AF, hypertension, prior stroke, ischemic heart disease, chronic kidney disease and obstructive sleep apnea. For this patient, the initial rhythm before cardiac arrest was FF/ventricular fibrillation. Another patient had been treated with ibrutinib for 55 months, developed AF 5 months prior to death, and had diabetes mellitus and hypertension as cardiovascular comorbidities. In this case, the initial rhythm was asystole. Finally, the third patient had been treated with ibrutinib for 11 months and had no comorbidities, the initial rhythm before cardiac arrest being asystole. No prior malignant arrhythmia was described in any of the patients. Of the two patients who died due to heart failure, both had AF at baseline which had not worsened during ibrutinib treatment. However, one of these patients was diagnosed with heart failure during ibrutinib treatment.
Overall, 47% of the patients (63/134) experienced at least 1 dose reduction during the time of study, and the majority of these (57/63) were still on a reduced dose when ibrutinib was discontinued or at last follow-up. The most common reason for dose reduction was bleeding events (25/63), most often low-grade (n = 21). All reasons for dose reduction are listed in Table S1.
3.4 Occurrence and management of atrial fibrillation
Of the 110 patients with no prior history of AF, 27 (24.5%) developed AF during ibrutinib treatment. The estimated cumulative incidence of AF at 1 year was 11.9% (95% CI 7–18), at 2 years 14.4% (95% CI 9–22), at 3 years 15.5% (CI 9.9–22), at 4 years 18.2% (95% CI 12–26) and at 5 years 22.7 (95% CI 15–31) as illustrated in Figure 3.
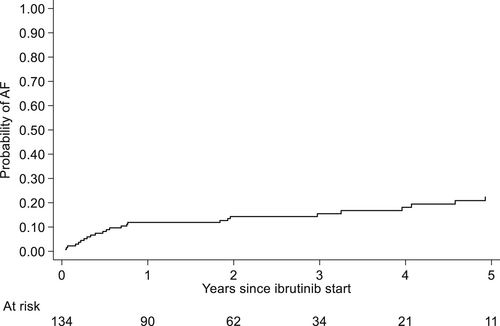
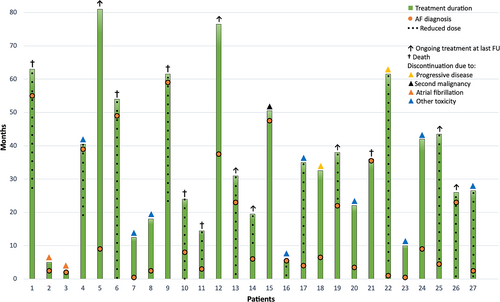
The median time to AF diagnosis from treatment start was 7 months (range 0.5–59) and median age at AF diagnosis was 77 years (range 44–89). Seventeen patients (63%) were diagnosed within the first year of treatment. At the time of AF diagnosis, 9 pts were on a reduced dose of ibrutinib. No dose reductions after the onset were due to AF. The time to AF diagnosis in relation to treatment duration, reason for discontinuation, and dose reductions for each individual patient is illustrated in Figure 4.
We performed a uni- and multivariate analysis comparing baseline clinical characteristics (that is, age > 65 years, male gender, hypertension, heart failure, diabetes mellitus, ischemic coronary heart disease, BMI >30, valvular heart disease, and obstructive sleep apnea) of the patients who developed AF and those who did not. No characteristic was significantly different between the groups.
Atrial fibrillation was diagnosed in different clinical settings and health care instances, most commonly at the emergency room (n = 14, 52%), followed by the hematology clinic (n = 4, 15%), during hospitalization (n = 4, 15%), other specialized clinics (n = 3, 11%) and primary health care (n = 2, 7%).
At time of diagnosis, the reason for seeking health care was infection for 8 patients (30%), symptomatic heart palpitations and irregular heart rhythm for 7 patients (26%), dyspnea for 3 patients (11%), chest pain for 1 patient, dizziness for 1 patient and circulatory shock for 1 patient. Six patients (22%) were asymptomatic and AF was diagnosed by routine heart auscultation and/or electrocardiogram (ECG). Fourteen patients did not require any frequency or rhythm-control medications while 13 patients did. Five patients were prescribed betablockers, 5 patients were prescribed a higher dosage of beta-blockers, 1 was prescribed a higher dosage of beta-blocker and continuously orally administrated Digoxin, 1 was treated temporarily with Digoxin intravenously and 1 was prescribed beta-blockers, temporary digoxin intravenously and cardioversion was performed.
Median CHA₂DS₂-VASc for the patients with newly diagnosed AF was 3 (range 0–6). Two patients had a score 0. Anticoagulant treatment was prescribed to 22 patients (81%). Direct oral anticoagulants (DOAC) were prescribed in 20 patients of whom 16 patients in reduced doses, which is the current practice at our clinic. The dose reduction was motivated in 12 patients by the additional bleeding risk given by concomitant ibrutinib. Of those 5 patients who were not prescribed anticoagulant treatment, 2 patients had CHA₂DS₂-VASc score 0 and 2 had an excessive bleeding risk and 1 patient refused treatment. No ischemic stroke was reported among the patients diagnosed with AF.
Six of 24 patients (25%) had worsening of pre-existing AF during ibrutinib treatment. The median time to worsening was 2 months (range 0.5–44). Of these, 3 had paroxysmal AF, and 3 persistent. After start of ibrutinib treatment, all 3 patients with paroxysmal AF developed persistent AF. The symptoms were heart palpitations and increased heart rate requiring either a new or increased dose of medication for rate and/or rhythm control.
Four patients discontinued treatment due to AF. Of these, 2 were diagnosed with AF shortly after treatment started and had symptomatic heart palpitations. Both changed treatment to acalabrutinib, as it was available and approved in Sweden at that time point. Two patients with previously known AF had multiple symptomatic AF episodes which required inpatient care for rate control. One of these patients initially reduced the dose without improvement of the AF. Initial strategy was to watch and wait but both experienced CLL progression within 3 months and next treatment was acalabrutinib and venetoclax, respectively.
3.5 Occurrence of other cardiovascular toxicities
Seventy-nine of the 134 patients had no history of hypertension at treatment start. Of these, 9 (11%) patients were diagnosed with hypertension during ibrutinib treatment. Of the 55 patients who had hypertension at start of treatment, 12 (22%) worsened during treatment. One patient discontinued ibrutinib due to hypertension and treatment was changed to acalabrutinib. Nineteen patients had heart failure at the time of ibrutinib initiation. Of these, 5 worsened during the course of treatment, one due to worsening, that is, increased heart frequency of AF that was newly diagnosed after the start of ibrutinib. Of the 115 patients without a previous history of heart failure, 10 were newly diagnosed during treatment. In 4 of these 10 patients, of whom all were diagnosed with AF during ibrutinib treatment, AF was considered as the underlying cause of heart failure. All cardiovascular and bleeding events are summarized in Table 3.
| Atrial fibrillation, total, n (%) | 33/134 | (24.6) |
| Newly diagnosed, n (%) | 27/110 | (24.5) |
| Worsening of pre-existing, n (%) | 6/24 | (25.0) |
| Hypertension, total, n (%) | 21/134 | (15.7) |
| Newly diagnosed, n (%) | 9/79 | (11.4) |
| Worsening of pre-existing, n (%) | 12/55 | (21.8) |
| Heart failure, total, n (%) | 15/134 | (11.2) |
| Newly diagnosed, n (%) | 10/115 | (8.7) |
| Worsening of pre-existing, n (%) | 5/19 | (26.3) |
| Ischemic heart disease, total, n (%) | 2/134 | (1.5) |
| Ischemic stroke, n (%) | 2/134 | (1.5) |
| Bleeding events, all grades, n (%) | 89/134 | (66.4) |
| Grade 1–2, n (%) | 79 | (59.0) |
| Grade 3–4, n (%) | 10 | (7.4) |
3.6 Occurrence of bleeding events
Bleeding events occurred in 89 patients (66%). Of these, the majority were grade 1–2 occurring in 79 patients (59%) of which the vast majority was bruising. Grade 3/4 events occurred in 10 (7%) patients: 6 upper gastro-intestinal bleedings requiring blood transfusion, 1 intracranial hemorrhage, 1 nose bleeding, and 2 post-operative bleedings after hip surgery and aorta dissection surgery, respectively. At the time of occurrence of the bleeding, 3 patients were receiving anticoagulant/antiplatelet medication: 1 with DOAC, 1 with low molecular weight heparin (LMWH), and 1 with acetylsalicylic acid. Bleeding events were the most common reason for permanent discontinuation (9/31 patients) as well as the most common reason for dose reduction (27/63 patients).
4 DISCUSSION
In this real-world study, we retrospectively gathered and analyzed data from a well-defined real-world cohort of consecutively identified patients with CLL treated with ibrutinib with a long follow-up. We focused on toxicity, in particular cardiovascular and bleeding events, and evaluated the reasons for treatment discontinuation and dose reduction. The aim was to provide a genuine description of the usage and side effects of ibrutinib outside clinical trials.
At a median follow-up of 32 months (up to 8 years), 68% of the patients had discontinued treatment permanently, which is a higher percentage compared to what reported by other real-world studies as well as randomized clinical trials. Indeed, at the long-time follow-up of the randomized clinical trials RESONATE and RESONATE 2, 54% and 41% of the patients discontinued treatment at a median follow-up of 41 and 60 months, respectively, the most common reason for discontinuation being progressive disease for previously treated patients and adverse events for patients receiving ibrutinib as first-line treatment.4, 5
In real-world studies with a median follow-up shorter than 2 years, discontinuation rates have been reported to range from 15% to 42%.6, 18-20 In one study conducted by Moldovianu et al, with a median follow-up time of 32 months (range 22–51), 44% of the patients discontinued treatment.21 However, only 9% of the patients discontinued due to toxicity while in our study 25% did, which could be explained by the older age and higher comorbidity of the patients in our cohort. Data from other studies are discordant, with either toxicity6, 20, 22 or progressive disease18, 19, 21 reported as the most frequent reason for discontinuation.
In our study, 47% of the patients experienced at least 1 dose reduction, the most common reason for dose reduction being low-grade bleeding events, pointing out that even lower-grade toxicity often leads to dose reductions.
We then focused on AF, which is probably the clinically most relevant ibrutinib-related adverse event. At the end of the follow-up, a total of 24.5% had developed AF and at a median time of 2.5 years, the cumulative incidence was 15%. In a pooled analysis from four large randomized clinical trials, the incidence of AF was 6.5% at a median follow-up time of 16.6 months, compared with 1.6% in the control group.23 In long-term follow-up of clinical trials, the incidence of AF has been reported in 11%–16% of the patients.4, 5
In previously published real-world studies with varying follow-up times, the majority shorter than ours, the incidence ranged between 3% and 17%. In these studies, AF was reported as cumulative incidence at the end of follow up, and therefore a direct comparison with our data is not always possible.6, 18, 20, 21, 24-26 Also, real-world data from long-term follow-up are limited. However, in a prospective study in which patients were monitored with ECG at least every third month, the cumulative incidence was reported to be 38% at 2 years.27 In our study, two-thirds of the newly diagnosed AF cases occurred within the first year of exposure of ibrutinib. Thereafter the rate plateaued, which is in line with long-term follow-up data from clinical trials.4, 5
Previously, clinical characteristics such as age > 65 years, male gender, hypertension, and other cardiovascular diseases have been associated with an increased risk of developing AF in patients treated with ibrutinib.23, 28, 29 Our analysis did not identify any significant differences with regard to baseline clinical characteristics (i.e., age > 65, male, hypertension, heart failure, diabetes mellitus, ischemic coronary heart disease, BMI >30, valvular heart disease, and obstructive sleep apnea) between the patients who developed AF and those who did not, probably due to the small size of the cohort. Therefore, based on our results, we cannot speculate that the higher incidence of cardiovascular comorbidities in our patient cohort at baseline might have contributed to the higher incidence of AF we observed compared to previous reports.
The incidence of both newly diagnosed and worsening of pre-existing hypertension in our study was 16%, similar to clinical trials with comparable ibrutinib exposure. Dickerson et al. reported a distinctively higher incidence of 78%30 with a cut-off for hypertension at 130/80 mmHg. In Sweden, in accordance to the guidelines from European Society of Cardiology,13 hypertension is diagnosed at a level of 140/90 mmHg. In addition, Dickerson et al. collected blood pressure numerically from medical records while in our study we reported hypertension when it was diagnosed by a physician. This might have contributed to the lower incidence of hypertension in our patient cohort since elevated blood pressure rarely causes clinical symptoms.
We then reported that three patients died due to sudden cardiac arrest during ibrutinib treatment. Two of these patients had been on treatment for more than 2 years and had significant cardiovascular comorbidities, including AF, while the third patient had none. With regard to the patients who died due to heart failure, both had AF at baseline which had not worsened during ibrutinib treatment and one was diagnosed with heart failure during ibrutinib treatment. Even if it noticeable that one patient experienced sudden cardiac arrest shortly after start of ibrutinib in the absence of previous cardiovascular comorbidities, it is not possible to draw any conclusion about the possible impact of ibrutinib treatment on this occurrence of sudden cardiac arrest in these patients.
Cardiovascular toxicity of ibrutinib is indeed a great concern in clinical practice. Second-generation BTKis, that is, acalabrutinib and zanubrutinib, demonstrated a non-inferior efficacy and a significantly lower incidence of AF and hypertension as well as fewer permanent discontinuations due to toxicity.31, 32
Last year, updated international cardio-oncology guidelines have been published with recommendations on the management of cardiovascular toxicity from different anti-cancer drugs. It is recommended that before initiation of treatment with BTKis, a thorough cardiovascular assessment should be performed including blood pressure measurement and ECG in all patients. In addition, an echocardiogram should be included in patients with high cardiovascular risk. For this latter high-risk group, established cardiovascular disease and/or age > 70, treatment with a second-generation BTKi is preferred. The guidelines recommend frequent monitoring, that is, once to twice weekly monitoring of blood pressure during the first 3–6 months of treatment and thereafter at every clinical check-up, when pulse palpation and/or ECG should also be performed.33, 34
In our cohort, 49% of the patients had either hypertension, AF, heart failure, valvular disease, and/or ischemic heart disease. In a recently published review, it has been suggested that in case the event of AF occurs during ibrutinib treatment, a CHA₂DS₂-VASc score ≥2 can be used as a guidance that BTKi treatment should be discontinued.34 In our cohort, 93% of the patients who developed AF had a CHA₂DS₂-VASc score ≥2. However, data that strongly support this recommendation are still lacking and there are no current international guidelines available on how to manage cardiovascular events during ibrutinib treatment. A less stringent approach based on the clinician's judgment rather than on defined criteria have also been suggested.35 In other words, the benefits of ibrutinib-treatment should be balanced for every individual patient against the cardiovascular risks.
The major limitation of our study is the relatively small size of the patient cohort. However, we were able to identify all patients treated in our region and performed a careful review of the patients' medical records from different clinics and physicians, which hopefully has contributed to providing a faithful picture of ibrutinib treatment in the real world.
We confirmed that the incidence of AF and bleeding events is high among patients with CLL treated with ibrutinib. However, the real incidence of AF and in particular hypertension might be even higher, considering the fact that both these conditions might be asymptomatic and therefore underdiagnosed. Even if we could not find any clinical characteristics significantly associated with increased risk of developing AF, a thorough cardiovascular assessment of all the patients, and of those with high cardiovascular risk in particular, is warranted before start of treatment with ibrutinib, as well as frequent monitoring of the blood pressure and pulse during the first months of treatment. This recommendation holds true also for second-generation BTKis, which have a more favorable, but still not irrelevant, cardiovascular toxicity profile.
Finally, in our patient cohort, two-thirds and almost half of the patients discontinued treatment or reduced the dose, respectively, mostly due to toxicity in the form of bleeding and infections. This prompts us to regard treatment-related toxicity of any grade as a concern of prolonged ibrutinib therapy since even low-grade toxicity can significantly affect the patients' quality of life.
AUTHOR CONTRIBUTIONS
Maria L. Andersson, Anders Österborg, Agneta Månsson-Broberg, Lotta Hansson, and Marzia Palma designed the study, analyzed the results, and wrote the draft manuscript. Hemming Johansson performed the statistical analyses and wrote the statistical part of the manuscript together with Maria L. Andersson. Maria L. Andersson, Marzia Palma, Lotta Hansson, Agneta Månsson-Broberg, and Anders Österborg interpreted the results. All authors reviewed and edited revisions of the manuscript and had final responsibility for the decision to submit for publication.
ACKNOWLEDGEMENTS
This study was supported by grants from the Swedish Blood Cancer Foundation, Region Stockholm, The Swedish Cancer Society, the Cancer- and Allergy Foundation, and the Cancer Society in Stockholm.
CONFLICT OF INTEREST STATEMENT
Lotta Hansson has received research grant support from Janssen-Cilag. Marzia Palma and Anders Österborg have received research grant support from Beigene Ltd and AstraZeneca. Agneta Månsson-Broberg has received research grant support from Amgen, and speaker's honoraria from BMS and Orion Pharma.
Open Research
DATA AVAILABILITY STATEMENT
All files with original data are available upon request from the corresponding author.




