Crovalimab treatment in patients with paroxysmal nocturnal haemoglobinuria: Long-term results from the phase I/II COMPOSER trial
Abstract
Objectives
This study reports long-term outcomes from the open-label extension (OLE) period of the Phase I/II COMPOSER trial (NCT03157635) that evaluated crovalimab in patients with paroxysmal nocturnal haemoglobinuria, who were treatment-naive or switched from eculizumab at enrolment.
Methods
COMPOSER consists of four sequential parts followed by the OLE. The primary OLE objective was to assess long-term crovalimab safety, with a secondary objective to assess crovalimab pharmacokinetics and pharmacodynamics. Exploratory efficacy endpoints included change in lactate dehydrogenase (LDH), transfusion avoidance, haemoglobin stabilisation and breakthrough haemolysis (BTH).
Results
A total 43 of 44 patients entered the OLE after completing the primary treatment period. Overall, 14 of 44 (32%) experienced treatment-related adverse events. Steady state exposure levels of crovalimab and terminal complement inhibition were maintained over the OLE. During the OLE, mean normalised LDH was generally maintained at ≤1.5× upper limit of normal, transfusion avoidance was achieved in 83%–92% of patients and haemoglobin stabilisation was reached in 79%–88% of patients across each 24-week interval. Five BTH events occurred with none leading to withdrawal.
Conclusions
Over a 3-year median treatment duration, crovalimab was well tolerated and sustained C5 inhibition was achieved. Intravascular haemolysis control, haemoglobin stabilisation and transfusion avoidance were maintained, signifying long-term crovalimab efficacy.
Novelty Statement
What is the new aspect of your work?
This work reports data on long-term treatment with the terminal complement inhibitor crovalimab in paroxysmal nocturnal haemoglobinuria (PNH).
What is the central finding of your work?
This work establishes the long-term efficacy and safety of crovalimab for the treatment of patients with PNH.
What is the specific clinical relevance of your work?
This work supports the continued clinical development of crovalimab, a potential new treatment option for symptomatic patients with PNH, that can be delivered in a low volume, subcutaneous injection every 4 weeks.
1 INTRODUCTION
Paroxysmal nocturnal haemoglobinuria (PNH) is a potentially life-threatening, acquired, clonal haematopoietic stem-cell disorder arising from dysregulation of the complement pathway.1, 2 PNH can manifest as haemoglobinuria, haemolytic anaemia, acquired thrombophilia and bone marrow failure,2 often resulting in a poor quality of life for patients.3 The presence of intravascular haemolysis predicts PNH-related mortality, especially when associated with clinical symptoms such as abdominal pain, chest pain or dyspnoea.4 Furthermore, disease complications and related conditions such as impaired renal function, thromboembolic events, infections, bone marrow failure with cytopenia, or evolution to myelodysplastic syndrome or leukaemia, have been found to be associated with increased risk of death compared with the general population.4-6 Prior to the approval of complement inhibitor therapies, the median survival of patients with PNH ranged from 10 to 22 years, with a 5-year survival rate of 67%.7, 8
Terminal complement inhibitors targeting C5, including eculizumab9 and ravulizumab,10 changed the PNH treatment landscape and are the current standard of care for patients with PNH in countries where they are available.8 C5 inhibitors have shown substantial long-term clinical benefits in overall survival, control of intravascular haemolysis and need for transfusions, and improvement in quality of life of patients with PNH7, 9-12; however, unmet medical needs persist for patients with PNH. For example, a significant proportion of patients treated with eculizumab experience residual intravascular haemolysis and breakthrough haemolysis (BTH), resulting in persistent anaemia and fatigue.13, 14
In addition, a proportion of patients treated with currently available C5 inhibitors continue to require transfusions despite treatment9, 10 and the requirement for intravenous (IV) administration at regular intervals with eculizumab can represent a significant treatment burden to patients.15, 16 Eculizumab and ravulizumab also lack efficacy in people with certain C5 germline polymorphisms.10, 17 More recently, a proximal complement inhibitor targeting C3, pegcetacoplan18 has also been approved in some countries.
Crovalimab is a novel anti-C5 monoclonal antibody engineered with Sequential Monoclonal Antibody Recycling Technology (SMART-Ig) and characterised by pH-dependent, target-binding enhanced recycling by the neonatal Fc receptor, and high subcutaneous (SC) bioavailability allowing for small-volume administration every 4 weeks.19, 20 Crovalimab binds to an epitope on the C5 β chain, in contrast to eculizumab and ravulizumab that bind to the C5 α chain,19 allowing crovalimab to be effective in patients with a C5 R885H polymorphism.10, 17 The safety, pharmacokinetics (PK), pharmacodynamics (PD) and efficacy of crovalimab have been investigated in the sequential, adaptive, four-part Phase I/II COMPOSER trial (NCT03157635). Part 1 of COMPOSER enrolled healthy volunteers. Parts 2, 3 and 4 of COMPOSER enrolled patients with PNH naive to treatment and switched from eculizumab for open-label treatment during a primary treatment period of 20 weeks, at the end of which patients were able to enter an open-label extension (OLE) period (Figure S1).
Results from the primary treatment period of Parts 1, 2 and 3 of COMPOSER have previously been reported.20, 21 The dose-ascending Part 1 of COMPOSER established the safety and tolerability of crovalimab in healthy volunteers.21 Results from the primary treatment period of Parts 2 and 3 of COMPOSER indicated the safety and tolerability of crovalimab in patients with PNH who were naive to C5 inhibitor treatment (Part 2) and those switching from eculizumab to crovalimab treatment (Part 3), with different dosing regimens of crovalimab administered during the primary treatment period through Week 20.20 In both treatment-naive and switched patients, an IV loading dose followed by SC maintenance dosing of crovalimab resulted in sustained complement inhibition and effective control of intravascular haemolysis over the initial 20-week treatment period.20
Part 4 of COMPOSER enrolled both switched and treatment-naive patients and aimed to evaluate an optimised dosing regimen of crovalimab developed based on data from Parts 1, 2 and 3. This dosing regimen was optimised to shift the size distribution of drug-target-drug complexes (DTDCs), which transiently form in the serum of patients who switch from eculizumab to crovalimab, to decrease the formation and circulation duration of large DTDCs. DTDCs form in patients switching between crovalimab and other currently available C5 inhibitors with distinct target epitopes, which permits the binding of both eculizumab or ravulizumab and crovalimab to C5.20 DTDC formation can potentially lead to a temporary increase in crovalimab clearance and, in a proportion of patients, cause self-limited, clinically mild Type III hypersensitivity reactions.20 Results from the primary treatment period of COMPOSER Part 4 are presented in the Supplementary Information (Supplementary Methods; Figures S2–S5; Table S1). Patients with PNH enrolled in Parts 2, 3 and 4 of COMPOSER who completed the primary treatment period through Week 20, and derived benefit from crovalimab per the investigator's judgement, were eligible to enter the OLE period.
This work focuses on the long-term safety, PK, PD and exploratory efficacy outcomes in patients with PNH who enrolled in the OLE period of the COMPOSER trial.
2 METHODS
2.1 Study design
The COMPOSER trial (NCT03157635) was conducted in compliance with the principles of the Declaration of Helsinki and in accordance with a written protocol approved by the institutional review board and/or independent ethics committees of each participating centre. Patients were enrolled at 14 sites in six countries (Germany, Japan, France, Hungary, Korea and Italy). COMPOSER's trial design has been previously described (Figure S1).20 The open-label COMPOSER trial enrolled healthy volunteers and patients with PNH. Part 1 (single ascending dose) included healthy volunteers, Part 2 (intra-patient dose escalation) included patients with PNH who were naive to C5 inhibitor treatment and Part 3 (dose ranging and regimen finding) included patients who switched from eculizumab to crovalimab. Part 4 evaluated the optimised dosing regimen developed using dose-optimisation simulations based on data from Parts 1–3 and included both C5 inhibitor-naive patients (Arm A) and patients who switched from eculizumab to crovalimab (Arm B). Following completion of the primary treatment period up to Week 20, patients from Parts 2, 3 or 4 had the opportunity to enter the OLE.
2.2 Patient population
All patients provided written informed consent. Parts 2, 3 and 4 of COMPOSER enrolled patients with PNH aged from 18 to 75 years who had a documented PNH clone size of ≥10% by red blood cells (RBCs) and/or granulocytes within 3 months prior to enrolment or randomisation. All patients must have had a platelet count of >30 × 109/L and absolute neutrophil count of >0.5 × 109/L in order to be enrolled. Patients enrolled in Part 2 and Arm A of Part 4 had not previously been treated with a C5 inhibitor or if previously treated, stopped treatment due to lack of efficacy based on a single missense C5 heterozygous mutation (treatment-naive patients), and must have had a lactate dehydrogenase (LDH) level of ≥1.5× the upper limit of normal (ULN) at screening. Patients enrolled in Part 3 and Arm B of Part 4 had been treated continuously with eculizumab for at least 3 months preceding enrolment in the trial (switched patients). All patients must have been vaccinated against Neisseria meningitidis.
2.3 Interventions
The crovalimab dosing used in the primary treatment period (baseline to Study Week 20) has previously been described (Figure S1).20 In Part 2, treatment-naive patients with PNH received three single ascending IV doses of crovalimab on Days 1 (375 mg), 8 (500 mg) and 22 (1000 mg), followed by 170 mg SC administered weekly from Day 36. In Part 3, patients with PNH who switched from eculizumab received a loading dose of 1000 mg crovalimab IV on Day 1, followed by a 1:1:1 randomisation into three arms to receive either 680 mg SC every 4 weeks, 340 mg SC every 2 weeks, or 170 mg SC every week starting on Day 8. In Part 4, patients with PNH who were treatment-naive (Arm A) or who had switched from eculizumab (Arm B) received loading doses of crovalimab (1000 mg IV on Day 1 followed by 340 mg SC on days 2, 8, 15 and 22), followed by maintenance dosing (680 mg SC once every 4 weeks [Q4W] from Day 29 onwards).
Patients who entered the OLE initially stayed on their originally assigned maintenance dosing schedule as described above. If the investigator assessed the patient/caregiver as able to self-administer/administer crovalimab SC at home, home administration by the patient/caregiver was allowed, following successful training. With the implementation of weight-based tiered dosing to ensure that all patients received a comparable drug exposure across the body weight continuum, all patients in the OLE who were not currently receiving a Q4W dosing regimen were required to receive crovalimab 680 mg SC Q4W (body weight 40 to <100 kg) or 1020 mg SC Q4W (body weight ≥100 kg).
2.4 Study objectives and endpoints
The objectives of the OLE of COMPOSER were to assess the long-term safety, immunogenicity, PK and PD of crovalimab. Exploratory long-term efficacy endpoints were also analysed.
Safety endpoints included, but are not limited to, assessments of the incidence and severity of adverse events (AEs), serious AEs and AEs leading to withdrawal.
PK and PD endpoints included assessment of serum concentrations of crovalimab, complement activity by liposome immunoassay (LIA; Wako Autokit CH 50; FUJIFILM Wako Chemicals Europe, Neuss, Germany), and levels of total and free C5.
Exploratory efficacy endpoints included mean normalised LDH by visit, proportion of patients by visit achieving LDH ≤1.5× ULN, proportion of patients achieving transfusion avoidance by 24-week intervals from Week 20 to clinical cut-off date (CCOD), proportion of patients achieving haemoglobin stabilisation (defined as avoidance of ≥2 g/dL decrease in haemoglobin from the interval's baseline in the absence of blood transfusion during that interval) by 24-week intervals from Week 20 to CCOD, and BTH (defined as ≥1 new or worsening symptom or sign of intravascular haemolysis in the presence of LDH ≥2× ULN after prior LDH reduction to ≤1.5× ULN on therapy).
The proportion of patients achieving transfusion avoidance and haemoglobin stabilisation was assessed sequentially over 24-week intervals in order to evaluate the stability of these endpoints over long-term follow-up. Transfusions were administered per the investigator's clinical judgement.
2.5 Analyses
Long-term safety analyses are reported by treatment-naive, switched and total populations cumulatively from study baseline up to the CCOD of 1 November 2021, and included all patients who were enrolled in Parts 2, 3 and 4 of COMPOSER.
Long-term PK and PD analyses are based on data from study baseline up to a CCOD of 1 November 2021 and included all patients who were enrolled in Parts 2, 3 and 4 of COMPOSER. Time profiles of total serum crovalimab, total and free C5 concentrations, and the relationships between crovalimab total concentration, free C5 and serum haemolytic activity following terminal complement inhibition were evaluated graphically.
Long-term efficacy endpoints were evaluated during the OLE period, from Week 20 up to the CCOD, 1 November 2021. At the time of entry into the OLE period, all patients had completed 20 weeks of treatment and were in the maintenance phase of treatment, and therefore were considered comparable from a safety and efficacy standpoint, irrespective of naive or switch status at time of enrolment into the study. Therefore, the long-term efficacy analyses were conducted on a pooled population of patients enrolled into the OLE from Parts 2, 3 and 4. Pooled analysis of the endpoints for LDH by visit was conducted employing time windows that defined corresponding visits across the study parts, which had non-identical visit schedules. Pooled analyses of the endpoints analysed over a defined time period, including transfusion avoidance and haemoglobin stabilisation, were conducted across sequential 24-week intervals. The analysis of a specific 24-week interval only included patients who have completed the entire 24-week interval at the time of CCOD.
3 RESULTS
3.1 Patient disposition and baseline characteristics
A total of 43 out of 44 patients from Parts 2, 3 and 4 who completed the primary treatment period up to Week 20 entered the OLE (treatment-naive, n = 18; switched, n = 25; Figure S6). One patient in Part 3 completed the primary treatment period but elected not to enter the OLE. Therefore, the OLE efficacy-evaluable population included 43 patients, whereas the PK, PD and safety-evaluable populations included 44 patients. At the CCOD of 1 November 2021, 38 patients were ongoing in the OLE (two patients discontinued due to lack of efficacy as determined by the investigator and three patients discontinued due to withdrawal by patient); no patients discontinued from the study due to AEs. The two patients who discontinued due to lack of efficacy switched to another C5 inhibitor. Both of these patients completed the 9-week safety follow-up and final follow-up visit.
Table 1 summarises the baseline characteristics of patients at the time of enrolment into the study. Of the enrolled patients, 70% were male and 50% were White (Table 1). Seventeen of the 44 patients (39%) had a history of RBC transfusions up to 1 year prior to starting study treatment and 10 (23%) had a history of aplastic anaemia (AA). From baseline up to the CCOD (1 November 2021), the median treatment duration was 3.0 years (range, 0.4–4.4).
| Characteristics | Part 2 (N = 10) Naive | Part 3 (N = 19) Switched | Part 4 (N = 15) | |
|---|---|---|---|---|
| Naive (N = 8) | Switched (N = 7) | |||
| Median age (range), years | 52.5 (35–74) | 46.0 (33–69) | 55.5 (42–73) | 44.0 (29–57) |
| Male, n (%) | 6 (60) | 13 (68) | 6 (75) | 6 (86) |
| Race, n (%) | ||||
| Asian | 3 (30) | 7 (37) | 4 (50) | 2 (29) |
| White | 7 (70) | 9 (47) | 3 (38) | 3 (43) |
| Unknown | 0 | 3 (16) | 1 (13) | 2 (29) |
| Median weight (range), kg | 66.9 (58.9–98.0) | 78.2 (40.6–131.5) | 80.1 (56.7–100.0) | 79.8 (60.4–114.0) |
| History of RBC transfusiona, n (%) | 3 (30) | 8 (42) | 4 (50) | 2 (29) |
| Median RBC units transfuseda, (range) | 2 (2–6) | 5 (1–21) | 7 (2–48) | 3 (1–5) |
| Median baseline PNH granulocytes clone size (range), % | 83.4 (34.8–94.4) | 92.4 (71.6–99.9) | 86.0 (37.0–97.0) | 92.3 (17.9–99.6) |
| Median baseline PNH erythrocytes clone size (range), % | 49.0 (14.3–71.4) | 64.8 (12.4–99.1) | 17.0 (5.0–58.4) | 23.4 (8.7–87.4) |
| History of aplastic anaemia, n (%) | 1 (10) | 4 (21) | 3 (38) | 2 (29) |
| Median baseline normalised LDH × ULN (range) | 4.8 (1.9–12.1) | 1.2 (0.9–4.8) | 5.2 (2.3–20.4) | 1.1 (0.7–1.3) |
| Median baseline haemoglobin (range), g/L | 96.0 (77–122) | 99.0 (77–149) | 89.5 (80–121) | 105.0 (78–145) |
- Abbreviations: LDH, lactate dehydrogenase; PNH, paroxysmal nocturnal haemoglobinuria; RBC, red blood cell; ULN, upper limit of normal.
- a Transfusion events that occurred within a year prior to first dose.
3.2 Long-term safety
Median treatment duration from baseline was 3.4 years (range, 0.9–4.4) for treatment-naive patients and 2.9 years (range, 0.4–3.9) for patients who switched from eculizumab (Table 2). A total of 42 of 44 (95%) patients experienced at least one all-cause AE from baseline to the CCOD of 1 November 2021; AEs were treatment-related in 14 patients (32%). Overall, the majority of these AEs (77%) were of mild or moderate severity. No meningococcal infections, deaths or AEs leading to withdrawal of treatment were reported.
| Naive (N = 18) | Switched (N = 26) | Total (N = 44) | ||
|---|---|---|---|---|
| Median treatment duration (range), years | 3.4 (0.9–4.4) | 2.9 (0.4–3.9) | 3.0 (0.4–4.4) | |
| Median number of doses received (range) | 83.5 (19–163) | 48.5 (11–129) | 51.0 (11–163) | |
| Median cumulative dose (range), mg | 31 880 (11949–41 485) | 28 030 (4400–42 480) | 28 880 (4400–42 480) | |
| Patients with ≥1 any-cause AE, n (%) | ||||
| Any-grade AE | 17 (94) | 25 (96) | 42 (95) | |
| AE leading to death | 0 | 0 | 0 | |
| AE leading to treatment withdrawal | 0 | 0 | 0 | |
| Serious AE | 6 (33) | 8 (31) | 14 (32) | |
| Serious AE leading to treatment modification/interruption | 1 (6) | 0 | 1 (2) | |
| Patients with ≥1 AE related to treatment, n (%) | ||||
| Any-grade AE | 4 (22) | 10 (38) | 14 (32) | |
| AE leading to treatment withdrawal | 0 | 0 | 0 | |
| Serious AE | 1 (6) | 1 (4) | 2 (5) | |
| Serious AE leading to treatment modification/interruption | 0 | 0 | 0 | |
- Abbreviations: AE, adverse event; CCOD, clinical cut-off date.
Serious all-cause AEs were reported in 14 (31.8%) of patients, of which four were infections (device-related infection, erysipelas, respiratory tract infection and upper respiratory tract infection). One event of myocardial infarction occurred (not related to crovalimab); no additional thrombotic events were reported. Serious treatment-related AEs occurred in two (5%) patients, including one event of BTH in a patient following discontinuation from crovalimab and switch to eculizumab, and one event of upper respiratory tract infection. AEs occurring in ≥20% of patients were nasopharyngitis (30%), pyrexia (23%), upper respiratory tract infection (23%) and headache (20%) (Table S2). Common treatment-related AEs included headache (occurring in 7% of patients), BTH (5%), urticaria (5%) and viral infection (5%) (Table S3).
Two of 26 patients (8%) who switched from eculizumab experienced an AE of special interest (AESI) of Type III hypersensitivity reactions. Both events were mild or moderate and resolved within 23 days of continued crovalimab treatment. To treat these Type III hypersensitivity reactions, one patient received topical betamethasone for 4 days, followed by fexofenadine, topical gentamicin and topical betamethasone for 7 days. The other patient received fexofenadine, topical gentamicin and topical betamethasone for 6 days and crotamiton for 4 days. Both events resolved with no change in crovalimab treatment. One patient in the treatment-naive group experienced an AESI of Type III hypersensitivity at the time of switch from crovalimab to eculizumab, after discontinuing from the study due to a lack of efficacy.
3.3 Long-term PK/PD
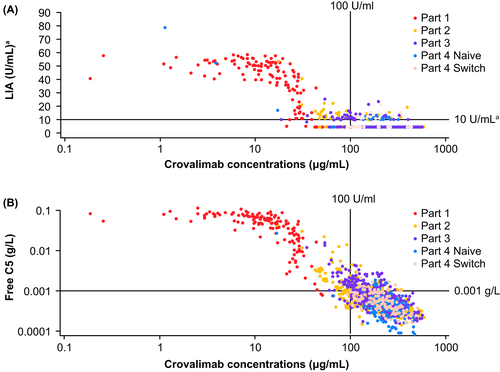
Integrated PK/PD analysis of the relationship between free C5 or LIA and crovalimab concentrations demonstrated that, over long-term follow-up in the 44 enrolled patients, crovalimab levels of above 100 μg/mL resulted in the inhibition of terminal complement activity (Figure 1). Long-term terminal complement activity by LIA was close to or below the lower limit of quantification (10 U/mL; Figure 1A) and free C5 levels were maintained at low levels (<0.001 g/L; a level that is associated with complete terminal C5 inhibition) in most study participants over the OLE period (Figure 1B).
3.4 Long-term exploratory efficacy
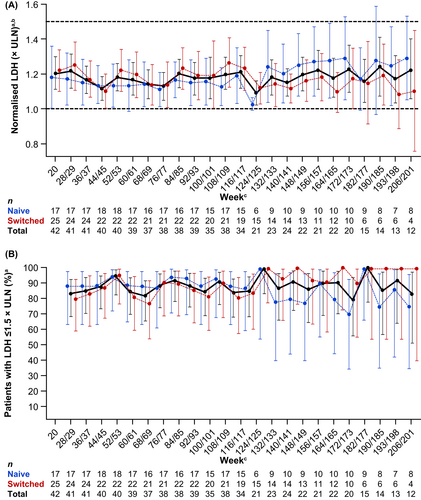
Mean normalised LDH was generally maintained at ≤1.5× ULN at each visit during the OLE in the 43 efficacy-evaluable patients (Figure 2A), and between 80% and 100% of patients had LDH ≤1.5× ULN at each visit during the OLE (Figure 2B).
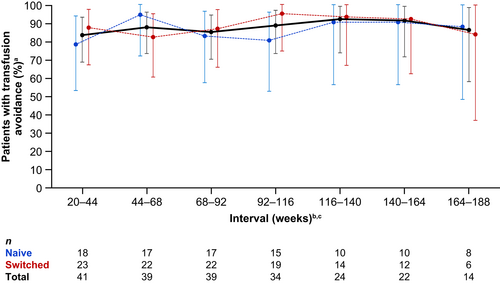
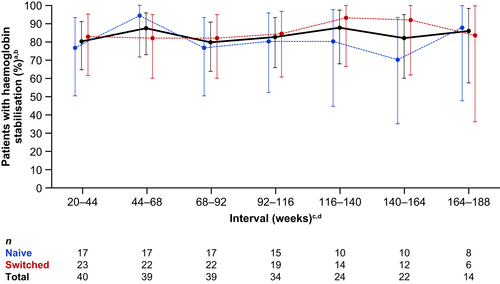
Between 83% and 92% of all patients were transfusion avoidant across the 24-week intervals during the OLE (Figure 3). Overall, 11 patients required transfusions during the OLE up to the CCOD. The proportion of patients with haemoglobin stabilisation was 79%–88% across the 24-week intervals during the OLE (Figure 4).
A total of five BTH events occurred that met the per protocol criteria for BTH (Table S4). These five BTH events occurred during the OLE over a cumulative 109.8 patient-years at risk, for an overall BTH rate of 0.05 events per patient-year (95% confidence interval [CI]: 0.01, 0.11). All BTH events were single occurrences in individual patients and resolved with continued treatment. For two of the BTH events transfusions were given, and for another BTH event rescue doses of IV crovalimab were given. Clinical data and related lab parameters at the time of each BTH event are given in Table S5. Three of the five BTH events occurred concurrently with an AE of infection (urinary tract infection, nasopharyngitis and otitis media), one BTH event was unrelated to either concurrent infection or loss of free C5 control/suboptimal complement inhibition per LIA, and another BTH event was considered to have an undetermined cause as there was no reported concurrent infection and data pertaining to free C5 levels and LIA were not available.
4 DISCUSSION
Results from the OLE period of the Phase I/II COMPOSER study establish the long-term safety, PK/PD properties and exploratory efficacy of crovalimab in a total of 44 patients with PNH, over a median treatment duration of 3 years. Crovalimab was well tolerated, with no new safety signals identified over long-term follow-up. Steady state crovalimab exposure levels and terminal complement inhibition were maintained over the long term in both treatment-naive and switched patients. Additionally, patients with PNH treated with crovalimab maintained disease control over long-term follow-up as indicated by efficacy endpoints related to haemolysis control (change in LDH), transfusion avoidance, haemoglobin stabilisation and BTH.
C5 inhibitors are generally well tolerated over the long term and appear to have a favourable risk–benefit profile in patients with PNH.12, 22 Consistent with this, results from the current study showed that crovalimab was well tolerated over long-term follow-up in both C5 inhibitor-naive and switched patients with PNH. Further, the safety profile of crovalimab was consistent with the known safety profile of C5 inhibitors. Over long-term follow-up, the majority of AEs were of mild or moderate severity and treatment-related serious or severe AEs were infrequent with crovalimab. There were no deaths or AEs that resulted in withdrawal from study. Although the inhibition of terminal complement activity with C5 inhibitors is known to increase risks of meningococcal infection,13, 22 no cases of meningococcal meningitis were reported in the long-term follow-up of COMPOSER. The most frequently reported AEs with crovalimab were headache, upper respiratory tract infection, pyrexia and nasopharyngitis; these were also among the most common AEs observed with eculizumab9 and ravulizumab.10, 11
PK and PD profiles were comparable during long-term follow up between patients who were naive to treatment and those switched from eculizumab at time of enrolment into the study. Free C5 levels and terminal complement activity were generally maintained at low levels through long-term follow-up. PK/PD relationships between free C5 or LIA with crovalimab concentrations confirmed that crovalimab levels of approximately 100 μg/mL resulted in inhibition of terminal complement activity (LIA close to or below the lower limit of quantification, 10 U/mL) through long-term follow-up, as seen in earlier studies.23
During long-term follow up, mean LDH levels were maintained at ≤1.5× ULN per visit and the proportion of patients with LDH ≤1.5× ULN per visit was also maintained during long-term follow-up. Haemolysis control was assessed using LDH ≤1.5× ULN as the threshold to evaluate maintenance of intravascular haemolysis control in this study, as it is considered a clinically relevant threshold to assess PNH disease control.3, 24, 25 LDH is considered a sensitive biochemical marker of intravascular haemolysis in patients with PNH and is linked to the clinical manifestations of disease.4
The long-term efficacy of crovalimab was further supported by endpoints related to transfusion avoidance, haemoglobin stabilisation and BTH. Transfusion avoidance is a clinically relevant measure to assess the clinical impact of C5 inhibitors.9, 11, 26 However, there are many factors that contribute to the need for a transfusion, including bone marrow failure, which is frequently concurrent with PNH, and may influence the level of haematological response in patients treated with C5 inhibitors. Studies of eculizumab suggest that patients with concurrent AA are likely to require continued transfusions to address underlying bone marrow failure, despite adequate haemolysis control with C5 inhibitor therapy.26 Seven of the 11 patients who received a blood transfusion during the OLE period reported a history of AA and/or anaemia. Additionally, all patients who received a transfusion during the OLE also had one or more transfusions during the primary treatment period, with the exception of one patient who received only a single transfusion during the OLE in the context of a BTH. During COMPOSER OLE, transfusion avoidance and haemoglobin stabilisation were assessed across 24-week intervals to evaluate the stability of these endpoints over long term follow-up. During long-term follow-up, the proportion of patients who achieved transfusion avoidance and haemoglobin stabilisation remained stable across the 24-week intervals.
Additionally, the rate of BTH over long-term follow up in the COMPOSER OLE was low with an overall rate of 0.05 events per patient-year (95% CI: 0.01, 0.11); all events were single occurrences in an individual patient that did not lead to study discontinuation. In general, in C5 inhibitor-treated patients, BTH occurs either due to incomplete terminal complement inhibition or due to AEs such as infection or inflammation that trigger complement activation.25 Although BTH incidence cannot be directly compared across studies due to differences in trial design, it has been reported that BTH events in patients that received eculizumab were associated with low plasma levels of eculizumab, and resultant suboptimal C5 inhibition has been reported in 10%–15% of patients.14, 25 Among patients receiving ravulizumab, BTH incidence was <10%, and no BTH events were temporally associated with suboptimal C5 inhibition.12, 27, 28 During the COMPOSER OLE, three BTH events occurred concurrently with an AE of infection. BTH episodes caused by such ‘trigger events’ tend to be self-limiting and can occur independently of the plasma level of the C5 inhibitor.25 During the COMPOSER OLE, data on free C5 levels and LIA were not available for all patients at the time of their respective BTH event, limiting assessment of suboptimal C5 inhibition in these patients. However, for the BTH events where free C5 and LIA data were available, no events were associated with concurrent elevated free C5 >0.001 g/L or LIA values above the lower limit of quantification.
Limitations of this Phase I/II, first-in-human study include the open-label single-arm design, the small sample size and the descriptive nature of the analyses. Additionally, LDH levels were assessed in local laboratories and the need for transfusion was based on investigator judgement only, potentially allowing for variability in the results.
Overall, the promising efficacy and safety data reported here support the further clinical development of crovalimab. The optimised weight-based dose regimen of crovalimab (680 or 1020 mg SC Q4W) evaluated here is being further validated in a series of Phase III clinical trials. Ongoing Phase III trials include the global, randomised COMMODORE 1 (NCT04432584) study evaluating crovalimab versus eculizumab in patients with PNH currently treated with C5 inhibitors; the global, randomised COMMODORE 2 (NCT04434092) study evaluating crovalimab versus eculizumab in people with PNH who have not previously received C5 inhibitor treatment; and the multicentre, single-arm COMMODORE 3 (NCT04654468) study studying crovalimab in C5 inhibitor-naive patients in China.
5 CONCLUSIONS
Over a median treatment duration of 3.0 years, the Phase I/II COMPOSER trial establishes that crovalimab treatment is well tolerated and results in sustained terminal complement inhibition in patients with PNH who are C5 inhibitor naive as well as in those switching from eculizumab. Intravascular haemolysis control, haemoglobin stabilisation and transfusion avoidance were maintained over long-term follow-up, signifying long-term efficacy with crovalimab. Steady state exposure levels of crovalimab and terminal complement inhibition were maintained over the long term. The results reported here support the continued clinical development of crovalimab.
AUTHOR CONTRIBUTIONS
Conception and design of the study: Alexander Röth, Yoshikazu Ito, Nadiesh Balachandran, Kenji Shinomiya, Alexandre Sostelly, Jun-ichi Nishimura. Recruited patients and/or collected data: Alexander Röth, Satoshi Ichikawa, Yoshikazu Ito, Jin Seok Kim, Zsolt Nagy, Naoshi Obara, Jens Panse, Hubert Schrezenmeier, Simona Sica, Juliette Soret, Kensuke Usuki, Sung-Soo Yoon, Jun-ichi Nishimura. Analysed and interpreted the data: Alexander Röth, Yoshikazu Ito, Jin Seok Kim, Zsolt Nagy, Jens Panse, Sung-Soo Yoon, Nadiesh Balachandran, Muriel Buri, Pontus Lundberg, Himika Patel, Alexandre Sostelly, Jun-ichi Nishimura. All authors contributed to the manuscript and provided final approval.
ACKNOWLEDGEMENTS
The authors would like to thank the patients who participated in the trial, the patients' families and the investigators and staff at all clinical study sites. This study was funded by F. Hoffmann-La Roche, Ltd and Chugai Pharmaceutical. Medical writing assistance for this manuscript was provided by Akshaya Srinivasan, PhD, of MediTech Media Ltd, and funded by F. Hoffmann-La Roche, Ltd. We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
CONFLICT OF INTEREST STATEMENT
Alexander Röth provided consultancy for Alexion, Amgen, Apellis, BioCryst, Bioverativ, F. Hoffmann-La Roche, Kira, Novartis and Sanofi; received research funding from F. Hoffmann-La Roche; received honoraria from Alexion, Bioverativ, F. Hoffmann-La Roche and Novartis; and sits on advisory committees for Alexion, Apellis, BioCryst, F. Hoffmann-La Roche, Novartis and Sanofi. Satoshi Ichikawa received research funding from AstraZeneca, and BeiGene and F. Hoffmann-La Roche. Yoshikazu Ito provided consultancy for Alexion. Jin Seok Kim received research funding from Alexion and F. Hoffmann-La Roche; and received honoraria from Alexion. Zsolt Nagy sits on advisory committees for Janssen, Novartis, Takeda, AbbVie and F. Hoffmann-La Roche. Naoshi Obara received research funding from Alexion, Kyowahako Kirin; and received honoraria from Alexion, Novartis, Bristol Myers Squibb, Chugai, Kyowahako Kirin and Janssen. Jens Panse serves on a speaker's bureau for Alexion, Boehringer Ingelheim, Novartis, Pfizer and Chugai; and sits on advisory committees for Apellis, Bristol Myers Squibb, Merck Sharp & Dohme, F. Hoffmann-La Roche, Alexion, Boehringer Ingelheim and Novartis. Hubert Schrezenmeier received research funding from Alexion, Novartis; received honoraria from Alexion, Novartis and Sobi; and sits on advisory committees for Alexion, Novartis and Sanofi (all to University of Ulm). Simona Sica received research funding from F. Hoffmann-La Roche. Juliette Soret received honoraria from Novartis. Kensuke Usuki provided consultancy for Alexion and Novartis; received research funding from Alexion, Novartis, Chugai, F. Hoffmann-La Roche and Apellis; serves on a speaker's bureau for Alexion. Sung-Soo Yoon provided consultancy for Amgen, Novartis and Janssen; received research funding from F. Hoffmann-La Roche, Genentech, Inc., Kyowahako Kirin and Yuhan Pharma; received honoraria from Novartis and Amgen. Nadiesh Balachandran is an employee of F. Hoffmann-La Roche. Muriel Buri is an employee of F. Hoffmann-La Roche. Pontus Lundberg is an employee of F. Hoffmann-La Roche. Himika Patel is an employee of Genentech, Inc. Kenji Shinomiya is an employee of Chugai; and holds a patent for crovalimab. Alexandre Sostelly holds shares in F. Hoffmann-La Roche. Jun-ichi Nishimura provided consultancy for Alexion; received research funding from Alexion and F. Hoffman-La Roche; received honoraria from Alexion; holds a patent for WO2020/027279 (anti-C5 antibody dosage regimen); and sits on advisory committees for Chugai, F. Hoffmann-La Roche, Sanofi K.K., BioCryst Pharmaceuticals, Apellis Pharmaceuticals and Novartis.
Open Research
DATA AVAILABILITY STATEMENT
For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.