The hematologic consequences of obesity
Abstract
The prevalence of obesity is increasing and progressively influencing physician-patient interactions. While there is a sizable amount of data demonstrating that obesity is a state of low-grade inflammation, to our knowledge, there is no single review summarizing its effects on hematologic parameters and thrombotic risk. We performed a literature search which largely surfaced observational studies, with a few systematic reviews and meta-analyses of these studies. We took care to review the mechanisms driving an inflammatory state and obesity's effect on white blood cells, red blood cells, platelets, and thrombotic risk. There is an observed relative, and sometimes absolute leukocytosis driven by this inflammatory state. Obesity is also associated with increased platelet counts and an increased risk for venous thromboembolism (VTE). Lastly, the association between obesity, iron deficiency (ID), and red blood cell counts may be present but remains uncertain. Recognizing the above associations may provide clinicians with reassurance regarding otherwise unexplained hematologic abnormalities in obese individuals. We hope this review will prompt future studies to further understand the underlying mechanisms driving these abnormalities and identify modifiable risk factors and potential therapeutic targets to prevent the development of probable obesity-associated conditions with significant morbidity and mortality, such as ID and VTE.
Summary Statements
- To our knowledge, there is no single review summarizing the effects of obesity on commonly measured hematologic parameters and thrombotic risk, this addresses that.
- Obesity is associated with a state of chronic low-grade inflammation and subsequently with an observed relative and sometimes absolute leukocytosis, as well as an increased risk for venous thromboembolism (VTE); its effects on iron deficiency (ID) and red blood cell counts remain unclear.
- Recognizing the above associations may provide reassurance regarding otherwise unexplained hematologic abnormalities in obese individuals as well as prompt future studies to identify modifiable risk factors and potential therapeutic targets to prevent the development of probable obesity-associated conditions with significant morbidity and mortality, such as ID and VTE.
1 INTRODUCTION
The pervasiveness of obesity in the United States has been dramatically increasing over the last several decades and shows no signs of slowing. In 2015-2016, the prevalence of obesity in the US population was 39.8%, accounting for about 93.3 million US adults,1 with new data predicting that by 2030 nearly 1 in 2 adults will be obese.2 As our patient interactions become progressively influenced by the health effects of obesity, it is becoming increasingly important to understand the consequences of the hematologic alterations observed in these individuals.
Obesity has been associated with a state of low-grade systemic inflammation characterized by an adipose tissue driven acute-phase response, with interleukin (IL)-6, IL-1, IL-8, and tumor necrosis factor (TNF)-α playing the largest role and resulting in subsequent elevations of acute-phase proteins such as c-reactive protein (CRP). This produces an environment defined by inflammation, with theoretical and established down-stream effects. This review will summarize our current understanding of this pro-inflammatory state and its alteration of hematologic parameters, particularly with regard to white blood cell, red blood cell, and platelet counts and perturbances to thrombosis.
2 THE PRO-INFLAMMATORY STATE OF OBESITY
2.1 Macrophages and adipose tissue
There is ample evidence that adipose tissue is a primary source of inflammatory mediators.3, 4 White adipose tissue is the most abundant type of adipose tissue and is composed of many cell types. Adipocytes are the most plentiful, but adipose tissue macrophages are also present, with a relative population size that correlates with the level of adiposity.5 Adipose tissue macrophages are estimated to comprise up to 40% of the cells in obese adipose tissue, compared with under 10% in lean individuals,6 and elevated macrophage infiltration is noted in visceral compared with subcutaneous fat.7 Although it has been reported that preadipocytes can function as macrophage-like cells,8 adipose tissue macrophages are most likely bone marrow-derived circulating monocytes that subsequently infiltrated adipose tissue.6 Leptin and other chemokines such as monocyte chemotactic protein-1 and leukotriene B49 contribute to this, and increasing concentrations of recombinant human leptin have been shown to result in an increased adhesion and transmigration of blood monocytes in a concentration-dependent manner,5 though all factors attracting immune cells into obese tissues are not yet fully understood. See Boutens et al (2016)10 for a full review of adipose tissue macrophages in obesity.
2.2 Adipose tissue and the acute-phase response
The acute-phase response that accompanies inflammatory states is characterized by increased production of acute-phase proteins, largely released by hepatocytes. The role of these acute-phase proteins varies from mediating inflammation, to inhibiting proteases, to scavenging free radicals. IL-6 is a principle regulator for most positive acute-phase proteins and stimulates the production of serum amyloid A, c-reactive protein, α1-acid glycoprotein, α1-antichymotrypsin, haptoglobin, α1-antitrypsin, fibrinogen, complement component C3, and ceruloplasmin.11 Human adipose tissue can independently produce and release cytokines that are known to be major inducers of the acute-phase response, including IL-6, IL-1, IL-8, and TNF-α.12 Both adipocytes and macrophages contribute to cytokine production, though there is some evidence that macrophages are the major source of white adipose tissue-derived IL-6 and TNF-α.6 Cottam et al (2004)13 comprehensively summarized all acute-phase reactants and inflammatory mediators observed in obesity.
IL-6 and TNF-α are most evidently increased in the serum and white adipose tissue of obese individuals,14-21 and Mohamed-Ali et al (1997)22 calculated that the whole body adipose tissue mass is responsible for the production of 15%-35% of systemic IL-6. IL-6 and TNF-α levels are also noted to decrease with weight loss,23-28 particularly secondary to bariatric surgery.29-31 Local adipose tissue IL-6 production is also noted to be greater in subjects with a higher waist-to-hip ratio.17, 19, 22 A higher waist-to-hip ratio often indicates increased visceral adipose tissue, which has been shown to have elevated inflammatory status, compared with subcutaneous adipose tissue.10 This supports the observation that omental adipose tissue has been shown to release 2-3 times more IL-6 than its subcutaneous counterpart.32, 33 As IL-6 regulates CRP production, it is not surprising that CRP elevation significantly corresponds to the level of adiposity present in the body17, 19, 21, 23, 34-36 and also decreases with weight loss24-26, 37, 38; this association has been shown to be stronger in women compared with men.35 There is even some evidence that CRP is produced by the adipose tissue itself.39
2.3 Conclusion
In summary, in obesity, leptin and other chemokines contribute to the transmigration of bone marrow-derived monocytes into adipose tissue, with a resultant elevation of IL-6, TNF-α, and other cytokines. This subsequently results in the rise in acute-phase proteins, such as CRP, and all of this in combination accounts for the resultant chronic low-grade inflammation which actively contributes to alterations in hematologic parameters and alters the risk of thrombosis, as discussed below. While there may be a cycle at play in which bone marrow-derived monocytes are likely not only the source but also the target of pro-inflammatory signals, this is not clear from the current literature. This is something that should be further researched as it could identify additional potential therapeutic targets upstream of the acute-phase response.
3 OBESITY AND WHITE BLOOD CELL COUNT
3.1 Obesity and leukocytosis
A significant number of patients referred for investigation of unexplained leukocytosis have no identifiable cause of a persistent and stable leukocytosis, other than their being obese. Multiple studies have worked to elucidate the connection between leukocytosis and body mass index (BMI). We discuss these studies below, as well as the hypothesized mechanisms driving this observation.
Herishanu et al (2006)12 analyzed patients (n = 327) referred to their outpatient hematology clinic for persistent leukocytosis and found that 15% were asymptomatic, obese, mostly middle-aged females with a mild leukocytosis (mean white blood cell count 13.05 ± 1.44109/L) characterized mostly by neutrophilia without bandemia and accompanied by elevated acute-phase reactants (CRP and erythrocyte sedimentation rate). No other recognized cause for the leukocytosis (eg, infection, inflammation, smoking, malignancy, etc) was identified, and the elevated count persisted through a mean follow-up of 45 months. In three individuals, reduction in weight was accompanied by a gradual reduction and normalization of the white blood cell count and accompanying CRP levels. To further validate this association, they performed a cross-sectional analysis of non-smoking individuals without underlying inflammatory or infectious conditions and showed that subjects found to have a leukocytosis (n = 62) had a higher BMI when compared with the population with normal white blood count ranges. Raghavan et al (2016)40 similarly noted that white blood counts are relatively high in obese women, meaning that while mean values for total leukocyte count did not exceed accepted physiological range, there was a relative leukocytosis between categories, with a progressive numerical increase in the values of the total leukocyte count as the BMI increased. Additionally, absolute neutrophil count and differential neutrophil count showed a progressive increase with increase in BMI. The absolute lymphocyte counts likewise showed statistically significant differences between the control group and obese group, but the strength of the association appeared to be less than that observed with absolute neutrophil count. Of note, the differential lymphocyte count displayed a progressive decrease with increase in BMI, suggesting that the increase in absolute lymphocyte counts was counterbalanced by a respectively greater increase in neutrophils. Although traditionally neutrophils have been acknowledged as key players in acute inflammation, their contribution to chronic inflammation is recently being appreciated, particularly in conditions such as chronic obstructive pulmonary disease, arthritis, neurodegenerative disease, and cardiovascular inflammation.41 Given this, the myeloid skewing observed in obesity is unsurprising. Additionally, evidence from animal models shows that high fat diets lead to myeloid hyperplasia, especially of the granulocytic compartment.42 Their findings are summarized in Table 1, adapted from their paper. Several other studies support the positive correlation between leukocyte count and BMI.21, 36, 43-45
|
Control BMI ≤ 24.9 Mean ± SD |
Overweight BMI ≥ 25 - ≤ 29.9 Mean ± SD |
Obese BMI ≥ 30 Mean ± SD |
Met. Syndrome BMI ≥ 30 Mean ± SD |
|
|---|---|---|---|---|
| Age |
42.41 ± 13.22 |
44.77 ± 10.41 |
45.95 ± 11.00 |
52.29 ± 10.30 |
| BMI |
22.15 ± 2.76 |
27.02 ± 1.44 |
34.29 ± 3.50 |
34.77 ± 4.44 |
| WC |
74.82 ± 6.44 |
83.92 ± 4.79 |
98.53 ± 9.53 |
101.7 ± 10.14 |
| TLC |
6590 ± 1608 |
7405 ± 1711 |
8759 ± 1882 |
10 110 ± 1947 |
| ANC |
3706 ± 1315 |
4268 ± 1269 |
5310 ± 1471 |
6148 ± 1499 |
|
ALC |
2203 ± 527 |
2340 ± 583 |
2554 ± 613 |
2916 ± 786 |
| PLT |
265.4 ± 50.02 |
279.7 ± 70.34 |
307.8 ± 87.01 |
320.1 ± 66.73 |
3.2 The role of cytokines and adipokines
Peripheral blood leukocytosis is a hallmark of inflammation and in obesity is likely driven by the chronic low-grade inflammatory state discussed in detail above. Pro-inflammatory cytokines such as IL-6 and IL-8 are important inducers of leukocytosis, particularly neutrophilia, through multiple mechanisms including demargination of intravascular neutrophils, acceleration of bone marrow neutrophil release, and enhancement of bone marrow granulopoiesis.46-50
Adipokines such as leptin may also be playing a role. Wilson et al (1997)51 showed a positive, though indirect, correlation between white blood cell count and fasting plasma leptin concentrations in obese Pima Indians. Laharrague et al (2000)52 developed a homologous system where purified CD34+ progenitors from adult human bone marrow were treated with recombinant human leptin. They found that leptin (at high levels, but levels which are observed in obesity) significantly stimulated the appearance of granulocyte-macrophage colonies, the precursor for monocytes, and granulocytes (Figure 1) and that plasma leptin concentration was significantly correlated with leukocyte count in overweight and obese subjects. They also found that leptin concentrations were significantly higher in women than men, a difference which persisted even after controlling for BMI. Furthermore, human and mouse hematopoietic stem cells have been shown to express the leptin receptor53 and a murine model has shown that treatment with a synthetic fragment of leptin resulted in a twofold increase in the number of hematopoietic stem cells with an associated increase in the number of granulocyte/macrophage colony-forming units produced by bone marrow cells.54 Leptin clearly has a role in regulation of hematopoiesis through a mechanism that is multifactorial and indirect. Zhou et al (2014)55 have shown that bone marrow mesenchymal stromal cells are leptin receptor positive and although these are defined as non-hematopoietic, as they rather differentiate into osteogenic, chondrogenic, and adipogenic progeny, they do give rise to bony ossicles that become invested with hematopoietic bone marrow as well as directly expressing hematopoietic stem cell niche factors such as stem cell factor and Cxcl12, suggesting they contribute to the organization of a hematopoietic microenvironment as important components of the hematopoietic stem cells niche. For an excellent review on the hematopoietic stem cells niche, see Sugiyama et al (2018).56
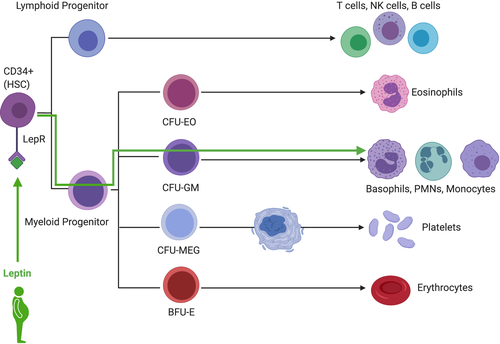
3.3 Sex differences
As there is a clear predominance of obesity-associated leukocytosis in women compared with men, it is important to discuss this observation. In women, even more than men, the etiology behind this leukocytosis is likely multifactorial. Higher levels of inflammatory mediators (eg, CRP) and leptin are likely contributing, though the underlying mechanisms driving this difference remain unclear. As obesity in women is sometimes accompanied by polycystic ovarian syndrome (PCOS), this could also be contributing. Some cytokines, including IL-6 and TNF-α, are greater in women with PCOS compared with women of similar BMI.57 Furthermore, total white cell count, due to a higher mean neutrophil count, has been found to be significantly greater in women with PCOS when compared to control subjects, despite matching for BMI and insulin resistance.58, 59 Also, as women with obesity and PCOS are at increased risk of developing obstructive sleep apnea (OSA),60, 61 this could be considered a contributing factor. However, while there are multiple studies that have shown a positive association between OSA and the neutrophil to lymphocyte ratio,62-65 a value sensitive to physiologic stress, few studies have independently associated OSA with leukocytosis, though it has been noted.66 While we can theorize that the known elevated levels of systemic inflammatory markers in OSA (including CRP, IL-6, and TNF-α),67 or increased sympathetic activity associated with OSA68 would result in a leukocytosis, further studies are needed to solidify or reject this claim.
3.4 Conclusion
In summary, the low-grade systemic inflammation that accompanies obesity results in elevated levels of cytokines (primarily IL-6 and IL-8) and adipokines such as leptin, which induce leukocytosis through multiple mechanisms, including demargination of intravascular neutrophils, acceleration of bone marrow neutrophil release, and enhancement of bone marrow granulopoiesis; leptin in particular promotes the differentiation of granulocytes from hemopoietic progenitor cells. Several studies support the positive correlation between leukocyte count and BMI, and there is a clear predominance of obesity-associated leukocytosis in women compared with men. Possible explanations include an amplified inflammatory state and associated comorbidities such as PCOS and OSA.
4 OBESITY AND RED BLOOD CELL COUNT
4.1 Obesity and iron deficiency
Wenzel et al (1962)69 were the first to report lower serum iron levels in obese compared with non-obese adolescents. Although it appears counterintuitive to expect iron deficiency (ID) in the setting of calorie and nutrient excess, lower concentrations of serum iron have been observed in relation to increasing BMI for many decades. Zhao et al (2015)70 conducted a metanalysis of 26 cross-sectional and case-control studies, including a total of 13,393 overweight and obese individuals and 26,621 non-overweight subjects. They found that compared with the non-overweight group, the serum iron and transferrin saturation percentage were significantly different in the overweight/obese populations, with only a marginally significant difference in the serum ferritin level between the same groups. The pooled odds ratio (OR) of developing ID in the overweight/obese subjects was 1.31 (95% CI, 1.01-1.68). Importantly, the method used to diagnose ID differed among the 15 studies that were included in that analysis, with only 8 studies using ferritin-based ID diagnosis. In the studies using a ferritin-based diagnosis, the association between ID and overweight/obesity was not significant (OR 1.04, 95% CI, 0.69-1.56). In contrast, the pooled OR calculated from the 7 studies that did not use a ferritin-based ID diagnosis was significant (OR 1.49, 95% CI, 1.19-1.85); using ferritin as the sole biomarker for assessment of ID may underestimate ID, due to the elevation of serum ferritin through obesity-related chronic inflammation and the possibility that in overweight and obese individuals, ferritin is a marker of inflammation rather than iron status.71 A review by Aigner et al (2014)72 reported similar findings, as well as reported some improvements in iron status in the setting of weight loss.
It is important to acknowledge that many of the investigations included in the above metanalysis and review were performed in children and adolescents, and that in adults, the results are even more complex. In their metanalysis, Zhao et al (2015) performed a subgroup analysis which revealed no association between obesity and ID in adults. Cheng et al (2012)73 conducted a systematic review of 25 studies, looking at the association in adults only. They noted a tendency for higher ferritin and lower transferrin saturations in obese individuals, compared with their non-obese counterparts. Additional studies in non-pregnant adults (or a mean age ≥18), not included in either Zhao et al (2015) or Cheng et al (2012), have conflicting results. Some studies suggest an association between obesity and ID,71, 74-78 while multiple others note no difference.79-82
Given the literature, a relationship between obesity and ID likely exists in children and adolescents but remains unclear in adults. The reviews and metanalysis available are limited by the number of available studies, high heterogeneity in these studies, lack of consistent non-obese control groups, frequently uncontrolled confounders (eg, acute infection, menopausal status, oral contraceptive use, iron supplementation, obesity-related comorbidities), and lack of standardization in diagnosing ID (eg, some studies used ferritin alone, others used serum iron and transferrin saturation, very few used soluble transferrin receptor). This is particularly important given that obesity is associated with chronic low-grade inflammation which will potentially elevate ferritin and may alter the sensitivity of ferritin as an indicator of iron status; the use of additional biomarkers is something to be considered in future studies. While an association may be present between obesity and ID, future higher quality studies are needed to solidify this claim, as well as establish if a causal relationship exists.
4.2 Obesity and red blood cell count
Given that ID can progress to iron deficiency anemia (IDA), one may expect to see an association between obesity and IDA. In the metanalysis by Zhao et al (2015), only 4 studies of the 26 analyzed looked at the risks of developing IDA in the obese population and the results differed, with only one reporting a higher risk of IDA in their obese group; the pooled OR of IDA in overweight/obese individuals was 1.09 (95% CI, 0.57-2.10). The review by Cheng et al (2012) actually noted higher hemoglobin concentrations in the obese compared with non-obese controls. There have been additional studies not included in the above analysis which show lower levels of hemoglobin in obese compared with non-obese controls,21, 71 while others show no correlation80, 81 or a protective effect.83, 84 Comorbidities associated with obesity, such as OSA or obesity hypoventilation syndrome, may be leading to a secondary polycythemia. Regardless, future studies are needed to look into this possible association.
4.3 Possible mechanisms driving iron deficiency
In children and adolescents, a possible explanation for the observed ID is higher iron demands, in the setting of accelerated body growth.85 Poor dietary intake may also be playing a role,86, 87 though multiple studies have shown that this is unlikely.88-91 Another theory is that increased iron requirements are due to a larger blood volume which increases iron requirements and confounds iron biomarkers by hemodilution.92, 93 The largest body of evidence actually points toward the chronic inflammatory state associated with obesity driving the observation, with hepcidin, and its role in iron homeostasis being the main player.
Iron homeostasis is regulated through hepcidin, a small peptide hormone largely produced by the liver, which controls the activity of ferroportin-1, an iron exporter. Increased serum levels of hepcidin result in reduced dietary iron absorption through downregulation of the exporter and increased iron sequestration within enterocytes, hepatocytes, and iron-storing macrophages, leading to reduced iron bioavailability94, 95 (Figure 2). It has been shown that obese subjects demonstrate impaired ability to absorb iron.76, 96-98
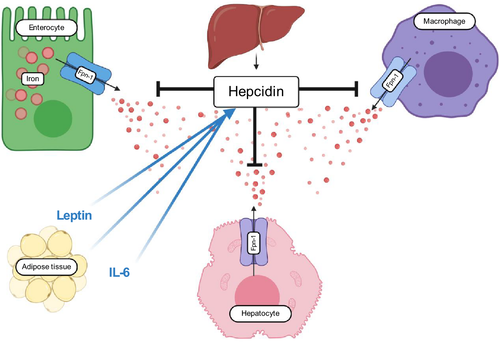
Hepcidin expression correlates with inflammation.99 Given that obesity is a state of chronic low-grade inflammation, it comes as no surprise that hepcidin expression has been shown to be increased in obese individuals.89, 100 This increase in hepcidin is likely driven by cytokines, with significant data pointing to IL-6 as a main driver.99, 101-103 There is also in vitro evidence that leptin also stimulates hepcidin expression.104 Additionally, adipose tissue has been shown to express hepcidin itself, marking hepcidin as an adipokine,102 though this has been questioned.105 It should also be noted that not all studies agree that hepcidin plays a role at all.106
4.4 Conclusion
In summary, a link between ID and obesity likely exists in children and adolescents but the association remains unclear in adults; a link between obesity and anemia remains even less certain. Given current limited studies of high heterogeneity and with conflicting results, future studies are needed to further assess this. Special care should be taken to include a non-obese control group, to include additional biomarkers of iron status which may not be as easily altered by the chronic inflammatory state accompanying obesity as is ferritin, and to control for confounders of iron status such as acute infection, menopausal status, and iron supplementation. Driving this possible association is the chronic inflammatory state associated with obesity, which through the secretion of cytokines such as IL-6, results in increased hepcidin expression and subsequent impaired duodenal absorption of iron. Leptin may also be playing a role in increased hepcidin expression and hepcidin itself may also be secreted by adipose tissue.
5 OBESITY, PLATELETS COUNT, AND THROMBOSIS
5.1 Obesity and platelet count
Obesity is also associated with increased platelet counts. Platelet counts have been found to be increased in obese non-diabetic rats107 as well as obese individuals, when compared to non-obese controls,12, 21, 40 though the correlation has sometimes only been noted in women.108 This correlation is further supported by observed reductions in platelet count in the setting of weight loss following bariatric surgery.109 IL-6 is likely the main driver, working synergistically with other interleukins to increase thrombopoietin and subsequently stimulate megakaryocytopoiesis.110-115 Visceral adipose tissue itself may also be an additional source of thrombopoietin.116
5.2 Obesity and thrombosis
There is substantial evidence that obesity as a pro-inflammatory condition, promotes a prothrombotic state, supporting arterial and venous thrombosis. A meta-analysis by Ageno et al (2008)117 on the influence of obesity on the risk of first episode of venous thromboembolism (VTE) estimated an overall odds ratio for VTE of 2.33 (95% CI, 1.68-3.24). Tsai et al (2002),118 included in that meta-analysis, found an even higher hazards ratio of 2.7 for patients with severe obesity (BMI ≥40 kg/m2). Di Minno et al (2005)119 reported the OR of obesity for deep vein thrombosis to be 1.97 (95% CI, 1.4-2.78) for males and 2.29 (95% CI, 1.85-2.84) for females. There are many additional studies not included in the meta-analysis but with similar observations.120-123 Borch et al (2010)124 and Hansson et al (1999)125 also noted associations with abdominal obesity and VTE. Eichinger et al (2008)126 reported obesity as a risk factor for VTE recurrence, a risk which may be even higher in women compared with men.127 In general, clinically men and women appear to experience different thrombotic phenotypes. As noted above, obese women appear to be at higher risk for VTE and data have shown, though not exclusive to obese individuals, that women appear to be at higher risk for stroke, while men are more likely to be affected by cardiovascular disease.128 The relative protection of women against atherosclerosis before menopause is poorly understood with some thought that a more beneficial lipid profile contributes or that there is some protection conferred by sex hormones.129
The biological mechanisms underlying this observed connection are multifactorial and the relationship is confounded by a frequently accompanied metabolic syndrome and associated lifestyle factors, whose individual components (hyperinsulinemia, hypertriglyceridemia, hypertension) have associations with thrombosis by mechanisms independent of obesity. However, despite this, there is notable and consistent evidence of an association between obesity and thrombosis through damage to the venous endothelial layer, increased platelet reactivity, enhanced coagulation, and impaired fibrinolysis, all discussed below and summarized in impressive reviews by Vilahur et al (2017)130 and Schafer & Konstantinides (2011).131 The physical aspects of an obese body habitus may also contribute to the risk by promoting limited venous return through chronically raised intra-abdominal pressure and decreased blood velocity through the femoral vein132; inactivity and poor gait would likely have a compounding effect.133
5.3 The role of leptin in thrombosis
Leptin is a suspected driver of these prothrombotic changes through multiple mechanisms. There are significant data surrounding leptin-mediated promotion of ADP-induced platelet aggregation,134-137 with the inhibition of leptin via a neutralizing antibody being protective against arterial and venous thrombosis in mice.138 However, Ozata et al (2001)139 found no significant increase in platelet aggregation, even at high concentrations of leptin in obese subjects or controls. Other studies have also reported a reduction in the pro-aggregatory effects of leptin from obese individuals, suggesting platelet resistance to leptin in obesity.140, 141 P-selectin, a significant player in platelet aggregation, has been shown to have leptin-mediated increased expression on human platelets in vitro.142 However, this observation was not consistent and is without correlation in vivo. Less convincing, but also noted in the literature, is a contribution to elevated levels of tissue plasminogen activator (tPA) antigen,143 which would promote thrombosis. Leptin also has been shown to induce elevated levels of plasminogen activator inhibitor-1 (PAI-1),144 which at higher concentrations is known to inhibit fibrinolysis and consequently promote a thrombotic state. Lastly, leptin is associated with increased factor levels, as noted below.
5.4 Obesity is associated with elevated levels of coagulation factors and von Willebrand factor
BMI has been shown to positively correlate with elevated factor levels and markers of fibrinolysis (fibrinogen, factor VII, factor VIII, factor IX, PAI-1, tPA antigen),120, 145, 146 with reductions in thrombin, tissue factor, PAI-1, and (prothrombin fragment 1.2) noted in weight loss.147 Plasma levels of tissue factor, a significant driver of thrombus formation, are increased in obese subjects compared with healthy controls.148 Leptin has been shown to increase tissue factor in humans,149, 150 and elevated levels of circulating tissue factor have been recognized as a contributor to the prothrombotic tendency of patients with high-grade obesity.31 Levels of fibrinogen, von Willebrand factor, and factors VII and VIII have all been found to be increased in obesity, with abdominal obesity noted to have a particular association.146, 151 Although elevations in factor levels are strongly associated with increased risk of VTE, one study did show that adjusting for clotting factor levels did not affect the risk estimate for obesity and VTE120; we did not find other studies that commented on this possibility. It remains unclear whether these factors are merely biomarkers of inflammation, or direct contributors to thrombosis and this is an area that would benefit from further research.
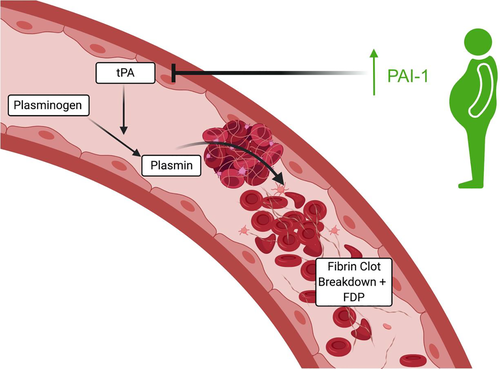
5.5 Obesity impairs fibrinolysis
There is evidence tying PAI-1, which blocks the activation of fibrinolysis by inhibiting tPA (Figure 3), to obesity-associated thrombosis, particularly through the metabolic syndrome and its interactions with insulin.152 PAI-1 levels correlate with BMI146 and body fat distribution plays a role, with an association noted between waist-to-hip ratio and PAI-1 levels.151, 153 It has also been shown to be produced in adipose tissue154, 155 and levels have been shown to decrease with weight loss.156, 157 As stated above, leptin may be a player in PAI-1 over expression, but TNF-α and transforming growth factor-β, which is produced in visceral fat, have also been proposed to be involved in the regulation of PAI-1 expression in adipose tissue.133 CRP may also participate by enhancing the expression of PAI-1 in human endothelial cells.158-160 Counter to this, tPA expression has also been associated with BMI,146 with noted reductions following weight loss.153, 161
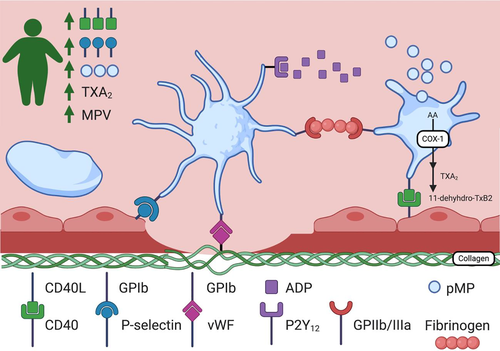
5.6 Obesity promotes platelet hyperactivity
Platelet activation is also a key component of thrombogenesis, and platelet hyperactivity has been noted in obese individuals. Particularly, android obesity (abdominal obesity characterized by increased waist-to-hip ratio) has been found to have higher levels of platelet activation, as reflected by urinary excretion of the major metabolite of TXA2, 11-dehyhdro-TxB2, when compared to gynoid obese (gluteal-femoral obesity) women and non-obese women.162 P-selectin expression is a marker of platelet activation, mediating the rolling of platelets on activated endothelial cells and promoting thrombogenesis by stabilizing initial GPIIb/IIIa-fibrinogen connections, permitting the development of large stable platelet aggregates in a venous thrombus. Epidemiological evidence has shown that platelet activation markers such as CD40L and P-selectin are increased in obesity and may reflect persistent in vivo platelet and endothelial activation,163 with increased P-selectin expression noted in genetically and diet-induced obese mice.164 CRP levels have been shown to increase P-selectin expression and platelet adhesion.165 Mean platelet volume, an element of platelet function, is an emerging risk factor for thrombotic disease166, 167 and has positively correlated with BMI.168 Platelet-derived microparticles are considered an index of platelet activation and have pro-inflammatory and prothrombotic effects. These too may be playing a role, as circulating levels of platelet-derived microparticles are elevated in obese subjects in comparison with age-matched non-obese subjects.153 Lastly, platelets from obese subjects have been found to retain greater reactivity after suppression by aspirin, compared with non-obese individuals after aspirin therapy.169, 170 These effects are summarized in Figure 4.
5.7 Obesity promotes endothelial dysfunction
Obese individuals exhibit endothelial dysfunction, and this is likely induced by the continuous exposure to inflammatory stimuli and oxidative damage. It has been shown that endothelial cells exposed to cytokines secreted by adipose tissue from obese individuals have enhanced expression of endothelial adhesions molecules.171 Leptin again may be contributing, promoting oxidative stress; leptin has been shown to increase levels of markers of endothelial cell dysfunction and activation.172 Individual components of the metabolic syndrome (ie, hyperlipidemia) which often accompany obesity also contribute to activate the endothelial layer by enhancing endothelin-1 production, a vasoconstrictor, and pro-inflammatory peptide, and conversely impairing nitric oxide and endothelium-derived hyperpolarizing factor-related vasodilation leading to endothelial dysfunction.130
5.8 Conclusion
In summary, obesity is associated with increased platelet counts. IL-6 is likely a main contributor to this phenomenon, increasing thrombopoietin and subsequently stimulating megakaryocytopoiesis, resulting in thrombocytosis. There is also substantial evidence that obesity as a pro-inflammatory condition significantly increases the risk of VTE. The mechanisms underlying this are multifactorial and confounded by lifestyle factors, as well as the individual components of a frequently accompanying metabolic syndrome. However, despite this, there is extensive literature discussing elevated factor levels, impaired fibrinolysis, platelet hyperactivity, and endothelial dysfunction, all driven by obesity and its associated inflammatory state; encouragingly, many of these abnormalities improve with weight loss.
6 CONCLUSION
There is a sizeable amount of data demonstrating that obesity is a state of chronic low-grade inflammation. Future studies to further understand the underlying mechanisms would be helpful; particularly, future investigations of leptin would be of high interest, given the high suspicion that it is involved in many of the perturbances to hematologic parameters and thrombosis. Also, future studies looking to unmask the underlying mechanisms driving an even higher inflammatory state in women compared with men would be important in identifying risk factors that could be intervened on. There is an observed relative, and sometimes absolute, leukocytosis driven by this inflammatory state. Recognizing this association may prompt clinicians to avoid unnecessary and extensive work-ups by providing reassurance regarding an unexplained, stable and mild, neutrophilic leukocytosis in obese individuals who have the demographic features present in the above studies (eg, middle-aged females). The association between obesity, ID, and red blood cell counts remains uncertain and future well designed studies are needed to accept or reject this association, as well as to establish if a causal relationship exists. Given the accompanying state of chronic inflammation, identifying abnormalities of iron metabolism is particularly challenging in the obese, but this presents an opportunity to use and validate additional and novel biomarkers of iron status in future investigations and to standardize the methods used to diagnose ID in obese individuals. Also, recognizing that in obesity there is impaired iron absorption via the gut, making oral supplementation insufficient, and encourages future studies to further explore and identify underlying mechanisms (eg, hepcidin) as potential therapeutic targets that could prevent the development of ID in the obese. Lastly, there is sufficient evidence associating obesity with VTE, and some evidence that weight loss resolves some of the underlying mechanistic abnormalities. Considering that the relative risk of first and recurrent VTE associated with obesity are comparable to those for other established risk factors such as hereditary thrombophilia and estrogen therapy,173 and given the high morbidity and mortality that VTE can carry, this presents an additional opportunity for clinicians to more vigorously encourage weight loss among their obese patients. This also further incentivizes future research in the area, particularly with regard to anti-coagulation and anti-inflammatory therapies to prevent thrombosis in obesity.
7 Conflict-of-interest
JJS is a consultant for Aronora, Inc The remaining authors declare no potential conflict of interest.
ACKNOWLEDGMENT
J Shatzel is supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (HL151367)
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




