The role of iron repletion in adult iron deficiency anemia and other diseases
Abstract
Iron deficiency anemia (IDA) is the most prevalent and treatable form of anemia worldwide. The clinical management of patients with IDA requires a comprehensive understanding of the many etiologies that can lead to iron deficiency including pregnancy, blood loss, renal disease, heavy menstrual bleeding, inflammatory bowel disease, bariatric surgery, or extremely rare genetic disorders. The treatment landscape for many causes of IDA is currently shifting toward more abundant use of intravenous (IV) iron due to its effectiveness and improved formulations that decrease the likelihood of adverse effects. IV iron has found applications beyond treatment of IDA, and there is accruing data about its efficacy in patients with heart failure, restless leg syndrome, fatigue, and prevention of acute mountain sickness. This review provides a framework to diagnose, manage, and treat patients presenting with IDA and discusses other conditions that benefit from iron supplementation.
1 BACKGROUND
Iron deficiency is the most common micronutrient deficiency affecting nearly one-third of the population and is the leading cause of anemia worldwide.1 Iron is consumed in heme (meat-derived) and non-heme (vegetable-derived) forms; heme form is more readily absorbed by the body, making vegetarians more prone to iron deficiency.2 Iron absorption occurs in the duodenum and is regulated by the divalent metal transporter (DMT1) in the enterocytes.3 Iron enters the circulation via ferroportin and is bound to transferrin for transport to the bone marrow and liver for red blood cell (RBC) production and storage, respectively. Stored intracellular iron is bound to ferritin to prevent cellular damage by generation of free radicals. Since there are no mechanisms for iron excretion by the body other than through blood loss or cell turnover,4 total body iron levels are strictly regulated. Iron excess and inflammatory states stimulate hepcidin production by the liver, which blocks iron absorption into the circulation via ferroportin degradation and inhibits iron release from storage.5 This process of iron metabolism plays a major role in the development, diagnosis, and management of iron deficiency anemia.6
Iron deficiency anemia is characterized by decreased hemoglobin synthesis leading to hypochromic and microcytic RBC production. Causes of IDA include reduced iron intake or absorption, increased iron demand during adolescence and pregnancy, bariatric surgery, heavy blood loss during menstruation, chronic gastrointestinal (GI) blood loss, polyps, or carcinoma.7 Affected individuals present with fatigue, dyspnea, pale conjunctiva, headaches, and pica. Reduced brain iron levels can manifest as cognitive dysfunction during infancy or restless leg syndrome later in life.8 While management of IDA with oral supplementation has been the mainstay of treatment for several decades, lack of evidence for optimal dosing quantity and frequency has made it challenging to standardize a treatment protocol. With newer and safer formulations of intravenous (IV) iron, studies have shown more efficacious and faster resolution of anemia.9 This paper will discuss the various indications and recommendations for oral and IV iron use.
2 DIAGNOSIS AND MANAGEMENT OF IRON DEFICIENCY ANEMIA IN ADULTS
Iron studies should be performed in patients with newly discovered anemia as well as in the workup of patients with fatigue or restless leg syndrome even in the absence of anemia. Some patients may not have overt anemia but have low iron stores, a state known as latent iron deficiency (LID). See Table 1 for laboratory parameters to evaluate iron status.10 Figure 1 provides an overview of our practice's schema guided by supporting literature for risk categorization, recommended workup, and treatment for various patient populations.10-12 In premenopausal women, heavy menstrual bleeding (HMB) is a frequently encountered cause for IDA and mild cases may be treated with a trial of oral iron depending on patient tolerability.13 Total iron absorption with daily oral supplementation is <10 mg/d such that moderate-to-severe anemia from HMB will require IV iron to keep up with the losses and achieve rapid and safe resolution of anemia.14-16 Men and women presenting with IDA in the absence of known pathology should be evaluated for other causes of blood loss and a gastroenterology referral should be considered. If no cause can be identified, alternative inflammatory, absorptive, or genetic etiologies should be considered.
| Normal values (adults) | Iron deficiency anemia (IDA) | Latent iron deficiency | Functional iron deficiency—adequate stores | ||
|---|---|---|---|---|---|
| Iron-refractory iron deficiency anemia | Anemia of chronic disease | ||||
| Serum iron (μmol/L) | 10-30 | ↓ | N/↓ | ↓ | ↓ |
| Percent transferrin saturation (TSAT), % | 17-44 | <16 | N/↓ | <10 | N/↓ |
| Serum ferritin (μg/L) |
20-200 (F) 40-300 (M) |
<12-50 | <30 | Variable | >100 |
| Hemoglobin (g/dL) |
>12 (F) >13 (M) |
↓ | N | ↓ | ↓ |
- Abbreviations: F, Female; M, Male; N, Normal.
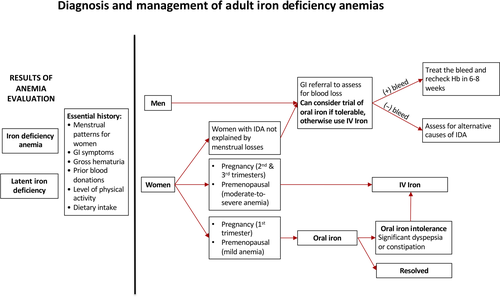
3 EVALUATION FOR GASTROINTESTINAL BLOOD LOSS
Occult GI bleeding is the most common cause of IDA in adults without a known source of blood loss; all men and women with unexplained IDA should receive thorough GI evaluation for a potential source.10 The choice and sequence of procedures can be made through clinical suspicion based on history and symptoms. Although esophagogastroduodenoscopy (EGD) and colonoscopy are sensitive procedures to locate the source of bleeds in a majority of patients, capsule endoscopy can be subsequently used to evaluate for bleeding sources originating from other parts of the GI tract.17 Once the source is identified and appropriate treatment is initiated, hemoglobin levels should be rechecked in 6-8 weeks to ensure the resolution of anemia. Depending on the severity of anemia, concomitant IV iron use can quickly and effectively rectify low iron levels.
Iron deficiency anemia is the most common non-intestinal complication of inflammatory bowel disease (IBD) affecting an estimated 16% of outpatient and 68% of hospitalized patients with IBD.18 The pathogenesis of IDA in IBD hinges on multiple factors including mucosal ulcerations causing blood loss, high hepcidin levels blocking iron absorption, and postbowel resection stress on iron stores.5, 19 Since the current first-line treatment, ferrous sulfate, is associated with significant GI side effects compared with IV iron, it is no surprise that 52% of IBD patients were found to reduce their dose or discontinue oral ferrous sulfate entirely.20 Ferric maltol is a new oral agent, which has shown better tolerability and good efficacy in patients with inactive IBD.21 A comparison of the impact of IV and oral iron supplementation in IBD patients found that oral iron increased luminal ferrous iron and altered the gut microbiome in ways that could worsen IBD symptoms.22 While some IBD patients may respond to oral iron, the decreased intestinal absorption and frequency of GI adverse effects make IV iron the preferred therapy in many cases.23
4 EVALUATION OF ALTERNATIVE GASTROINTESTINAL CAUSES OF IRON DEFICIENCY ANEMIA
Environmental and autoimmune forms of gastritis have both been implicated as occult causes of iron deficiency anemia. A meta-analysis in 2008 revealed that Helicobacter pylori infection was associated with an increased risk for IDA.24 Causality is suggested by successful improvement in iron stores and red cell indices after H pylori eradication in multiple studies; anti-H pylori therapy is recommended by some experts for treatment of otherwise unexplained IDA in the setting of positive H pylori testing.25
There is significant overlap in occurrence, course, and distinction between H pylori gastritis and autoimmune gastritis. In both adult and pediatric populations, unexplained or refractory IDA is often one of the earliest presentations of autoimmune gastritis.26
Celiac disease is another observed cause of unexplained IDA. It is an immune-mediated disorder characterized by intestinal inflammation and reduced absorptive surface area in the duodenum causing IDA by iron malabsorption which is also likely worsened by the iron sequestration known to occur in chronic inflammatory states.25, 27 One study observed sustained anemia remission in 92% of affected individuals after implementation of a gluten-free diet and iron supplementation.28
Administration of IV iron is the preferred route for patients with these GI pathologies in order to ensure rapid correction, circumvent decreased iron bioavailability in pathologic settings, and to prevent further GI upset often accompanying oral iron treatments.
Drugs may also cause iron deficiency by way of decreased iron absorption or increased iron losses. Gastric acid inhibitors have been considered as possible causes of decreased absorption given that they decrease gastric acid available for conversion of dietary non-heme ferric iron to the absorbable ferrous form.29 A recent community-based case-control study performed by Kaiser Permanente in Oakland found that long-term use of proton pump inhibitors (PPIs) or histamine-2 receptor antagonists was associated with an increased risk for iron deficiency and that this risk decreased in the case of PPIs after discontinuation of the medication.30
In two separate double-blind randomized controlled trials, NSAIDs were found to cause a clinically significant decrease hemoglobin by presumed mechanism of GI blood loss, though no data on occult GI blood loss was collected in these studies.31
5 MICROCYTIC ANEMIAS WITH ADEQUATE IRON STORES
While IDA is the most commonly encountered microcytic anemia in clinical practice, differential diagnosis for microcytic anemias includes various other causes of reduced hemoglobin synthesis including anemia of chronic disease (ACD), thalassemia, rare genetic disorders, and lead poisoning.
Anemia of chronic disease is a diagnosis made by exclusion of other possible etiologies. Malignancies, autoimmune disorders, and infections upregulate inflammatory cytokines that stimulate production of the acute-phase reactant hepcidin, which reduces iron distribution to erythrocyte precursors and decreases dietary iron absorption.11, 12 These inflammatory conditions also cause decreased erythropoietin (EPO), a renally produced hormone that drives RBC production.32 Therefore, laboratories supporting a diagnosis of ACD therefore includes an EPO that is not appropriately elevated for the severity of the patient's anemia, low transferrin saturation (TSAT), and a normal ferritin.12 Soluble transferrin receptor (sTfR) and reticulocyte hemoglobin content (CHr) are also emerging as useful diagnostic tools in differentiating between IDA and ACD. The sTfR level is a measure of erythropoietic activity; it rises in states of low cellular iron but remains unaffected by inflammation.33 In comparison, CHr measures the amount of iron available for hemoglobin synthesis, which decreases in IDA but not in ACD.34 Treating the underlying pathology of functional iron deficiency should always be the primary goal when possible. Exogenous EPO is an option for certain patient populations including those with chronic kidney disease, malignancy with chemotherapy-associated anemia, and low/intermediate-risk myelodysplastic syndromes with <500 mIU/mL EPO levels.35 Parenteral iron should be administered in patients with absolute iron deficiency and in those who did not respond to EPO.36
Thalassemia syndromes are heritable disorders with a broad range of clinical manifestations caused by mutations in genes responsible for alpha- or beta-globin synthesis, ultimately leading to ineffective erythropoiesis, hemolysis, and splenic sequestration of erythrocytes.37 Management of severe thalassemia generally consists of chronic blood transfusions and iron chelation therapy when serum ferritin exceeds 800-1000 ng/mL.38 Splenectomy may be indicated, particularly in beta-thalassemias if the patient experiences poor growth, increased transfusion demands, or sequestration crises.39 Bone marrow transplant is a curative option that should be considered for all thalassemic children if an HLA-matched donor is available.40
Iron-Refractory Iron Deficiency Anemia (IRIDA) is an autosomal recessive disorder which leads to a microcytic anemia. It involves a mutation to the transmembrane serine protease 6 gene (TMPRSS6) which normally downregulates hepcidin.25 Most IRIDA presents as hypochromic microcytic anemia in childhood that is refractory to oral iron and improves with age.41 Oral iron is often ineffective, while IV iron administration can lead to gradual partial correction of hemoglobin in most patients.42 Care should be taken not to tailor treatment to target normal reference hemoglobin ranges in these patients as this confers higher risk for of iron overload.41
Lead toxicity can rarely cause microcytic or normocytic anemia in children through decreased hemoglobin synthesis and hemolysis by generation of reactive oxygen species.43, 44 Lead is known to adversely affect hemoglobin synthesis through inhibition of multiple enzymes involved in porphyrin synthesis and iron incorporation into the porphyrin ring.44 Both capillary blood testing and venous sampling can accurately detect elevated blood lead levels in children.45 Management involves elimination of the lead source with chelation therapy if blood lead concentrations exceed 45 µg/dL.46
6 IRON DEFICIENCY ANEMIA IN PREGNANCY
Iron deficiency anemia in pregnancy is widespread and can lead to increased maternal and fetal morbidity and mortality.47 Chronic blood loss (heavy menstrual bleeding or GI bleeding), increased iron demand, reduced iron intake, and reduced iron absorption all may contribute to the development of IDA in pregnancy.23, 48-50 Serum ferritin level of <30 µg/dL and hemoglobin of <11 g/dL in pregnant women meet the diagnostic criteria for IDA.51 The first trimester IDA should be treated with oral iron if tolerated.51 Compared to oral iron, daily oral bovine lactoferrin has been shown to have fewer GI side effects with improved hematologic parameters in moderate anemia.52
In second and third trimesters, IV iron therapy is safe and generally favored over oral formulations due to fewer side effects and rapid correction of anemia.53, 54 Ferric carboxymaltose is commonly used when treating second and third-trimester pregnancy IDA given its comparable safety profile to iron sucrose while requiring fewer repeated applications due to higher iron dose with each administration.55-57 Since hypophosphatemia is a known side effect of ferric carboxymaltose, care should be taken to mitigate any exacerbating factors like vitamin D deficiency.58 Data are increasing on the safety of other formulations in pregnancy including low-molecular-weight iron dextran.59 As such, other IV iron formulations are likely efficacious and choice of drug can vary based on availability, cost, and clinician preference.
7 ANEMIA IN CHRONIC KIDNEY DISEASE
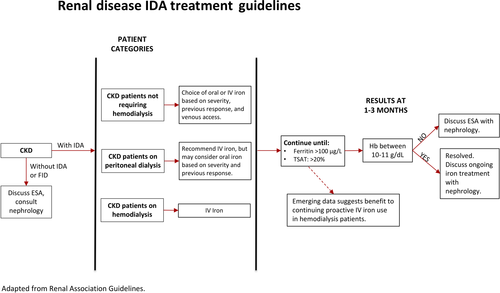
The prevalence of anemia in patients with chronic kidney disease (CKD) is 15.4% compared to 7.6% in the general population, but prevalence rises up to 53.4% in patients with End-Stage Renal Disease (ESRD).60 Major factors contributing to anemia in CKD include reduced EPO production, chronic inflammation leading to poor iron absorption and availability, blood loss (GI or hemodialysis associated), and nutritional iron deficiency.61 These factors may lead to functional and/or absolute iron deficiencies, which need to be clinically differentiated for optimal treatment. While serum ferritin concentration <30 µg/L or TSAT <20% is indicative of an absolute iron deficiency, no current laboratory test accurately diagnoses functional iron deficiency in CKD patients. The Renal Association Guidelines recommend initiation of iron treatment for serum ferritin <100 μg/L and TSAT <20%.62 New evidence from the 2019 PIVOTAL trial, however, indicates that proactively administered high-dose IV iron regimen is superior to reactively administered low-dose IV iron regimen in patients undergoing hemodialysis and it also decreases the necessary erythropoiesis-stimulating agent (ESA) dosing.63, 64 There is also emerging literature that suggests higher ferritin levels can be tolerated before treatment initiation to reduce exogenous EPO requirements and improve anemia.65
Treatment options for anemia in CKD include oral iron, IV iron, and ESAs (Figure 2). IV iron is the standard-of-care treatment for hemodialysis patients, and should be strongly considered in patients on peritoneal dialysis (PD). In patients with NDD-CKD, IV iron has been shown to rapidly correct anemia and reduce need for ESAs relative to oral iron without evidence of renal toxicity, cardiovascular, or infectious events.66 Rates of acute infusion-related hypersensitivity reactions are low with IV iron use in NDD- and PD-CKD patients and the safety of short-term IV iron is established by multiple trials.67-69 A trial of oral iron may however be considered in NDD-CKD patients based on the severity of their anemia, previous response to oral iron, venous access, and cost considerations.61 Use of ESAs increases utilization of iron stores for hematopoiesis; they should only be used after functional or absolute iron deficiency has been corrected.62 The benefit of ESA initiation should be weighed against an increased risk of stroke.62, 70
8 IRON TREATMENTS
8.1 Oral iron
Oral iron is generally the favored therapy for uncomplicated iron deficiency. There are multiple oral iron preparations available over the counter for treatment of anemia (Table 2).21, 71 Ferrous sulfate is considered first-line given its favorable bioavailability and efficacy.72 Most common side effects include nausea, epigastric pain, diarrhea, constipation, and dark discoloration of stools, all of which often lead to suboptimal adherence.20 Conventional consecutive day high-dose iron supplementation was demonstrated to elevate hepcidin levels and reduce iron bioavailability, particularly when dosed multiple times daily.73 There is mounting evidence to support the efficacy of lower daily doses between 15 and 20 mg elemental iron in a variety of iron-deficient patient populations including elderly and pregnant women.74, 75 Alternate-day dosing is also being investigated as it is found to have fewer GI side effects and reduced hepcidin levels compared to conventional daily dosing.16, 76 A prospective study carried out by Stoffel et al measured total and fractional absorption in 40 women randomly assigned to either 14 consecutive doses or the same number of doses spread across 28 days and found improved fractional and total absorption in the alternate-day group after the completion of those 14 doses. However, this comparison is made relative to the number of doses received rather than the time period over which they are taken. When compared over the same amount of time, that is, 2 weeks, consecutive day dosing actually demonstrated greater total iron absorption.16 At this time, there is a need for more studies to clarify this relationship. Dosing regimen decisions should weigh the need for rapid anemia correction against the increased side effect profile of daily dosing. Meat protein and vitamin C maintain iron in its soluble ferrous form and can aid in iron absorption.5 Concomitant consumption of tea and coffee should be avoided as polyphenol compounds present in these drinks can form an insoluble complex with iron, making it unavailable for absorption.77
| Oral iron formulations | Dose |
|---|---|
| Ferrous sulfate | 350 mg |
| Ferrous sulfate anhydrous | 200 mg |
| Ferric maltol | 60 mg |
| Ferrous gluconate | 325 mg |
| Ferrous fumarate | 325 mg |
| Polysaccharide iron complex | 50 mg |
| Heme iron polypeptide | 24 mg |
| Carbonyl iron |
45 mg (Feosol) 66 mg (Ircon) |
| Ferric citrate | 210 mg |
| Ferrous ascorbate | 200 mg |
| Ferrous succinate | 200 mg |
8.2 Intravenous iron
Intravenous iron should be considered in patients with known GI malabsorption, IBD, ongoing heavy blood loss, moderate-to-severe CKD and ESRD, or those who have previously not tolerated trials of oral iron. IV iron has been found to be more efficacious than oral iron in IDA correction and is generally better tolerated, but universal administration is limited by availability and cost.78 Table 3 lists all the current available IV iron formulations.9, 51 All formulations have equivalent effectiveness and similar safety profiles.79, 80 Infusion reactions with IV iron are rarely encountered (1 in 200 administrations) and are often self-limited.9 In the setting of a mild reaction, IV iron can be resumed at slower infusion rates with relative safety.5, 9, 81
| Trade name | INFeD-US CosmoFer | Feraheme | Injectafer, US Ferinject | Monofer-Europe only | Venofer | Ferrlecit |
|---|---|---|---|---|---|---|
| Generic name | LMW iron dextran | Ferumoxytol | Ferric carboxymaltose | Iron isomaltoside | Iron sucrose | Sodium ferric gluconate |
| Manufacturer | Allergan/Watson Pharma | AMAG Pharmaceuticals | Vifor Pharma | Pharmacosmos | American Regent Inc | Sanofi Aventis Inc |
| Carbohydrate | Low-molecular-weight iron dextran | Ferumoxytol | Carboxymaltose | Isomaltoside | Sucrose | Gluconate |
| Total dose infusion | Yes | No | Yes | Yes | No | No |
| Test dose required | Yes | No | No | No | No | No |
| Approved dose | 100 mg per dose | 510 mg over 15 min | 750 mg over 15 min | 20 mg/kg (1000 mg if >66 kg) | ||
| Recommended dose | 1000 mg | 510 mg × 2 | 750 mg × 2 | 1000 mg | 200-300 mg | 125-187.5 mg |
| Infusion time | 1 h | 15 min | 15 min | 15 min | 15 min | 1 h |
| Black box warning | Yes | Yes | Yes | NA | No | No |
| Pregnancy category | C | C | C | Not listed | B | B |
9 ADDITIONAL APPLICATIONS FOR IRON SUPPLEMENTATION
9.1 Heart failure
Iron deficiency, with or without anemia, is thought to affect between 37% and 61% of patients with chronic heart failure (HF) and contributes to exercise intolerance, fatigue, decreased quality of life, increased hospitalization rates, and increased risk of mortality by 40%-60%.82 The etiology is likely multifactorial including reduced absorption from GI edema, increased GI blood loss from antiplatelet and anticoagulant therapies, and iron sequestration from chronic inflammation.82 Multiple large trials have established a role for the use of iron repletion in patients with HF. The FAIR-HF trial found IV iron use was associated with significant improvement to both patient scored measures of disease burden and New York Heart Association (NYHA) functional class relative to placebo.83 Secondary endpoint measures of 6-minute walk testing (6-MWT) and quality of life (QoL) also improved. The CONFIRM-HF trial replicated improvements in QoL, 6-MWT, and NYHA class with additional findings of reduced risk of rehospitalization due to worsening HF at 1-year follow-up.84 The evidence has led the American College of Cardiology and American Heart Association to recommend IV iron replacement in NYHA HF class II or III patients who are also iron deficient (ferritin <100 ng/mL or ferritin between 100 and 300 ng/mL with TSAT <20%).85 Iron repletion can be accomplished with initial IV iron dosed to account for body weight and followed at 12 weeks with measurement of hemoglobin levels and ferritin to assess whether additional maintenance therapy is indicated.82
9.2 Fatigue in adults with latent iron deficiency
A randomized controlled trial in 2011 found evidence that administration of 800 mg of iron (III)-hydroxide sucrose improved patient-reported fatigue scores in a subgroup analysis of premenopausal women with ferritin values below 15 ng/mL but otherwise normal hemoglobin values.86 A more recent systematic review and meta-analysis including both non-anemic iron-deficient men and women observed that subjective measures for scoring fatigue improved in trials of both oral and IV iron interventions; however, most studies did not observe a concordant improvement to objective measures of physical capacity such as maximal rate of oxygen consumption, time to exhaustion, or time trials.87-89
9.3 Bariatric surgery
The rising prevalence of obesity has led to increased rates of bariatric surgery which has known complications of decreasing body iron and vitamin B12 stores, even with oral supplementation.90 Rates of anemia rise from a baseline of 12.2%-25.9% at 24-months postsurgery.90 This may be due to multiple factors including reduced gastric acid secretion needed for iron conjugation and absorption, bypassing the duodenum which is the site of iron absorption, GI blood loss, as well as reduced dietary intake.91 Correction of anemia is most rapidly and safely achieved by IV iron in patients who have undergone Roux-en-Y or biliopancreatic bypass and should also be considered in patients with gastric sleeve or banding procedures in order to simplify care and reduce GI side effects.9
9.4 Restless leg syndrome
Central nervous system iron deficiency is thought to be implicated in the pathophysiology of restless leg syndrome63; patients with RLS were observed to have significantly lower CSF ferritin and elevated CSF transferrin levels compared to controls.92 A systematic review of 10 studies and 428 individuals with restless leg syndrome 63 found IV iron therapy provides a low-to-moderate benefit for patients with RLS even with normal ferritin at the time of therapy.93 Iron repletion therapy has become one of the first-line treatments for RLS and consensus guidelines established in 2017 now recommend use of oral iron (ferrous sulfate supplemented for 12 weeks) when serum ferritin ≤75 µg/L, or IV iron (ferrous carboxymaltose 1000 mg over 15 minutes) in the case that serum ferritin is between 75 and 100 µg/L or oral therapy is contraindicated, poorly tolerated, or more rapid symptom relief is desired.94 Both forms of iron therapy should be reserved for patients with TSAT <45% to prevent iron overload.94
9.5 Acute mountain sickness prophylaxis
Acute mountain sickness (AMS) may present in as many as 50% of people who travel to high altitudes each year.95 Prophylactic IV iron treatment has been shown to significantly reduce the incidence of AMS in healthy volunteers.96 Although the mechanism is not fully understood, it is postulated that iron loading reduces the production of hypoxia-inducible factor (HIF), which is implicated in the development of AMS.9
10 CONCLUSION
There are many distinct causes of microcytic anemia, with IDA being the most common and treatable. Patients should be evaluated appropriately to identify the cause of their anemia in order to determine the best treatment options. Clinical management involves correction of the underlying cause of absolute or functional iron deficiency when possible and iron repletion through oral or IV therapy. The landscape for IDA treatment is actively shifting in favor of IV therapies as they provide rapid iron repletion with fewer adverse effects relative to oral therapies. There is accumulating evidence to suggest important physiologic roles for iron beyond its requirement for hematopoiesis as demonstrated by the positive effects of iron repletion for the treatment of some forms of fatigue, acute mountain sickness, restless leg syndrome, and heart failure patients, further broadening the scope of IV iron therapy.




