Influence of platelet and white blood cell counts on major thrombosis – analysis from a patient registry in essential thrombocythemia
Abstract
Objectives
Although guidelines recommend normalization of platelet counts as an appropriate endpoint for treatment in high-risk essential thrombocythemia (ET), retrospective studies could not prove a correlation of diagnostic platelet counts with an increased thrombotic rate. There is, however, an increasing evidence that leukocytosis is an important risk factor for arterial thrombosis in myeloproliferative neoplasms.
Methods
This study considers the Austrian cohort of a European registry regarding the platelet-lowering therapeutic anagrelide. Influence of platelet and white blood cell (WBC) counts on thrombotic risk was assessed.
Results
Using the calculated cutoffs of 574.5 G/L for platelets and 8.48 G/L for WBC counts, respectively, the Cox regression analysis revealed a clear influence of elevated platelets (P = 0.008) and WBC counts (P = 0.011) on the occurrence of major thrombotic events. The time to a major thrombotic event was shortest (P < 0.001) and the frequency related to 100 patient-years was highest (P = <0.001) when both platelet and WBC counts ranged above the calculated cutoffs.
Conclusion
Our data add evidence to the impact of platelet and WBC counts on thrombosis in ET. We suspect a particular interaction between platelets and WBC which might be based on a biological interplay depending on particular cell counts.
Pathogenesis of thrombosis in myeloproliferative neoplasms (MPN) is very complex, and beside clinical factors, the increase of blood cell counts may contribute to the increased risk of thrombosis to a certain extent 1. It is evident that cytoreductive therapy decreases the risk of vascular complications in high-risk essential thrombocythemia (ET), but it is not clear which particular threshold for the platelet count is appropriate as endpoint for treatment 2. Although several retrospective studies could not prove a benefit from achievement of a complete platelet response 3, 4, former and also current guidelines recommend normalization of the platelet count 5, 6. This is supported by several prospective investigations including two randomized studies where platelet-lowering therapy was associated with a low thrombosis rate 7-9, in particular in ET classified according to the WHO criteria. Moreover, there is increasing evidence that leukocytosis is an important risk factor for arterial thrombosis especially in WHO 2008-classified ET 10. Leukocytosis is even more pronounced in prefibrotic PMF compared to WHO 2008-classified ET 11-13. Patient cohorts that were diagnosed prior to the WHO 2008 classification likely consist of a mixture of ET and prefibrotic primary myelofibrosis (PMF) 14. Some authors assume an interplay between leukocytes and platelets in patients with myeloproliferative diseases 15, 16.
Patient registries offer the opportunity to observe blood counts and efficacy of treatment under real-life conditions and with long follow-up. In 2001, the platelet-lowering therapeutic anagrelide was registered in Austria as first European country for the treatment of ET. This study considers the Austrian cohort of the registry and focuses on the impact of platelet as well as leukocyte counts on the occurrence of a major thrombotic event during the course of treatment with anagrelide.
Patients and methods
All patients of the registry were considered for this analysis. Inclusion criteria consisted of a valid diagnosis of ET according to PVSG or WHO criteria and a sufficient follow-up time of at least one month. Registry entry was the start of treatment with anagrelide. Concurrent treatment with aspirin and other cytoreductive treatment when indicated were at the discretion of the treating physician.
Considered fatal and non-fatal major thrombotic events included stroke, transitory ischemic attack, myocardial infarction, coronary artery disease/angina pectoris, peripheral arterial disease, deep vein thrombosis, pulmonary infarction/embolism, and splanchnic vein thrombosis/Budd–Chiari syndrome.
Influence of platelet and white blood cell (WBC) counts on thrombotic risk was assessed by correlating the first major thrombotic event with the median from all blood counts recorded during the course of the disease until an event or until end of observation. Due to the non-normal distribution of the relevant data sets, subgroup comparisons (patients with major thrombotic events vs. patients without such events) of metric variables were performed by the Mann–Whitney U-test. The suitability of median platelet and WBC counts for the prediction of major thrombotic events was checked by means of receiver operating characteristic curves (ROC), cutoffs were assessed by the Youden index. Time to occurrence of major thrombotic events was depicted by Kaplan–Meier plots and analyzed by log-rank tests as well as by Cox regression (median platelet and WBC counts dichotomized according to the corresponding Youden index, age dichotomized with a cutoff of 60 yrs). The type I error was not adjusted for multiple testing. Therefore, the results of inferential statistics are descriptive only. Statistical analysis was performed using the open-source R statistical software package, version 3.1.2 (The R Foundation for Statistical Computing, Vienna, Austria). Unless otherwise mentioned, descriptive data in the text reflect medians and quartiles [in square brackets]. The study was approved by the local Ethical Committee and conducted in accordance with the current version of the Helsinki Declaration.
Results
The original registry included 845 patients, 225 patients were excluded because of missing variables for an exact diagnosis, lack of data or too short observation period (shorter than 30 days which was the time to the occurrence of the first major arterial or venous event). Median follow-up in the remaining 620 patients is 37.2 [14.52; 68.76] months, corresponding to 29 133 patient-months and 2428 patient-years, respectively. The majority (95%) of patients were primarily classified or later reclassified according to the WHO classification system (before 2008 according to WHO 2001, and after 2008 according to WHO 2008, respectively). In the remaining 5% of patients, information about the applied classification system is missing. Four hundred and eighteen patients (67%) were females. Patients' age at time of study entry was 62 [51; 72] years. Median platelet count at entry into the register was 776.50 [579.50; 1000.50] G/L (this unit should be given in capitalized letters throughout the text, as marked), median WBC count at study entry was 8.70 [6.80; 10.90] G/L. Median platelet count of the entire cohort during the time of observation (until major event or end of follow-up) was 509.00 [411.50; 623.25] G/L, median WBC count was 8.58 [7.08; 10.70] G/L. Thirty-four patients (5.5%) experienced at least one major arterial or venous event until data analysis corresponding to 1.40 events per 100 patient-years. Comparison of patient's characteristics with and without a major thrombotic event, respectively, are depicted in Table 1. Time to first major event was median 1.50 [0.80; 2.93] years. Age of patients at their first major event was 70 [56; 78] years compared to 66 [55; 76] years at the end of observation period in patients without any major event (P = 0.359).
| Variable | Patients with MTE; n = 34 | Patients without MTE; n = 586 | P-value |
|---|---|---|---|
| Gender | |||
| Female, n (%) | 26 (76.5) | 392 (66.9) | 0.346 |
| Male, n (%) | 8 (23.5) | 194 (33.1) | |
| Age at study entry | |||
| Years, median (quartiles) | 66 (55; 78) | 62 (51; 72) | 0.258 |
| Age at MTE or last follow-up | |||
| Years, median (quartiles) | 70 (56; 78) | 66 (55; 76) | 0.359 |
| WBC count at study entry | |||
| G/L, median (quartiles) | 10.1 (8.5; 12.9) | 8.6 (6.7; 10.7) | 0.052 |
| WBC count at MTEa or last follow-up | |||
| G/L, median (quartiles) | 9.8 (8.4; 12.3) | 8.6 (6.9; 11.0) | 0.089 |
| Platelets at study entry | |||
| G/L, median (quartiles) | 794 (635; 1067) | 776 (578; 1000) | 0.606 |
| Platelets at MTEa or last follow-up | |||
| G/L, median (quartiles) | 495 (361; 632) | 438 (346; 599) | 0.303 |
- MTE, major thrombotic event; WBC, white blood cells.
- a Last available value prior to or at an event.
The median platelet counts until the first event occurred and until end of observation (in patients without an event) were 595.75 [495.00; 743.00] G/L and 500.00 [410.50; 619.50] G/L, respectively, and thus substantially different (P =0.008). The median WBC counts until the first event occurred and until end of observation (in patients without an event) were not statistically different with 9.60 [8.70; 10.95] and 8.42 [7.05; 10.70] G/L, respectively (P = 0.084). However, the difference might be interpreted as trend for a higher WBC count in patients experiencing a major thrombotic event.
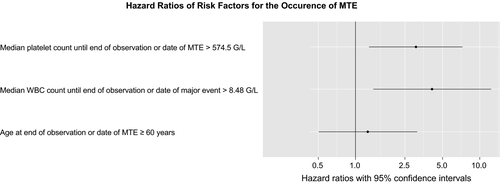
Using the calculated (Youden index) cutoffs of 574.50 G/L for platelets and 8.48 G/L for WBC counts, respectively, the Cox regression analysis revealed a clear influence of elevated platelets (P = 0.011) as well as WBC counts (P = 0.011) above the cutoffs on the occurrence of a major thrombotic event (Fig. 1).
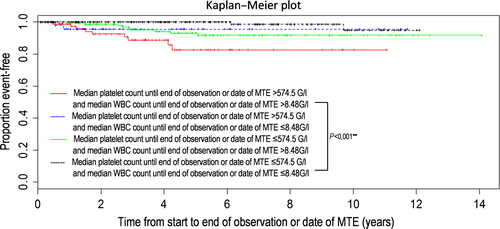
A Kaplan–Meier plot was applied for pair-wise subgroup comparison and revealed a markedly shorter time to a major thrombotic event for patients who displayed both high platelet counts and high WBC counts compared to patients with low platelet as well as low WBC counts (P = < 0.001)(Fig. 2).
The frequency of a major event related to 100 patient-years was highest when both platelet and WBC counts ranged above the calculated cutoffs. This frequency was substantially different from the frequency when both platelet and WBC counts ranged below the cutoffs (Table 2).
| Major thrombotic events/100 patient years | |||
|---|---|---|---|
| WBC > 8.48 G/L | WBC ≤ 8.48 G/L | Total | |
| Plts. > 574,5 G/L (95% CI) | 3.05 (1.12;4.98) | 1.05 (0.01;2.53) | |
| Plts. ≤ 574,5 G/L (95% CI) | 1.15 (0.41;1.89) | 0.20 (0.01;0.48) | |
| Total (95% CI) | 1.40 (0.94;1.86) | ||
- WBC, white blood cells.
Discussion
Using the calculated (Youden index) cutoffs of 574.5 G/L for platelets and 8.48 G/L for WBC counts, respectively, the Cox regression analysis revealed a clear influence of platelets (P = 0.011) as well as WBC counts (P = 0.011) above the cutoffs on the occurrence of a major thrombotic event (Fig. 1). However, lack of information on important known factors such as JAK2 mutational status, history of thrombosis, and cardiovascular risk factors as a result of incomplete resource data must be considered when evaluating these results. Pair-wise comparison shows a markedly shorter time to a major thrombotic event and major thrombotic events were substantially more frequent when both platelet and WBC counts ranged above the calculated cutoffs (Table 2, Fig. 2). These data clearly support the relevance of lowering platelet as well as WBC counts below a certain cutoff in order to prolong the time to and reduce the risk of a major thrombotic event in ET. Certainly, we cannot provide proof of the positive effect of normalization of WBC and platelet counts in ET. However, elevated WBC and platelet counts were strongly associated with thrombotic risk. In the present investigation, clearly anagrelide proved sufficient in lowering the median platelet count from median 776.5 G/L at treatment start to median 509.0 G/L during observation. However, the impact of platelet counts on major thrombosis is still being discussed controversially. Certainly, a very high platelet count (>1.000 G/L) is a risk factor for bleeding and thus inversely protects against thrombosis due to an acquired von Willebrand syndrome 10, 17, 18. Different platelet targets were investigated in the context of the thrombotic risk mainly in retrospective studies 19-21. However, achievement of a complete platelet response according to ELN criteria (even if mainly developed for usage in clinical trials) does not seem to be associated with a clinical benefit concerning the thrombotic risk 3, 4. In this study, the calculated cutoff for median platelet counts during the course of the disease was 574.5 G/L and herewith clearly above normalization. However, the calculated cutoff for median WBC counts for an increased risk for thrombosis was 8.48 G/L and therefore lower than the definition for CR according to the ELN criteria with <10 G/L 22. A very recent analysis of the Czech part of the registry revealed a higher platelet count prior to a thrombotic event compared to time points without an event (454 G/L vs. 400 G/L) despite a lack of any prognostic value of platelet counts at diagnosis 23. In our cohort, the clinical impact of median platelet counts during the course of the disease and median platelet counts to a greater or lesser extent directly prior to a thrombotic event was comparable (data not shown). As pretreatment prior to registry entry was allowed and diagnostic data were not retrospectively collected in our registry, we cannot comment on the importance of platelet count at diagnosis. However, the results of the Czech registry analysis support our observation of the importance of elevated platelet counts during the course of the disease on the risk of major thrombosis.
In addition, our analysis focused on the impact of median WBC counts on the risk of major thrombosis during follow-up, and we identified a cutoff for risk of major thrombosis just below the upper limit of normal WBC count (8.48 G/L).The impact of WBC counts on the risk of thrombosis becomes increasingly evident in Philadelphia-negative myeloproliferative neoplasms 24. However, a reasonable explanation for the still controversially discussed topic might be the fact that the studies are based on different classification systems. Strictly, WHO 2008 classified ET is not typically characterized by leukocytosis in contrast to prefibrotic PMF 8, 13, 25, 26. Patient cohorts that were diagnosed prior to the WHO 2008 classification likely consist of a mixture of ET and prefibrotic PMF 14. Our registry comprises patients diagnosed by different classification systems and therefore likely also included patients with prefibrotic PMF. Molecular genetic information could not be included in the analysis due to lack of comprehensive data. Moreover, our registry concentrated on patients treated with an exclusively platelet-reducing drug, namely anagrelide; this is mirrored by the fact that median WBC counts at study entry and during the course of the disease were quite similar (8.7 and 8.58 G/L, respectively). As hydroxyurea was not used in our registry, elevated WBC counts remained high, which gave us the opportunity to study the impact of elevated WBC counts on thrombophilia. Analog to our study, an analysis of the PVSG-classified PT1 study cohort 7 also revealed a significant influence of higher WBC counts during follow-up on thrombosis risk 27. Furthermore, a time-dependent analysis of another study on PVSG-classified ET patients that considered the last WBC count measured before a vascular event could show that treatment with hydroxyurea (a platelet- and WBC-lowering drug) reduces the strength of the association between an increased WBC count and the risk of major thrombosis 28. The recent analysis of the Czech registry also investigated WBC counts and detected significantly higher WBC counts preceding an arterial thrombotic event compared to median levels of all entries during follow-up (9.7 G/L vs. 8.6 G/L) 23.
Our data clearly indicate an interaction of platelets and WBC counts on thrombotic risk in MPN. Thrombotic risk is highest when both platelet and WBC counts range above the calculated cutoffs of 574.5 G/L for platelets and 8.48 G/L for WBC counts and lowest when both range below the cutoffs. This interaction could either result from simple addition of the thrombotic risk or represent a direct interplay between platelets and WBC. However, another study showed an even inverse relationship between diagnostic WBC and platelet counts. The lowest rate of vascular events (1.59% patients/yr) was observed in ET patients with a WBC count below 11 G/L and a platelet count above 1000 G/L; the highest rate (2.95% patients/yr) was observed in patients with WBC count above 11 G/L and platelet count below 1000 G/L. As most likely explanation for this finding, an acquired von Willebrand syndrome was stated by the authors 16.
It is well known that a significant number of polymorphonuclear leukocytes (PMN) circulate in an activation status in patients with MPN, which have a role in the thrombophilic state by releasing their intracellular granule content 29. Activated PMN can bind to platelets in a dynamic process, which reflects the activation of both platelets and leukocytes 30. A greater percentage of mixed aggregates was found in patients with ET, but no significant correlation existed with platelet number 29.
In conclusion, our data add evidence to the impact of platelet as well as WBC counts on time to and frequency of thrombosis in ET. Moreover, we suspect a particular interaction between platelets and WBC which might not be only the result of simple addition of risk, but might rather be based on a biological interplay. We speculate that this interaction depends on particular cell counts of platelets and WBC which we tried to define. Further studies are warranted in WHO-defined and prospectively observed cohorts in order to validate our findings.




