Thrombosis in thrombocythemic Ph- myeloproliferations is associated with higher platelet count prior to the event: results of analyses of prothrombotic risk factors from a registry of patients treated with anagrelide
Abstract
Controversies still exist regarding definition of the thrombotic risks in Ph- (BCR/ABL1-) myeloproliferative disorders with thrombocythemia (MPD-T). Platelet counts at diagnosis are currently not taken as a risk factor of thrombosis. In our cohort of 1179 patients with MPD-T, prospectively registered for anagrelide treatment, we found that the median platelet count prior to the thrombotic event was significantly higher than at time points without any ensuing thrombosis (453 vs. 400 × 109/L, P < 0.001), albeit higher platelet counts at diagnosis tended to be connected with fewer thrombotic events (in contrast to WBC counts at diagnosis). The JAK2V617F mutation predicted both arterial and venous events, while age >65 yr, hypertension, diabetes mellitus, smoking, elevated triglyceride and homocysteine levels predicted arterial events only. For venous events, the specific thrombophilic risk factors (factor V ‘Leiden’ and others), antiphospholipid antibodies, and elevated factor VIII levels played a major role. During anagrelide treatment (± aspirin), we documented a decrease in both venous (6.7-fold) and arterial events (1.8-fold), while bleeding (mostly minor events) increased twofold compared to history. Our results suggest that keeping platelet counts at low levels may be a meaningful therapeutic measure to prevent thrombosis, although their counts at diagnosis lack any prognostic value.
Ph- (BCR/ABL1-) myeloproliferative disorders (MPDs) encompass three main clinical entities: essential thrombocythemia (ET), polycythaemia vera (PV), and primary myelofibrosis (PMF). All three have in common that in their early stages, the initial laboratory presentation may involve thrombocythemia. According to the Polycythemia Vera Study Group (PVSG) criteria, their initial stages are difficult to differentiate and all of them are usually classified as ET 1. However, when a histopathology-based classification is used, such as WHO, European clinical and pathological (ECP), or the Czech Group for Ph- Myeloproliferative Disorders (CZEMP) criteria 2-4, these entities, currently called according to WHO 2 myeloproliferative neoplasms (MPNs), may be mutually differentiated. They differ in survival characteristics 5, albeit the most important, permanently present risks—thrombosis and bleeding—are common (to a varying power) for ET, PV, and PMF.
Treatment guidelines for ET issued by various groups 6-9 are based on the patients’ risk assessment for the major complications of the disease, that is, arterial, venous or microcirculatory thrombosis, and bleeding. The guidelines have in common to recognize age and previous thrombosis as major risks for thrombosis, clearly evidenced in the study from Bergamo 10. However, these guidelines have not yet incorporated additional prothrombotic risk factors demonstrated by the International Prognostic Score of thrombosis in WHO-defined ET (IPSET-thrombosis) study 11: in addition to the two factors mentioned above, also the JAK2V617F mutation and the cardiovascular risk factors (comprising hypertension, diabetes mellitus, and smoking) were included into the IPSET scoring system 11. Only according to the CZEMP guidelines, also the presence of additional thrombophilic states (hereditary and acquired) along with the degree of thrombocythemia and the JAK2V617F mutation has been used to define the high risk for thrombosis, respectively 4, 9. A set of other factors may be also of importance for thrombotic risk assessment (albeit not yet been included into the treatment guidelines): for example, WBC counts, hyperlipidemia (in addition to the IPSET definitions of cardiovascular risk factors), and others 6, 11-13. The treatment guidelines also differ with respect to which drug is recommended as first choice for cyto-/thromboreduction.
Excessive thrombocythemia (above 1500 × 109/L) has been regarded an indication for starting thromboreductive therapy in all the guidelines mentioned. The rationale is largely to prevent bleeding due to the secondary von Willebrand syndrome 14, 15. However, in the meta-analysis of the risk factors, Michiels et al. 16, 17 have shown that not only bleeding, but also the thrombotic risk is a function of the platelet count (peaking at ca 1000 × 109/L platelets). It has been also shown in another study from Bergamo that lowering the platelet count using HU (randomized to placebo) significantly reduces the thrombotic risk 18.
The recent studies of prognostic factors in ET indicate that WBC count at diagnosis, rather than platelet count, determines the risk of thrombosis. In fact, quite surprisingly, the platelet count at diagnosis correlated inversely with arterial thrombotic events 13, 19. We have studied the blood cell count parameters and an array of other risk parameters (some of them were mentioned above) with regard to their impact on the incidence of thrombosis and bleeding. The patient cohort was recruited from the Czech segment of an International registry of patients treated with anagrelide (‘Registry’).
Patients and methods
A total of 1179 patients prospectively assigned into the Registry of patients treated with ANG (Thromboreductin®, Austrian Orphan Pharmaceuticals—AOP, Vienna, Austria) were studied. The Registry covers all patients treated with ANG in the whole country since 2001 until the end of 2013 by 135 hematologists (listed in Table S1) in 70 centers. The male to female ratio was 2 : 3, and the median age at diagnosis was 52 (6–91) years. The majority of patients (N = 751, 63.7%) was pretreated with other cytoreducing drugs. According to PVSG criteria 1, the initial diagnoses were as follows: ET – 921 (78.1%), PMF – 87 (7.4%), or PV – 171 (14.5%) patients. In total, 812 patients could be evaluated according to the WHO 2008 or CZEMP modified criteria 2, 4, 20, 21. The diagnosed entities according to WHO/CZEMP were as follows: ET – 445 (54.8%), PMF – 206 (25.4%), PV – 107 (13.2%), and other (mostly MPN-unclassifiable) – 54 (6.7%) cases. The majority of the histopathological diagnoses were verified by second reading of the pathologists within the CZEMP group (V.C. and L.K.).
Data were collected monthly during the first 6 months and in 3-month intervals thereafter. Full blood cell counts (FBC) and basic biochemical parameters were filed. At the entry of the study, patient history of thrombosis and bleeding was recorded (and FBC counts at thrombotic events in history), along with the diagnostic conclusion according to PVSG 1 or WHO/CZEMP criteria 2, 4 both at diagnosis and at entering the Registry. The patients gave informed consent to collecting their data according to the Declaration of Helsinki.
Data from the time of diagnosis, time of Registry entry, and from the time of the thrombotic event (i.e. the FBC preceding the event no longer than 100 d) were evaluated. The median follow-up since Registry entry was 42 (0–150) months and the follow-up comprised 4742 patient-yr (while the time from diagnosis till entering the Registry comprised 4149 patient-yr). In general, patients were treated according to the Czech Hematological Society (CHS) guidelines, as first published in 2005 9, 22 and updated by the CHS working group CZEMP in 2011 4. All patients were treated with ANG and in 54.4% of follow-up reports, acetylsalicylic acid (ASA) was mentioned to be given in parallel. In 18.1% of entries (from registration and follow-up), administration of another cytoreducing drug (mainly HU or IFN) in combination with ANG was recorded. The median dosage of ANG was 1.5 mg/d (range 0.1–5.0 mg/d).
JAK2V617F mutation analysis
In 1114 patients, the JAK2V617F mutation was screened by the real-time RT-PCR assay (wt and mutated alleles being discriminated by specific TaqMan probes positioned in between the common primers) as described by Marková et al. 23. In a minority of these cases, the commercial MutaScreen PCR kit from Ipsogen (Marseille, France), marketed currently by Qiagen (Düsseldorf, Germany), was used.
Definition of thrombotic/bleeding events
Diagnostic criteria of thrombotic and hemorrhagic events were in accord with a previous publication 24. Thrombotic events were separated into arterial, venous, and microcirculatory ones. Major arterial events were ischemic stroke, myocardial infarction (with typical ECG and elevated cardio-specific enzymes), and other major peripheral arterial events. Minor arterial events were transitory ischemic attacks (TIAs) of the central nervous system and angina pectoris (including unstable angina) with acute symptomatology. Microcirculatory events were erythromelalgia, ocular, and neurological symptoms not fulfilling the definitions of stroke or TIA 25, 26. Major venous events were the following: pulmonary thromboembolism, splanchnic thrombosis (portal, lienal, mesenteric, or the Budd-Chiari syndrome), iliofemoral and other forms of deep vein thrombosis. As a minor venous event, typically superficial thrombophlebitis was classified.
As a major bleeding event, either hemoglobin drop of ≥1 g/dL or RBC transfusion need (both if attributable to bleeding) was recognized; other hemorrhagic events were classified as minor.
‘Thrombophilia’ work up
Specific thrombophilic predisposition factors, comprising both hereditary and acquired states, were studied. Protein C and protein S deficiencies and antithrombin levels were assayed using classical coagulation methods (thus, even acquired deficiencies were registered – e.g. decreased protein S levels due to hormonal contraception), whereas factor V ‘Leiden’ R506Q (G1691A) and prothrombin gene G20210A hereditary mutations were screened by DNA sequencing. The additional acquired states also registered in the study included widespread infections and tumors, major tissue damage (surgery, injury), pregnancy, and/or any other hypercoagulation state.
In addition to these classical thrombophilic conditions, also antiphospholipid antibodies were assayed by ELISA, and fibrinogen, factor VIII levels, and the lupus anticoagulant were determined by the standard coagulation methods.
Statistical analyses
Incidence of thrombotic events was counted as number of thrombotic events per 100 yr of all patients’ history or follow-up (‘patient-years’). Comparisons of two incidence rates were assessed using two-sample binomial test. Potential risk predictors for thrombotic events were determined by univariate logistic regression, followed by the multivariate one, in which all significant factors from the univariate model were included and analyzed further. Correlation of blood cell counts (leukocyte counts, hematocrit, hemoglobin levels, and platelet counts) with the thrombotic risk was assessed by the Mann–Whitney test. Blood cell count values from the time of the individual thrombotic events were compared to overall median of the respective levels of all entries from all patients without thrombotic event during follow-up using the Wilcoxon test.
Results
Incidence of thrombosis and its general predictors
Of 667 thrombotic events reported, 458 (68.7%) occurred in history (i.e. before Registry entry) and 209 (31.3%) during follow-up. The number of arterial, venous, and microcirculatory events in history were 209, 176, and 73, respectively. Of the 209 thrombotic events in 145 patients during follow-up (4.41 events/100 patient-years), 95 were classified as major (78 arterial and 17 venous ones). Altogether, there were 130 arterial, 30 venous, and 49 microcirculatory events. During ANG ± ASA therapy (i.e. follow-up), the incidence of venous events decreased 6.7-fold in comparison with historical events (prior to Registry entry and starting ANG therapy), while arterial and microcirculatory events were reduced 1.8-fold and 1.7-fold, respectively. Hemorrhagic events (minor ones in the vast majority of events) increased twofold compared to events in history (Fig. 1). A thrombotic event in history predicted thrombosis during follow-up (P < 0.001). Arterial or venous events in history predicted the same type of event during follow-up (not shown).
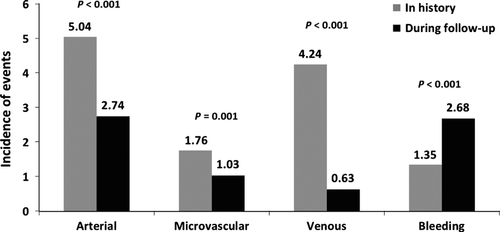
At diagnosis, univariate analyses (Table 1) revealed that the strongest predictors of all thrombotic events (of any type jointly) in history and/or during follow-up were JAK2V617F mutation (P < 0.001), age >65 yr (P < 0.001), hypertension (P = 0.003), presence of antiphospholipid antibodies (P = 0.001) and lupus anticoagulant (P = 0.023), diabetes mellitus (P = 0.012), elevated triglyceride levels (P = 0.045), the specific thrombophilic states (P = 0.037), of which factor V ‘Leiden’ mutation reached statistical significance by itself (P = 0.026), and also factor VIII elevation (P = 0.044). Only the JAK2V617F mutation and factor VIII elevation predicted both the arterial and venous events (P values 0.002 and 0.030 for JAK2V617F and 0.045 and 0.010 for factor VIII) (Table 1). Some of the factors, such as age, hypertension, increased homocysteine levels, elevated triglycerides, diabetes, and smoking, powerfully predicted rather arterial events, whereas others (presence of antiphospholipid antibodies, the specific thrombophilic markers, especially factor V ‘Leiden’ mutation, and protein C deficiency) were connected preferentially with venous events (Table 1).
| Any thrombosis | Major thrombosis | Arterial thrombosis | Micro-vascular events | Venous thrombosis | |
|---|---|---|---|---|---|
| Age > 65 yr | <0.001 | 0.046 | <0.001 | 0.510 | 0.059 |
| Overweight (BMI >25) | 0.403 | 0.181 | 0.536 | 0.918 | 0.132 |
| Smoking | 0.135 | 0.293 | 0.038 | 0.322 | 0.158 |
| Hypertension | 0.003 | 0.022 | <0.001 | 0.457 | 0.501 |
| Diabetes mellitus | 0.012 | 0.047 | 0.011 | 0.606 | 0.060 |
| Cholesterol elevation | 0.793 | 0.060 | 0.202 | 0.775 | 0.045 a |
| Triglycerides elevation | 0.045 | 0.141 | 0.001 | 0.301 | 0.469 |
| JAK2 mutation | <0.001 | <0.001 | 0.002 | 0.273 | 0.030 |
| Specific thrombophilic markers jointlyb | 0.037 | 0.005 | 0.835 | 0.949 | 0.003 |
| AT deficiency | 0.805 | 0.060 | 0.304 | 0.900 | 0.790 |
| Prothrombin gene mutation | 0.155 | 0.035 | 0.481 | 0.999 | 0.122 |
| Factor V ‘Leiden’ mutation | 0.026 | 0.005 | 0.265 | 0.343 | 0.001 |
| Protein C deficiency | 1.000 | 0.073 | 0.339 | 0.509 | 0.007 |
| Protein S deficiency | 0.194 | 0.357 | 0.761 | 0.006 | 0.865 |
| Lupus anticoagulant | 0.023 | 0.028 | 0.007 | 0.977 | 0.362 |
| Antiphospholipid antibodies | 0.001 | 0.018 | 0.379 | 0.021 | <0.001 |
| Factor VIII elevation | 0.044 | <0.001 | 0.045 | 0.603 | 0.010 |
| Homocysteine elevation | 0.254 | 0.674 | 0.011 | 0.720 | 0.139 |
| Fibrinogen elevation | 0.258 | 0.558 | 0.423 | 0.586 | 0.859 |
- a Only in this case, the relationship between the parameter value (cholesterol elevation) and the event was inverse.
- b Prothrombin gene and factor V ‘Leiden’ mutations and proteins C and S and antithrombin deficiencies.
As the thrombotic risks for arterial and venous events differed substantially in univariate analysis (Table 1), multivariate analyses of the predictive factors for arterial and venous thromboses were executed separately. For arterial events (Table 2), the following parameters independently predicted thrombosis: hypertension, JAK2V617F mutation, age >65 yr, and elevated triglyceride levels (P values 0.001, 0.006, 0.012, and 0.012, respectively). The venous events (Table 3) could be independently predicted by the specific thrombophilic states (evaluated jointly), JAK2V617F mutation, and normal (unincreased) cholesterol levels (P values 0.009, 0.032, and 0.050, respectively).
| Prognostic factor | OR | CI (95%) | P |
|---|---|---|---|
| Hypertension | 1.813 | 1.295–2.538 | 0.001 |
| JAK2 mutation | 1.606 | 1.145–2.254 | 0.006 |
| Elevated triglycerides | 1.609 | 1.109–2.333 | 0.012 |
| Age >65 yr | 1.626 | 1.111–2.378 | 0.012 |
- OR, odds ratio; CI, confidence interval (lower – upper).
| Prognostic factor | OR | CI (95%) | P |
|---|---|---|---|
| Specific thrombophilic markers jointlya | 1.797 | 1.159–2.786 | 0.009 |
| JAK2 mutation | 1.562 | 1.040–2.347 | 0.032 |
| Cholesterol elevation | 0.617 | 0.381–1.000 | 0.050 |
- OR, odds ratio; CI, confidence interval (lower – upper).
- a Prothrombin gene and factor V ‘Leiden’ mutations and proteins C and S deficiencies. Antithrombin levels were not incorporated into the analysis due to low numbers of out of range results and lack of impact in univariate analysis.
Blood cell counts at diagnosis as risk factors for thrombosis
At diagnosis (Table 4), WBC, hematocrit and hemoglobin levels were positively correlated with the incidence of all thrombotic events in history, that is, before Registry entry and starting ANG treatment (P = 0.001, 0.006, and 0.011, respectively), whereas platelet counts showed a trend toward an inverse relationship with the incidence of all thrombotic events (P = 0.080). The same blood cell count parameters at diagnosis were also evaluated with respect to the incidence of major thrombotic events in history. Only WBC at diagnosis predisposed to major thrombosis in history (P = 0.006), while the platelet counts had a vigorous inverse relationship (P = 0.001) with the major events that occurred in history (Table 4).
| FBC parameter at diagnosis | Thrombosis | All thrombotic events | Major thrombosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In history | During follow-up | In history | During follow-up | ||||||||||
| N a | Median | P | N a | Median | P | N a | Median | P | N a | Median | P | ||
| Plt (109/L) | No | 826 | 902 | 0.080 | 1022 | 885 | 0.421 | 965 | 904 | 0.001 * | 1101 | 890 | 0.561 |
| Yes | 353 | 850 | 157 | 902 | 214 | 810 | 78 | 859 | |||||
| WBC (109/L) | No | 826 | 9.4 | 0.001 | 1022 | 9.5 | 0.101 | 965 | 9.5 | 0.006 | 1101 | 9.6 | 0.436 |
| Yes | 353 | 10.2 | 157 | 9.9 | 214 | 10.2 | 78 | 9.9 | |||||
| Hct | No | 826 | 0.42 | 0.006 | 1022 | 0.43 | 0.090 | 965 | 0.43 | 0.158 | 1101 | 0.43 | 0.083 |
| Yes | 353 | 0.44 | 157 | 0.44 | 214 | 0.44 | 78 | 0.44 | |||||
| Hb (g/dL) | No | 826 | 14.3 | 0.011 | 1022 | 14.4 | 0.241 | 965 | 14.4 | 0.311 | 1101 | 14.4 | 0.273 |
| Yes | 353 | 14.7 | 157 | 14.6 | 214 | 14.7 | 78 | 14.6 | |||||
- *P value marked with asterisk is given for patients who had significantly lower values of the parameter at diagnosis compared to those without thrombosis. P values not marked with asterisk given in bold denote patients who had significantly higher values of the parameter at diagnosis compared to those without thrombosis.
- a N denotes patient number.
Most importantly, none of the blood cell count parameters at diagnosis was able to predict thrombotic events during follow-up (Table 4). Only WBC counts and hematocrit showed a weak tendency to predict thrombosis occurring on treatment after Registry entry (P = 0.101 and P = 0.090, respectively).
Blood cell counts preceding the thrombotic events
Blood cell counts from the time of the thrombotic events (the last blood count before the event, not exceeding 100 d before the event) were studied and compared to median levels of all entries during follow-up. We detected significantly higher platelet and WBC counts preceding the thrombotic event (454 vs. 400 × 109/L, P < 0.001 and 9.7 vs. 8.6 × 109/L, P = 0.001, respectively; Table 5). The correlation of the platelet count preceding the event was significant for all types of thrombosis: arterial, microcirculatory, and venous (P values being 0.001, 0.004, and 0.018, respectively). The WBC values at the time of the event correlated significantly with arterial events only (P < 0.001), but did not correlate with microcirculatory (P = 0.126) and venous ones (P = 0.286). The red blood cell parameters—hematocrit and hemoglobin levels—were significantly higher prior to venous thrombotic events (P = 0.021 and P = 0.017, respectively).
| FBC parameter | Median from all entriesa | No of entries | All thromboses | Major thrombotic event | Arterial events | Microvascular events | Venous thrombosis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | P | N | Median | P | N | Median | P | N | Median | P | N | Median | P | |||
| Plt (109/L) | 400 | 21309 | 158 | 454 | <0.001 | 31 | 365 | 0.001 | 97 | 437 | 0.001 | 35 | 472 | 0.004 | 26 | 482 | 0.018 |
| WBC (109/L) | 8.6 | 21278 | 158 | 9.7 | 0.001 | 31 | 10.2 | 0.037 | 97 | 10.3 | <0.001 | 35 | 9.0 | 0.126 | 26 | 7.4 | 0.286 |
| Hct | 0.39 | 21212 | 157 | 0.38 | 0.383 | 31 | 0.34 | 0.052 | 96 | 0.38 | 0.316 | 35 | 0.40 | 0.094 | 26 | 0.37 | 0.021 |
| Hb (g/dL) | 13.0 | 21238 | 158 | 12.9 | 0.499 | 31 | 11.7 | 0.055 | 97 | 12.9 | 0.553 | 35 | 13.4 | 0.144 | 26 | 12.3 | 0.017 |
- a Median value from all entries from time points without any event during the subsequent follow-up period (median 3 months). Statistically significant P values are given in bold.
Discussion
According to all current guidelines 6-9, patients with MPD-T are treated by cytoreduction (by HU, IFN, or ANG) when the platelet count is in excess of 1000–1500 × 109/L, in line with the rationale to prevent bleeding at higher counts due to the secondary von Willebrand syndrome 14, 15. However, the CZEMP guidelines recommend lowering the platelet count in all high-risk patients (the majority of cases in fact). The high-risk status is characterized by any of the following features: previous thrombotic event, age >65 yr, JAK2V617F mutation, any inherited or acquired form of thrombophilia, or symptomatic disease 4. The rationale for this attitude was largely based on the results of meta-analysis performed by Michiels et al. 16, 17 showing that the thrombotic risk is tightly dependent on platelet count—the highest incidence of events peaking at ca 1000 × 109/L platelets. However, evidence from large prospective randomized trials supporting this attitude is lacking, although the role of platelet counts for thrombosis in MPDs has been suspected for decades 27. Moreover, the data by Carobbio et al. 13, 19 showed nearly an opposite thing: the platelet counts of ET patients at diagnosis correlated inversely with arterial thrombotic events, whereas they found a positive correlation between thrombosis and WBC counts at diagnosis 13, 19. The inverse correlation of the platelet count at diagnosis and thrombotic events in ET might even infer that thromboreducing therapy in these patients (unless they are at risk of bleeding at platelet counts of 1500 × 109/L and higher) may be counterproductive.
The paradox mentioned above is probably explained by the current study. Our study shows that the platelet count is critically important at the time of the thrombotic event: patients prior to the thrombotic event had actually higher median platelet counts than other patients. This holds true for all types of thrombotic events, that is, arterial, microcirculatory and venous—the highest median platelet count was documented prior to the latter events (482 × 109/L – see Table 5). However, in the same cohort of patients, we were able to show, in line with the results of Carobbio et al. 13, 19, a trend toward an inverse relationship of the platelet count at diagnosis and thrombosis in history, that is, before starting ANG treatment. We suspect that this apparent paradox may result from treatment: patients with higher platelet counts at diagnosis may receive more cytoreducing and/or antiaggregation therapy compared to patients with lower platelet counts (according to the treatment guideline used). Patients without treatment have then gradually increasing platelet counts leading to a higher propensity to thrombosis. Our observation thus points to that there is a firm rationale to reduce platelet counts in patients with MPD-T to diminish not only the bleeding, but also the thrombotic risk, in accord with the concept of Michiels et al. 16, 17. They have shown that both ASA treatment, as well as reduction of platelet counts using busulphan, are effective to reduce microvascular disturbances 25, 26. This is also in line with the findings of the randomized study by the Bergamo group showing that HU treatment reduces the thrombotic risk in these patients 18. Of course, it may be argued that this was rather due to lowering the WBC counts. But at least one of the two randomized studies of HU vs. ANG, ANAHYDRET 28, has shown that ANG is equally potent to prevent thrombosis without affecting WBC counts [which was, however, in contrast to the results of the other randomized study, the British MRC PT-1 29]. In the current study, the impact of higher platelet counts preceding the thrombotic event seemed to be statistically more profound than that of the WBC counts, especially when the venous and microvascular events are concerned (Table 5). Our results also point to the appropriateness of the CZEMP treatment guidelines 4, 9, recommending the reduction of the platelet count below 400 × 109/L in high-risk patients. True, thrombotic events in MPD may occur, less frequently, even at normal platelet counts according to the literature 30, 31, in line with our own observations.
In accord with the Italian studies 13, 19, our results show that higher WBC counts at diagnosis predicted thrombosis in history (i.e. before starting ANG) and also revealed a very loose trend to predict thrombosis even during follow-up. Also hematocrit and hemoglobin levels at diagnosis could predict thrombosis in history (before Registry entry). Above that, also prior to thrombotic events, the WBC counts were significantly higher (however, this holds true only for the arterial, but not for other types of thrombotic event). It is not surprising that WBC counts retained their predictive value even during treatment (follow-up), as lowering of the WBC count has not been a primary target for therapy in this study (and in any of the existing guidelines so far). For thrombosis, some interaction between the activated WBC (mononuclears as well as neutrophils) and platelets has to occur, so that both types of cells (WBC and platelets) are likely important prior to the thrombotic event. Of course, their activation may be potentiated by JAK2V617F mutation 32-34.
Our study also indicates that during ANG ± ASA therapy, the incidence of thrombosis was very low in MPD-T. In particular, the rate of venous events was extraordinarily low (0.63 events per 100 patient-yr), while the bleeding tendency was increased, in line with previous studies 24. However, this result was nearly exclusively on the account of clinically not serious minor hemorrhagic events.
This study also evaluated the general predictors of thrombotic events in the setting of MPD-T. At univariate analysis, the JAK2V617F mutation along with age >65 yr was the strongest predictor of any thrombosis in history and during follow-up. The mutation affected significantly the incidence of both the arterial and venous events (including the major events), while age was a stronger predictor of arterial thrombosis, compared to venous. The predictive value of JAK2V617F was preserved in multivariate analyses of the risk factors, again for both the arterial and venous events. The importance of the mutation toward the thrombotic risk has been suggested since its discovery in 2005 35, albeit it has not yet been incorporated into the treatment guidelines except for the CZEMP ones 4. However, the JAK2V617F mutation has been recently validated as a major prothrombotic constituent in the recent IPSET risk factor study in WHO-defined ET 11. For arterial thrombosis, hypertension and age >65 yr seemed to be important both in univariate and multivariate analyses in our study. For venous thrombosis, we provide clear evidence in the largest cohort analyzed so far that the specific thrombophilic states represent a major prothrombotic risk in MPD-T. Evidence of their role exists since the 90s in the literature 36-41. Their evaluation has been incorporated to the CZEMP treatment guidelines as one of the factors establishing a high-risk status since 2005 9, 22. However, they have not been evaluated by the IPSET study 11. Quite interesting results were obtained by studying the impact of hyperlipidemia. In historical cohorts, hypercholesterolemia predicted thrombosis 12. In our study, elevated levels of triglycerides could predict arterial thrombosis, while normal (in contrast to elevated) levels of cholesterol predicted venous events. The reason is difficult to explain. We speculate that the majority of patients with hyperlipidemia (especially those with hypercholesterolemia) currently take statins, which can inhibit JAK-STAT-dependent signaling and cell growth, and thus, their use may be highly advantageous in the setting of Ph- MPDs 42.
Identification of the risk factors for thrombosis in our study also validates the use of parameters such as age, previous thrombosis, JAK2V617F mutation, thrombophilic states, and platelet counts as factors determining the high thrombotic risk status in MPD-T deserving more aggressive cytoreducing (or at least thromboreducing) treatment. A therapeutic approach based on multiple risk factors has been already used in the CZEMP guidelines for nearly a decade 4, 9, 22, 43. In the near future, we plan to adopt also the remaining risk factors: the ‘cardiovascular’ risks and WBC.
Taken together, we have shown that the platelet counts at diagnosis have little prognostic relevance in patients treated by platelet-reducing therapy. This does not mean, however, they are not an important predictor of thrombosis—patients suffering thrombosis have a higher (above normal) median platelet count before the event. We also verified that patients with JAK2V617F mutation, another thrombophilic state, or those having the cardiovascular risk factors are more prone to thrombosis.
Acknowledgements
All authors except P.O., V.C., and L.K. contributed to the Registry with the highest numbers of patients. J.S., M.P., P.D., and P.O. designed the study. P.O. performed the statistical analyses. J.S and P.O. wrote the manuscript. V.C. and L.K. made the histopathological examinations. All authors (including other CZEMP members) approved publishing the manuscript. The authors are indebted to J. J. Michiels, P. E. Petrides and J. Marková for careful reading and criticisms of the manuscript.
Conflict of interest
J.S. and M.P. received consultation honoraria from AOP Orphan.




