Evaluation of Methods for Resistance Testing of Fraxinus excelsior Genotypes Against Hymenoscyphus fraxineus
Funding: This work was supported by a grant from the Swiss Federal Office for the Environment (project SURVIVASH).
ABSTRACT
Ash dieback (ADB), caused by the invasive fungus Hymenoscyphus fraxineus, has spread extensively across Europe, resulting in considerable economic and ecological damage to European forests. Resistance screening is crucial for selecting and breeding common ash genotypes that are resistant to the ADB pathogen. This requires a standardised and effective method for infecting ash trees with the pathogen and subsequently measuring the infection success. However, to date, there is a lack of scientifically sound comparisons of available methods. In this study, three different methods for testing the resistance of ash genotypes against H. fraxineus (i.e., stem infections, rachis infections, and spore germination assays) were compared and benchmarked against crown defoliation and stem infection assessments. All three resistance assays were performed on 12 ash genotypes covering a wide intraspecific variation in ADB resistance. Stem infections correlated best with crown defoliation observed in the field. However, this method requires a long preparation time and experimental duration. The easiest and fastest method in terms of preparation and execution was the rachis infection assay. Although not completely equivalent to stem infections, the results of the rachis infection assay significantly correlated with the stem lesion lengths measured. Spore germination assays were also quick to carry out, but the timing of sporulation and the sampling of the leaves, followed by agar-medium preparation, proved to be very difficult to synchronise. Following the very small variations in spore germination rates, there was no significant correlation with the stem lesion lengths measured. To obtain meaningful results on the resistance of ash genotypes to ADB, the stem infection method still proved to be the best and most accurate assay. Although new indirect methods for testing resistance in ash trees are arising (e.g., chemistry, spectroscopy, or genetics), direct resistance testing of ash genotypes remains the basis for resistance and breeding research for ash conservation.
1 Introduction
Ash dieback (ADB) has occurred in Europe for over 30 years and has caused severe damage to the European common ash Fraxinus excelsior L. The ADB causal agent Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz & Hosoya (Helotiaceae, Leotiomycetidae) is a fungus native to East Asia, where it usually behaves as a harmless leaf coloniser (Carroll and Boa 2024). Symptoms of ADB were first observed in Poland in the early 1990s (Przybył 2002), and ADB has since spread to almost the entire distribution range of F. excelsior (Cleary et al. 2016; Gil et al. 2017; Gross et al. 2014; Stroheker et al. 2021; Timmermann et al. 2011). George et al. (2022) estimated that ADB will have caused an overall mean defoliation of 50% in the F. excelsior populations in Europe by 2030. After almost three decades of infection, the survival probability of ash trees is estimated to have already reached a value of 0.51, albeit with regional differences ranging from 0.20 to 0.86, which is an alarming result (George et al. 2022). Phytosanitary measures in forests are almost useless as the fungus overwinters and forms fruiting bodies in the leaf litter, and its spores are wind-dispersed over large distances. No effective method of control or containment has yet been found, although different studies discovered viruses in H. fraxineus (Bengtsson et al. 2021; Gross et al. 2014; Haupt et al. 2022; Turczański et al. 2021). Breeding resistant ash trees to accelerate the development of resistance appears to be one of the few viable options to help Europe's ash populations cope with the fungus. Successful resistance breeding requires a sufficient number of resistant ash trees. In many studies, high levels of ADB-resistance trait heritability have been observed, which can facilitate ash resistance breeding (McKinney et al. 2011; Mckinney et al. 2014; Stener 2018; Plumb et al. 2020; Stocks et al. 2019).
However, the selection of ADB-resistant ash trees in the field is not trivial. The ash-fungus interactions are determined not only by resistance components but also by surrounding environmental conditions (Grosdidier et al. 2020; Klesse, Abegg, et al. 2021; Marçais et al. 2016). A high ADB prevalence is, for instance, related to elevated ash density, precipitation, and soil moisture (Grosdidier et al. 2020; Klesse, Abegg, et al. 2021; Marçais et al. 2016). The influence of the environment on the observed disease resistance or susceptibility can be reduced by selecting apparently ADB-resistant genotypes that are surrounded by heavily ADB-infected genotypes on the same site (Gossner et al. 2023). As other still unknown factors may influence ash susceptibility in the field (e.g., microbiome, soil type, and neighbour trees), efficient and reproducible test methods under controlled conditions (common garden) are needed to select resistant ash genotypes for breeding purposes carefully.
The first and most commonly used approach to test ash resistance against ADB is based on stem infections (Kowalski and Holdenrieder 2009). In this approach, young ash trees are infected through an artificial wound either directly with the mycelium of H. fraxineus grown on agar or with H. fraxineus-inoculated wood chips (Gossner et al. 2023; Gross and Holdenrieder 2015; Kowalski et al. 2015; Kowalski and Holdenrieder 2009; Lobo et al. 2015; Nielsen, McKinney, Hietala, and Kjær 2017; Vemić et al. 2021; Wiersma et al. 2022). This approach was initially chosen mainly for historical reasons, as many other well-studied infectious tree diseases, such as Dutch elm disease or chestnut blight, start directly in woody tissues. However, infecting ash trees directly via the stem does not reflect the infection pathways in nature, where tree shoots are infected by the growth of the fungus through leaflets and rachis up to young shoots. As such, the stem infection approach might fall short in thoroughly assessing a tree's resistance as resistance mechanisms at the leaf and rachis levels are bypassed. Therefore, another rather new approach was developed to infect trees via the leaves or rachises with spores or fungal mycelium (Kowalski et al. 2015; Loop 2020; Nielsen, McKinney, Hietala, and Kjær 2017, 2022; Orton et al. 2019; Schwanda and Kirisits 2016). An additional approach, which is less commonly used, is based on measuring spore germination of the ADB pathogen on ash leaf malt extract agar made of leaf extract from the different ash genotypes. The latter approach aims to investigate potential inhibitors in the leaf tissues that could prevent the spores from germinating (Brühwiler and Sieber 2021; Carrari et al. 2015; Mansfield et al. 2018).
However, these three resistance screening approaches have not been benchmarked against each other. As such, it remains unclear if the most commonly used stem infection approach is indeed the most suitable method for quantifying ADB resistance in ash.
To address this knowledge gap, the three most commonly used methods, namely stem infection, rachis infection, and spore germination assays, were tested on the same ash genotypes (grafted replicates). Stem lesion lengths (as the most frequently used resistance measure) were statistically compared to field observations (crown defoliation), rachis lesion lengths, the area under the disease pressure curve of the rachis infections, and spore germination rates. Additionally, differences in the duration of the tests, effort, and result accuracy were evaluated and compared.
2 Material and Methods
2.1 Sampling Locations and Grafting of Ash Scions
Ten sites were selected from a previous monitoring project (Future of Ash, 2018), containing healthy-looking ash trees distributed throughout Switzerland. All sites are characterised by the fact that both apparently resistant and susceptible ash genotypes co-occur and cover a broad geographical range (Figure 1, Tables 1 and 2). Additionally, sites were selected that have a slight incline and are easily accessible by car. The crown defoliation of the ash genotypes tested in this study was assessed using six different crown defoliation categories during the summer of 2020 (definition of the defoliation classes: 0 = 0%–5% defoliation, 1 = 6%–25%, 2 = 26%–50%, 3 = 51%–75%, 4 = 76%–99%, 5 = 100% defoliation ~ dead tree) slightly modified from Lenz et al. (2012a), Lenz et al. (2012b). Two trees were selected at each site: one with a lower crown defoliation rate (categories 0, 1, 2 (only once)), corresponding to a “healthy” genotype, and one with a higher crown defoliation rate (categories 3 and 4), corresponding to “susceptible” genotype. Because of losses following grafting, we did not get enough replicates of some selected genotypes, so we ended up with a final selection of 12 trees (Table 2).
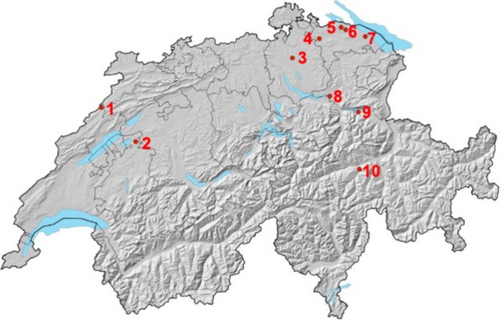
| Location Nr. | Site Nr. | Commune | Total number of ash trees | Number of ash trees belonging to each crown defolitation category on the plot in 2020 | Mean DBH (cm) | Altitude (m.a.s.l.) | Exposition | Slope | Forest community | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat. 0 | Cat. 1 | Cat. 2 | Cat. 3 | Cat. 4 | Cat. 5 | |||||||||
| 1 | 1025_8 | La Chaux-de-Fonds | 19 | 0 | 1 | 2 | 4 | 11 | 1 | 42.1 | 798 | w | 35 | 12s Cardamino-Fagetum typicum |
| 2 | 1049e_4 | Murten | 21 | 0 | 1 | 5 | 6 | 7 | 2 | 26.3 | 557 | ne | 2 | 7s Galio odorati-Fagetum typicum |
| 3 | 1006_9 | Bassersdorf | 21 | 2 | 2 | 3 | 5 | 2 | 7 | 32.9 | 522 | e | 2 | 7g Galio odorati-Fagetum typicum |
| 4 | 1060a_3 | Frauenfeld | 14 | 0 | 1 | 5 | 3 | 5 | 0 | 34.8 | 386 | Flat | 0 | 29a Ulmo-Fraxinetum listeretosum |
| 5 | 1060k_1 | Ermatingen | 21 | 0 | 0 | 4 | 6 | 7 | 4 | 33.6 | 566 | n | 4 | 29 Ulmo-Fraxinetum listeretosum |
| 6 | 1060l_4 | Kemmental | 14 | 0 | 0 | 2 | 4 | 7 | 1 | 35.5 | 436 | Flat | 0 | 29e Ulmo-Fraxinetum listeretosum |
| 7 | 1060n_4 | Kesswil | 19 | 1 | 1 | 3 | 4 | 8 | 2 | 37.4 | 802 | Flat | 0 | 26w Aceri-Fraxinetum |
| 8 | 1058_2 | Tuggen | 21 | 1 | 4 | 3 | 8 | 1 | 4 | 33.5 | 470 | s | 10 | 7s Galio odorati-Fagetum typicum |
| 9 | 1055d_1 | Quarten | 21 | 1 | 5 | 2 | 6 | 6 | 1 | 28.4 | 455 | n | 26 | 8a Milio-Fagetum |
| 10 | 1029_13 | Ilanz/Glion | 16 | 4 | 2 | 2 | 3 | 3 | 2 | 30.1 | 865 | w | 40 | 51 Galio-Abietetum |
| Location Nr. | Genotype | Site Nr. | Tree Nr. | Canton | Commune | X-coordinates | Y-coordinates | DBH (cm) | Crown defoliation category 2020 |
|---|---|---|---|---|---|---|---|---|---|
| 10 | G1 | 1029_13 | 1 | GR | Ilanz/Glion | N 46° 45.195 | E 9° 13.348 | 24 | 0 |
| 7 | G2 | 1060n_4 | 20 | TG | Kesswil | N 51° 47.266 | E 9° 27.860 | 30 | 4 |
| 9 | G3 | 1055d_1 | 8 | SG | Quarten | N 47° 6.666 | E 9° 13.396 | 33 | 4 |
| 8 | G4 | 1058_2 | 20 | SZ | Tuggen | N 47° 12.854 | E 8° 57.892 | 50 | 1 |
| 10 | G5 | 1029_13 | 16 | GR | Ilanz/Glion | N 46° 45.195 | E 9° 13.348 | 30 | 2 |
| 6 | G6 | 1060l_4 | 0 | TG | Kemmental | N 47° 37.724 | E 9° 7.411 | 28 | 3 |
| 1 | G7 | 1025_8 | 16 | NE | La Chaux-de-Fonds | N 47° 9.192 | E 6° 51.211 | 26 | 3 |
| 3 | G8 | 1006_9 | 7 | ZH | Bassersdorf | N 47° 27.455 | E 8° 37.500 | 32 | 4 |
| 5 | G9 | 1060k_1 | 15 | TG | Ermatingen | N 47° 38.708 | E 9° 4.989 | 37 | 4 |
| 4 | G10 | 1060a_3 | 7 | TG | Frauenfeld | N 47° 34.574 | E 8° 52.746 | 59 | 4 |
| 7 | G11 | 1060n_4 | 0 | TG | Kesswil | N 51° 47.266 | E 9° 27.860 | 42 | 1 |
| 9 | G12 | 1055d_1 | 9 | SG | Quarten | N 47° 6.666 | E 9° 13.396 | 29 | 1 |
To obtain biological replicates (clones) of each selected ash genotype (Table 2), 50 scions per tree (1-year-old, healthy shoots) were harvested from the selected trees in February–March 2019. To prevent the shoots from drying out, they were wrapped in plastic bags and stored at 4°C in the dark for 2 weeks until grafting. The shoots were then grafted onto rootstocks of two- to 3-year-old ash saplings (60–80 cm height) from the tree nursery Josef Kressibucher AG (Berg, Switzerland). The root-stock saplings originated from seeds collected on different mother trees from the two provenances Arni (AG) and Taegerwilen (TG) in Switzerland. All grafted ash trees were kept in greenhouses and in foil tunnels after the grafting (to prevent late frost damage after grafting and ADB infections during summer) for a whole year. Diseased and dead specimens were regularly discarded.
For the trials in 2020, 12 genotypes and 11 replicates per ash genotype were available for the experiments (6 for the stem infection experiment and 5 for the rachis infection experiment). Grafted trees on rootstocks from the two provenances were evenly distributed among the genotypes and experiments.
2.2 H. fraxineus Isolates
Since ADB strains appear to degenerate rapidly in laboratory conditions, experiments must use only fresh ADB isolates (V. Queloz, personal communication). One fresh H. fraxineus strain was used for the infection experiments: the strain was freshly isolated from natural shoot infections of young ash trees from the local population in Birmensdorf (ZH), Switzerland (VQ_200217_8), in February 2020. We used a single ADB strain for all experiments. Different ADB strains may exhibit varying levels of virulence (Kosawang et al. 2020). However, our study was limited by the available number of grafted replicates and therefore focused on only one strain.
The strain was grown on ash leaf malt agar (50 g fresh, sterilised F. excelsior leaves, 15 g agar (Plant Propagation Agar, Condalab, Spain), 20 g malt extract (DiaMalt “trocken”, Hefe Schweiz AG, Switzerland), 1000 mL tap H20) based on the recipe described in Gross et al. (2012).
2.3 Stem Infections
For the stem infection experiment, the strain VQ_200217_8 of H. fraxineus was cultivated (at room temperature) on ash leaf malt agar plates for 3 weeks. Subsequently, 17 ash wood pieces (0.5 mm × 5 mm × 5 mm) previously autoclaved in tap water were added to each agar plate on top of the fungal mycelium and cultivated again for another 3 weeks. At the end of August 2020, the colonised wood pieces (showing a black, pseudosclerotic layer on it) were then individually inserted into a superficial sterile (cambium) cut (0.5 mm deep × 4 mm wide × 6 mm high) on the main stem of ash replicates and sealed with parafilm. Six replicates of each ash genotype were used for the infection. Five trees were infected with the overgrown wood chip, and one was used as a negative control infected with a sterile wood chip previously placed on ash leaf malt agar without fungus for 6 weeks. The experiment was conducted in the Biosafety Level 3 greenhouse (WSL, Birmensdorf, Switzerland) at a temperature range of 20°C–25°C and a humidity range of 50%–75%.
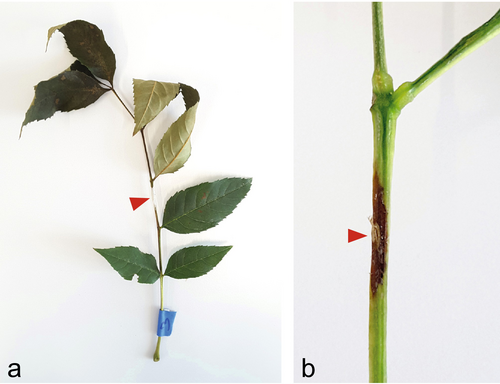
The proximal length of necroses was measured at the end of November (after 14 weeks) (Figure 2). The fungus was re-isolated on ash leaf malt agar to ensure that the observed lesions were caused by H. fraxineus. All samples with proximal necrosis length ≥ 0.5 cm were re-isolated. The isolates were morphologically grouped, and the groups showing high similarity to H. fraxineus were then genetically determined by sequencing the ITS region, according to Queloz et al. (2011).

2.4 Rachis Infections
From June to July 2020, rachis infection assays on leaves from the 12 ash genotypes were conducted following the method described by Orton et al. (2019). The trial took place in the Biosafety Level 3 greenhouse (WSL, Birmensdorf, Switzerland) at a temperature ranging from 20°C to 25°C and a humidity ranging from 50% to 75%. The grafted trees were introduced into the greenhouse 1 week prior to the infection, allowing them to gradually acclimatise to the new environmental conditions. Five replicates per ash genotype were used for the rachis infection assay. Three rachises distributed around the whole crown were infected on each ash tree replicate with the strain VQ_200217_8 and one rachis was used as a negative control (infected with a sterile ash leaf malt agar plug).
To prepare the infection assay, a 1 cm wide, 1 cm long and 0.3 cm thick piece of the H. fraxineus culture was cut out on ash leaf malt agar and placed in a sterile 1.5 mL tube. It was then crushed with sterile forceps and homogenised as well as possible. For inoculation, the upper surface of the rachis was carefully cut with a sterile scalpel (cut size approximately 0.3 mm depth and 5 mm length). The homogenised fungal mycelium (approx. 0.2 cm3) was carefully placed in the wound and closed hermetically with parafilm (for details, see Orton et al. 2019).
Inoculations were performed on 13 July 2020. The development of symptoms caused by fungal infection and spread was observed and recorded after 10, 18, 21, and 28 days. The classification of symptoms was based on five infection classes (0: no necrosis, 1: necrosis visible, 2: single wilted leaflets, 3: the tip of the leaf is dead, 4: the leaf has fallen off; Orton et al. 2019). These infection classes were used to calculate the Area Under Disease Pressure Curve (AUDPC), which is described in the following statistical analyses section. Additionally, at the end of the experimental period (28 days), the proximal length of the necroses on the rachis was measured (see Figure 3).
To be sure that the lesions were caused by H. fraxineus, 49 re-isolations on ash leaf malt agar were made from the resulting necroses of infected rachises. The isolates were morphologically grouped and the groups showing high similarity to H. fraxineus were then genetically determined by sequencing the ITS region, according to Queloz and Gossner (2019).
2.5 Spore Germination
Spore germination experiments were carried out in July 2020. The germination rate of H. fraxineus spores was measured on growing media made from leaf extracts of the grafted ash genotypes. Around 50–100 g healthy leaves from each ash genotype were sampled over at least 10 tree replicates. A cold extraction method was used to obtain ash leaf extracts in order to retain the potentially important metabolites that could inhibit fungal growth. The leaves were weighed and washed under deionised water. The leaves were then mixed with tap water at the ratio of two parts water to one part leaflets and mashed in a mixer (High-Performance Blender, Vitamix, Cleveland, USA). After coarse filtration through a filter paper with a pore size of 20–25 μm (Whatmann, Huberlab, Aesch, Switzerland) using a nutsche filter, smaller particles were filtered out with a pore size of 0.45 μm (Sartorius Spritzenvorsatzfilter Minisart NML, Huberlab, Aesch, Switzerland). This was followed by sterile filtration of the extract with a 0.2 μm pore filter (Sartorius Spritzenvorsatzfilter Minisart NML, Huberlab, Aesch, Switzerland) in a sterile bench. The leaf extracts were then added to an autoclaved malt-agar solution (35 g/L agar: Plant Propagation Agar, Laboratorios Conda S.A, Madrid, Spain; 37.5 g/L malt: DiaMalt, Hefe Schweiz AG, Stettfurt, Switzerland) with proportions of 60% leaf extract and 40% malt-agar (Brühwiler and Sieber 2021; Carrari et al. 2015). In order to mix it well, the extract was slowly heated up to 50°C in a water bath and added to the autoclaved malt-agar, which had previously been cooled to 50°C. For negative controls, simple water-agar (1.5% agar) was used. The prepared plates were stored at 4°C for 9 to 14 days until use.
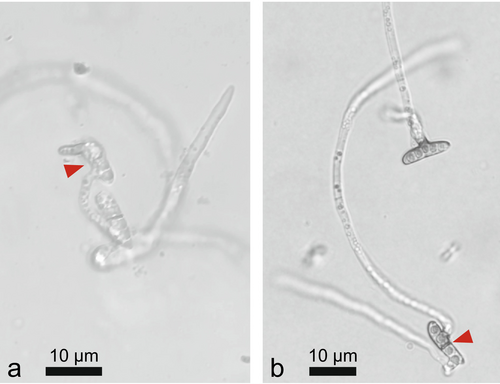
For spore production, in June, infected rachises (showing black pseudosclerotic layer) were collected in La Chaux-de-Fonds (NE; N 47.152544, E 6.854101) and Frauenfeld (TG, N 47.576431, E 8.878528) and kept on humid paper towels in big round sealed dishes (diameter 26 cm, height ca. 4 cm) at 20°C under UV and visible light (12-h-photoperiod) until fruiting bodies of H. fraxineus formed. Spores were collected as described by Schlegel et al. (2016). In brief, spores were captured using a water-agar Petri dish (15 g/L agar, deionised water, 90 mm diameter) moistened with 0.5 mL Milli-Q H2O. After spore collection, 3 mL Milli-Q water was added to each plate to bring the spores into solution. The spores present in the solution were counted using a Fuchs-Rosenthal haemocytometer, and the solution was then brought to approx. 100 spores/μL by centrifugation (1000 rcf). Approximately 100 spores (1 μL) were added to the prepared 0.4 mm diameter agar plugs of the leaf-extract agar (Brühwiler and Sieber 2021). Per ash genotype and rachis sample site for the spore production, three independent replicates and one negative control media were prepared. The inoculated agar plugs were incubated at 20°C for 72 h and then stored in the refrigerator at 8°C. The germinated spores were counted under the microscope (Figure 4).
2.6 Statistical Analyses
All analyses were conducted in R 4.2.3 (R Core Team 2023). Linear models (LMs) were fitted with R-base, and linear mixed effects models (LMMs) were fitted with the lme4 package v.1.1-32 (Bates et al. 2015). Robust LMMs were fitted using the package robustlmm v. 3.3-2 (Koller 2016). Fitted LMs and LMMs were subjected to type II analyses of variance (ANOVAs) with Kenward–Roger's method to produce a summary of the F- and P statistics (car package, v.3.1-2, Fox and Weisberg 2018). Response variables were log-modulus (John and Draper 1980) or square root transformed to meet the assumptions for normality and homoscedasticity. Beta-binomial models, which are robust against overdispersion, were fitted with the glmmTMB package (Brooks et al. 2017). All models were checked for multicollinearity with the performance package (Lüdecke et al. 2021).
First, the validity of ADB-stem lesion length as an ADB-resistance proxy was explored (Kowalski and Holdenrieder 2009; Lobo et al. 2015; McKinney et al. 2012). To this end, a LMM was used to explore the relationship between ADB-stem lesion length measured on trees in the infection assay and ADB-related crown defoliation category measured on field trees. The lesion length of individual trees was used as a response variable, whereas crown defoliation category, rootstock provenance (Arni/Tägerwilen), and tree DBH (Diameter at breast height) were included as fixed model effects. DBH was included in the model because of the observed significant negative correlation of DBH and the severity of the disease in the forest (Klesse, Abegg, et al. 2021). Individual tree replicates nested in tree genotypes were modelled as random intercepts since multiple lesion length values per tree were available.
For the statistical comparison of the rachis infections with other methods, the Area Under Disease Pressure Curve (AUDPC) was calculated for each rachis according to the disease progression test (Jeger and Viljanen-Rollinson 2001) with R-script information from the American Phytopathological Society (2024). The infection classes of the rachis and time points (days) for calculating the AUDPC are mentioned above in the section on the rachis infection. The aim of this approach is to calculate the integral progression of leaf infection to obtain a continuous variable that can then be compared with the crown defoliation category and the stem lesion length.
The crown defoliation category and the mean stem lesion length for each genotype measured at the end of the experiment were compared against three alternative ADB-resistance proxies: (i) mean length of ADB-caused rachis lesions measured on each genotype, (ii) the mean AUDPC value per genotype and (iii) ADB spore germination rate.
LMs were used to test the effects of ADB-stem lesion length and DBH on either ADB-rachis lesion length or AUDPC. For the LMs, the mean stem lesion lengths (± SE) were calculated from one subset of trees per genotype, while the mean rachis lesion lengths or AUDPC (± SE) values were calculated from different subsets of trees per genotype. Since different tree subsets were used, tree individuals could not be modelled as random intercepts. A LMM or a robust LMM was used to model the relationship between either ADB-rachis lesion length or AUDPC as response variables and crown defoliation category, rootstock provenance, and tree DBH as fixed effects. Individual trees were modelled as random intercepts. A mixed effects model with beta-binomial distribution was used to explore the relationship between ADB-spore germination success as a response variable and stem lesion length or crown defoliation category and tree DBH as fixed effects. The ash genotype was modelled as a random intercept since multiple spore germination values were measured per genotype.
3 Results
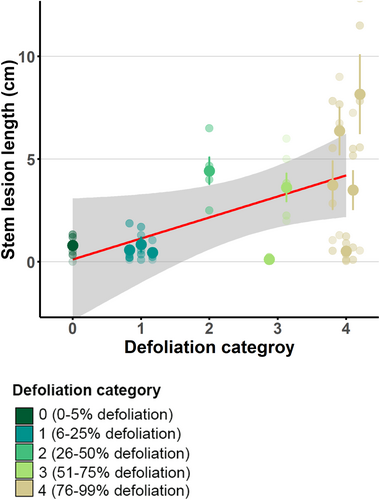
The genotypes tested were distributed across all crown defoliation categories in the field assessment. One genotype was assigned to category 0 and three to category 1. These genotypes can be considered “healthy” from a forester's point of view. As our selection focused mainly on healthy and susceptible ash genotypes, only one tree fell into category 2, a moderately defoliated genotype. Finally, two and five genotypes fell into categories 3 and 4, respectively. Categories 3 and 4 represent susceptible ash genotypes and were slightly overrepresented in our study. This can be explained by the higher grafting success of susceptible ash trees, which tend to produce epicormic shoots that are easier to graft (Oliver Sheridan, personal communication, data not shown).
The measured stem lesion lengths varied between 0 to 22 cm 3 months after infection. The reisolation of H. fraxineus was successful in 60% of cases (18 out of 30 isolation attempts revealed to be H. fraxineus). The measured rachis lesion lengths varied between 0 and 8 cm after 1 month. In the case of rachis infection, the reisolation rate of H. fraxineus was 21 out of 23 (91%). Comparing the resistance assessments of the ash genotypes using both stem and rachis infections, we observed a strong genotypic variation (stem lesion length: variance 6.99 ± 2.64 SD, rachis lesion length: variance 0.39 ± 0.62 SD).
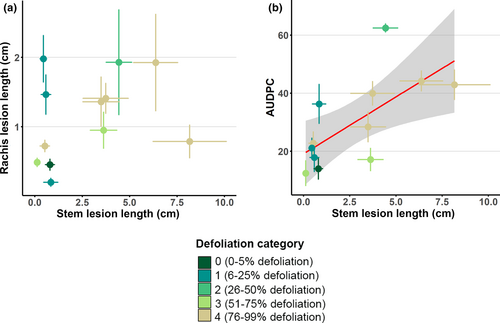
A significant linear correlation (p = 0.039) was found when comparing the results from the stem infection tests and crown defoliation categories (Figure 5). However, no significant correlation was found when comparing the rachis lesion lengths (p = 0.387), the AUDPC (Area Under Disease Pressure Curve, see methods, p = 0.115) and the spore germination rates (p = 0.147) with the crown defoliation categories observed in the field. Based on these results and the fact that stem infection is the most commonly used method for pathological testing in ADB, all the resistance assessment methods used were subsequently directly compared with stem lesion lengths. No significant correlation was found between the ADB stem and rachis lesion lengths (p = 0.132; Figure 6a). In contrast, a significant correlation between stem lesion lengths and the AUDPC for the rachis infections (p = 0.006) was detected (Figure 6b).
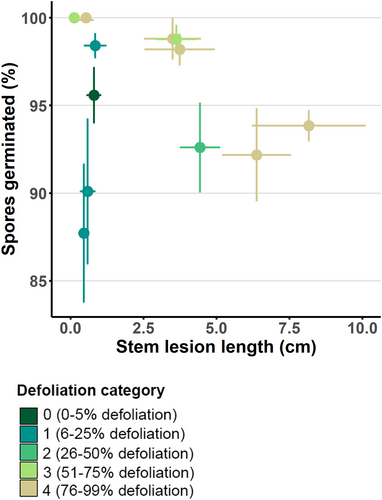
There was no significant relationship between the ADB stem lesion lengths and the spore germination rates (p = 0.191; Figure 7). All spores were germinated at high rates (95.5% ± 6.2%) after 72 h, so the maximum difference in mean spore germination rates between ash genotypes is not more than 12%. The germination rate on water agar, used as the negative control, was 98.2% ± 2.3%.
In summary, of the three resistance assessment methods tested under in vitro conditions on the same ash genotypes, two proved to reflect crown defoliation observed in the field (Table 3). The stem lesion lengths correlated with the field assessments considering crown defoliation, and the AUDPC of the rachis infections correlated strongly with the stem lesion lengths.
| Crown defoliation | Stem infection | ||
|---|---|---|---|
| Crown defoliation | — | P = 0.039, R-squared = 0.61 | |
| Rachis infection | Lesion length | P = 0.387 | P = 0.132 |
| AUDPC | P = 0.115 | P = 0.006, R-squared = 0.53 | |
| Spore germination | P = 0.147 | P = 0.191 |
- R-squared values (conditional for mixed-models) are provided for significant p-values (bold), that is, p ≤ 0.05.
4 Discussion
This study compared three in vitro methods for testing the resistance of ash trees to ADB. Stem lesion lengths and AUDPC of the rachis infections proved to be the most suitable measures of ADB resistance of ash genotypes. The advantages and disadvantages of the methods tested are summarised in Table 4 and will be discussed in detail in the following.
| Infection assay | Time required | Execution | Correlation with the stem lesion length | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Stem infection | 6–8 months | Grafted ash trees (in vitro) | — |
|
|
| Rachis Infection | 2–3 months | Grafted ash trees (in vitro) | Yes (only the AUDPC) |
|
|
| Spore germination | 1–2 months | Leaves of grafted ashes (in vitro) | No |
|
|
4.1 Crown Defoliation
Besides mortality, crown defoliation is the most commonly used parameter for monitoring disease progression and a fast method to search for resistant ash trees in the field (Enderle et al. 2018; George et al. 2022; Klesse, Von Arx, et al. 2021). Defoliation is measured by an estimate of the percentage of branches that no longer have leaves. In our study, we used six crown defoliation categories to determine the severity of ADB (slightly modified from Lenz, Pöllner, et al. 2012a; Lenz, Straßer, et al. 2012b). However, consistent crown defoliation assessments in the field are rather difficult to reach because they are highly dependent on the local conditions and on the experience of the observer (Dobbertin et al. 2004). In our case, crown defoliation was always assessed by the same person during a short period in summer, so we assume that the influence of the observer may be minimal.
However, we observed few genotypes that appeared to be healthy in the field but showed a low level of resistance in vitro and vice versa. One reason for this discrepancy between field and laboratory observations may be attributed to the fact that environmental conditions also influence crown defoliation in the field. In a favourable environment for tree growth, trees with the same infection status are likely to look healthier than in conditions where the trees are stressed and also fungi other than H. fraxineus will find favourable conditions to grow (Burns et al. 2022; Klesse, Abegg, et al. 2021). This means that a significant disadvantage of crown defoliation in the search for resistant trees is that the phenotype of the tree does not always match the genotype of the tree in terms of resistance to ADB.
4.2 Stem Infections
Stem infection assay is the most commonly used in vitro method to test for resistance of an ash genotype against H. fraxineus. In order to obtain tree replicates (clones) for in vitro resistance testing, scions of the ash genotype of interest must first be grafted. It usually takes more than a year for the stress of grafting to be overcome (Oliver Sheridan, personal communication) and for the trees to be large enough for the experiments. Once the ash tree replicates have been infected, stem lesions require 3–12 months to develop (Gross and Sieber 2017; Kowalski et al. 2015; Kowalski and Holdenrieder 2009; Lobo et al. 2014). Therefore, stem infections are the most time-consuming in vitro method for testing ash resistance. However, the finding that stem lesion lengths correlate strongly with crown defoliation categories of trees observed in the field favours this method.
4.3 Rachis Infections
The rachis infection assay focuses on the leaves and rachises of ash trees. Among the three tested methods, this approach most closely mimics the infection pathway, as infections in the field occur primarily through the leaves (Gross et al. 2014). Inhibiting the growth of the fungus on or in the leaf could prevent the fungus from spreading to the shoot, thus preventing entry into the tree's permanent woody tissues. Rachis infection assays, as described in this study, were not often used to test the resistance of ash genotypes and species to ADB. Natural infections of leaves in an orchard or in the greenhouse are more commonly used (Cleary et al. 2013; Nielsen, McKinney, Hietala, and Kjær 2017). However, it is difficult to make a quantitative assessment of the progression of infection by determining the exact time and the starting point of infections by natural infections. In contrast, this is possible with a targeted infection of the rachis in the laboratory. The length of the necrosis was usually measured at the end of such an experiment (Kowalski et al. 2015; Kowalski and Holdenrieder 2009; Nielsen et al. 2022; Orton et al. 2019). With this method, many replicates can be carried out on a single tree and the duration of the experiment is only about 2 months. However, the length of the necrosis must be measured before leaf shedding. Otherwise, the leaves will dry out and turn brown so that the necrosis caused by the fungus can no longer be distinguished from dried tissues. Another difficulty with this method is that it is sometimes difficult to distinguish the necrotic tissue from the healthy tissue due to the texture and small size of the rachises.
To properly quantify the progress of the infection, a method called the “Area under disease pressure curve (AUDPC)” was used. It is a mathematical model that is often used in agriculture for determining the resistance of plants against fungal pathogens (Bräunlich et al. 2021). It has never been used before in literature for analysing the rachis infection of ash trees. Nevertheless, it is an easy-to-obtain continuous variable for assessing the disease progression and severity of the rachis infection. In contrast, the rachis lesion length measured at the end of the experiment is only a snapshot of the severity of the infection at a given time and probably does not reflect reality. The results of our study confirm this statement. No correlation was observed between rachis lesion lengths and stem lesion lengths, but AUDPC values were strongly correlated with stem lesion lengths. To conclude, rachis infection assays can be informative if AUDPC values are analysed.
4.4 Spore Germination Rates
Several studies also focus on the inhibitory effect of ash leaf extracts against the fungal growth of H. fraxineus (Carrari et al. 2015; Nemesio-Gorriz et al. 2020; Vemić et al. 2024). Most of these studies measured the mycelium growth on agar plates. They detected differences in the growth rate of H. fraxineus between agar plates amended with leaves of different tree species. In other studies, the germination rate of fungal spores on leaf extract agar provided evidence for inhibitory or stimulating metabolites in the leaves for different ash species or genotypes. Therefore, the germination of H. fraxineus spores was tested on different media based on ash leaf extracts (as described in Brühwiler and Sieber 2021; Schlegel et al. 2016). It is a much faster method than the stem or rachis infections. The method is not destructive, as only a few leaves are required.
However, the biggest challenge of this method was to synchronise the production of culture media and spore solutions. In general, it should be easy to collect rachises with fresh fruiting bodies from surrounding forests, but long, hot, and dry summers prevented fruitbody formation for several consecutive years in Switzerland. We were, therefore, forced to produce fruiting bodies in vitro (see methods). During the 2020 trials, the fruiting bodies developed very slowly, and it was difficult to predict when they would sporulate. As a result, the prepared culture media with leaf extracts were stored in the refrigerator for several weeks before the spores were harvested and the experiments carried out. This may have led to the evaporation or conversion of specific metabolites from the agar, which could have had inhibitory or stimulating effects on spore germination (Brühwiler and Sieber 2021). Another challenge was to measure the germination rate exactly 72 h after inoculation. As it took around half an hour to count the germinated spores of one sample under the microscope, some of the samples had to be stored in the fridge overnight at 8°C before being measured.
4.5 Comparison of the Methods
The stem lesion length measurements correlated strongly with the field assessment of crown defoliation. This seems logical as crown defoliation is usually related to multiple stem infections that can be seen from a distance. This result is supported by several other studies that have also found a correlation between crown defoliation and stem lesion lengths (Lobo et al. 2015; Vemić et al. 2021; Wiersma et al. 2022). However, no significant correlation was found between crown defoliation categories and rachis infection measurements. This suggests that the severity of ash dieback is primarily linked to stem rather than leaf susceptibility.
We also found a strong correlation between the stem lesion lengths and the AUDPC for rachis infections, while there is no correlation between the stem lesion lengths and the final rachis lesion lengths. This indicates that AUDPC is a better measure of ash resistance than rachis lesion length because it reflects the progression of infection throughout the experiment rather than taking just a snapshot at the end. No genotype showed a low rachis infection (AUDPC) rate but showed long stem lesions. This supports the hypothesis that inhibiting fungal growth from the leaf to the branch is an important part of the resistance mechanism (Gross et al. 2014; Nielsen et al. 2022). Comparing stem lesion lengths with crown defoliation, there were some trees with a higher crown defoliation category that performed well in the stem infections assay (no or short lesions). This and also the fact that rachis infection measurements are not correlated to the crown defoliation categories refer to the assumption that crown defoliation categories must be verified in a laboratory setting, where all environmental conditions are the same and a genetic link can be determined. Also, the spore germination rates do not correlate with stem lesion lengths or crown defoliation categories. This could be due to a methodological problem, as described above. Alternatively, the inhibition of spore germination may not be important for tree resistance against H. fraxineus, since the fungus is described as an endophyte or a harmless leaf pathogen in its native range (Nielsen et al. 2017).
5 Conclusions
Our results suggest that the most consistent and useful method for resistance testing of ADB remains the stem infection assay, despite its time-consuming nature. Our results also indicate that there are only a few ash tree genotypes that show resistance to ADB in vitro but may be overlooked if only crown defoliation categories are monitored in the field. Conversely, some ash tree genotypes show no resistance in vitro but have a healthy crown in the field due to environmental factors or other influences. It is therefore important for the conservation of ash that some trees with moderately damaged crowns are not excluded or harvested too early. Resistance mechanisms of ash are not entirely understood, and natural selection takes time to become effective in the forest.
Acknowledgements
Our special thanks go to Oliver Sheridan, Gabor Reiss, Ludwig Beenken, Flavia Mäder, Jolanda Klaver, and Mario Sahli for their help with the grafting of the ash genotypes. We would like to especially thank the trial garden team at the WSL Birmensdorf for harvesting the ash scions and letting us use their tools and facilities. Also, many thanks are going to Irina Vögtli and Maurice Moor for their advice and help in the lab. We also want to thank the Swiss Federal Office for the Environment for their financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




