Epicuticular wax content of western white pine is involved in Cronartium ribicola resistance
Abstract
The introduction of Cronartium ribicola, the causative agent of white pine blister rust, has been devastating for white pines in North America, including western white pine. Among the observed partial resistance responses to white pine blister rust is a mechanism referred to as difficult-to-infect (DI), which is characterized by lower frequency of infection spots on needles and a lower incidence of branch and stem cankers than susceptible host plants. Parents with the DI trait were selected and bred to produce nine full-sib families. Progeny were propagated and cloned using in vitro techniques in controlled environmental conditions. Explants were inoculated with a single strain of C. ribicola and characterized by using a disease assessment index (DAI), which was used to compare DI full-sib families to several other full-sib families (from Idaho) selected for other partial resistance traits. Most DI families had significantly lower DAI scores and higher epicuticular wax content. When the wax was removed from the surface of needles, the DAI of all full-sib families increased. Scanning electron microscopy revealed that stomata in DI full-sib families are mostly occluded by epicuticular wax. The results of this study suggest that the DI resistance mechanism of western white pine is related to needle surface epicuticular wax, which likely hinders the penetration of stomata by fungal hyphae. The occurrence and magnitude of this trait in the breeding population remains unknown, and special assessment technique for large-scale screening will have to be developed.
1 INTRODUCTION
White pine species, such as eastern white pine (Pinus strobus L.), western white pine (P. monticola Dougl. ex D. Don), and sugar pine (P. lambertiana Dougl.), were historically among the most valuable timber species in North America. In the 19th century, massive harvesting of white pines and high demand for stock for replanting spurred significant nursery imports from Germany, France, and the Netherlands. These importations continued until a quarantine was established in 1914 following the introduction of Cronartium ribicola J.C. Fisch.in Rabh the causal agent of white pine blister rust (WPBR). White pine blister rust probably originated in Central Eurasia (Hunt, 2009). The primary infection sites for C. ribicola are the stomata of needles. The infection spreads from needles down the twig and into the tree stem, then girdling cankers finally develop on the main stem and mortality occurs. Although it has a complex life cycle involving five different spore stages, alternate hosts, and exacting environmental conditions, C. ribicola has proved remarkably successful in colonizing most of the native ranges of white pines (Geils et al., 2010; Kinloch, 2003). Many control efforts have been and continue to be, applied (Geils et al., 2010; Zeglen, 2010), of which genetic approaches have been most successful to the management of white pine blister rust (King et al., 2011). Large-scale screening and breeding trials for resistance have been conducted in the United States and Canada (Bingham, 1983; Hunt, 2009; King & Hunt, 2004; Kinloch & Davis, 1996; McDonald et al., 2004; Sniezko & Kegley, 2003) and in Eastern North America (Daoust & Beaulieu, 2004; Lu & Derbowka, 2009; Patton & Riker, 1966). Examples of both qualitative and quantitative resistance have been discovered during these screening programs. Vertical resistance (also known as R-gene resistance), which provides high levels of resistance based on a clearly defined hypersensitive response, is an example of the classic gene-for-gene system, which is common in rust diseases and as such, is susceptible to breakdown (Flor, 1946, 1971). The host genetics in the R-gene complex and corresponding virulence in C. ribicola are now well described for both sugar pine and western white pine (Kinloch Jr., 1992; Kinloch & Dupper, 2002; Kinloch Jr., Sniezko, & Dupper, 2003, Kinloch Jr., Sniezko, & Dupper, 2004). Most of the effort over the decades, though, has been devoted to screening for partial resistance, often broadly categorized as slow rusting resistance or low-level resistance, as R-gene resistance is relatively rare and found mostly in the more southern populations of white pine species (Kinloch Jr., Sniezko, & Dupper, 2003). Partial resistance refers to the ability of host plants to, in some way, limit rust development or tolerate infection without entirely excluding the pathogen. Various seedling response categories were noted through the inoculation and screening process which include slow canker growth, difficult to infect, needle-shed, and various categories of bark reaction (Hunt, 2004b). Most of these seedling response categories are presumably multigenic, although some, such as needle shed, may be controlled by individual recessive genes (Hoff & McDonald, 1971; Mahalovich, 2005).
The western white pine breeding program in British Columbia began by screening progeny of selected parent trees in controlled inoculation experiments. Several qualitative and quantitative resistance traits have been described over time (Yanchuk et al., 1994; King et al., 2010), and because of the difficulty in assessing any of the many quantitative mechanisms at play, we now generally score some genotypes as “difficult-to-infect” (DI), which is characterized by seedlings with little to no infection spots on needles, when assessed the following springtime after inoculation (Hunt, 2004). Given that symptoms of infection are absent from the needles of inoculated seedlings, the DI mechanism of resistance might be at the leaf surface. For instance, Smith et al. (2006) reported that epicuticular wax played a role in the resistance of some genotypes of eastern white pine (Pinus strobus) to white pine blister rust and showed that removal of wax from the surface of needles of resistant genotypes resulted in infection levels that resembled susceptible genotypes. The constitutive (passive) physical barrier is an intrinsic property of the plant (i.e., here, a physical barrier of wax across the stomata).
Interestingly, Woo et al. (2001) characterized resistant and susceptible families of western white pine based on a resistance trait that was phenotypically similar to the DI trait. They measured several needle morphology traits and found no difference in the occlusion of stomata by epicuticular wax of resistant and susceptible groups. They found that stomata of the susceptible group were wider and greater in area than the resistant group. Without invoking all the different means a plant in natural field screening sites can avoid infection by constitutive properties, scoring trees as ‘escapes’ (i.e., no spores landing near the stomates), it is unlikely these plants have escaped infection due to the heavy spore loads used in artificial inoculations. Moreover, there are considerable challenges in screening these low frequency ‘traits’, in their natural sites. These challenges include genetic variation in both the host and the pathogen, variation in the characteristics of resistance symptoms among inoculated plants (Hansen & Patton, 1977; Jurgens et al., 2003; Kinloch & Davis, 1996), variation in susceptibility of needles of different ages (Patton, 1961; Hunt, 2004a), large enough populations to get meaningful genetic parameters, the environmental variation where the plants were raised (Woo et al., 2002), and various endophytes in leaves of white pine have been shown to confer some resistance (Ganley et al., 2008). To overcome some of these challenges in characterization of resistance mechanisms, we investigated the use of clonal replication using in vitro techniques. In vitro screening techniques use somatic tissues or organs (explants) for controlled disease evaluation. To study mechanisms of resistance, we used wax removal techniques and electron microscopy to investigate characteristics of epicuticular wax and its impact on stomata's morphology in our resistant families, as utilized by previous researchers (Smith et al., 2006). These techniques can offer a powerful alternative for defining specific types of resistance vs. avoidance and identifying valuable resistant genotypes (Klimaszewska et al., 2007; Noshad et al., 2007; Park & Klimaszewska, 2003).
In this study, we used in vitro clonal propagules from the progeny of F1 crosses that were created in the British Columbia western white pine blister rust resistance breeding program to identify the underpinning resistance and to better understand related resistance mechanisms with special reference to epicuticular wax. Two sets of crosses were used: one set with parents that had been identified as ‘difficult-to-infect’ or DI (Hunt, 2004b), and another set created using parents that originated from the Idaho breeding program and were selected for high level of some partial resistance traits. Our primary objectives were as follow: (1) develop an efficient and consistent in vitro method for evaluating resistance or avoidance of western white pine to C. ribicola; (2) screen western white pine for resistance to C. ribicola using in vitro techniques in a controlled environment (i.e., to identify the role of wax content in these resistance mechanisms), and (3) investigate plant–pathogen interaction and better understand resistance mechanisms in the white pine trees with DI partial resistance trait using axenic techniques.
2 MATERIALS AND METHODS
2.1 Test population
British Columbia's western white pine breeding program for resistance to C. ribicola is composed of two sub-programs: the ‘coastal group’ and ‘interior group’ based on the geo-referenced area in the province. Initially, it was estimated that one out of six trees with resistance phenotypes (i.e., canker free) in natural stands would yield useful genetic resistance, so a target of 300 candidates was selected to yield 50 parents for each breeding program (Hunt, 2004b). In total, progeny from 507 parent trees selected in in 1983 British Columbia (291 and 216 for the coastal and interior programs, respectively) were screened by the joint effort of the Canadian Forest Service and the Ministry of Forests, Research Branch, over an approximate 15-year period. Progeny of these 507 parent trees were screened in controlled environments and field trials to identify resistance and tolerance trees against WPBR (Hunt, 2004b).
Furthermore, in collaboration with the USDA Forest Service, 50 trees were selected from a 17-year-old full-sib family screening trial at the Priest River Experimental Station in Idaho, to increase our selection intensity for resistance to WPBR in the ‘interior’ group. These full-sib families were created by crossing parents from the first generation that was selected for resistance to blister rust (Bingham, 1983, Yanchuk et al., 1994, Hunt, 2004b) (Table 1). Full-sib crosses were made between coastal parents that were ranked high for the DI partial resistance trait based on results from controlled inoculation experiments of open-pollinated (OP) families (Carlson, 2010; McDonald et al., 2004). The experimental material used in this research project comprised of 15 full-sib families: nine coastal DI full-sib families and six interior full-sib families. (Table 1). One additional full-sib family that was known to be highly susceptible to blister rust infection, included as positive control for comparison (total = 16 families).
| Female\male parents | 65 | 145 | 252 | 394 | 408 | 461 | 1406 | 1407 | 1417 | 1424 | 1427 | 1447 | 1448 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 65 | DI | DI | DI | ||||||||||
| 145 | DI | DI | |||||||||||
| 252 | DI | ||||||||||||
| 394 | |||||||||||||
| 408 | DI | DI | |||||||||||
| 461 | DI | ||||||||||||
| 1406 | SRxSS | ||||||||||||
| 1407 | CD | CD | |||||||||||
| 1417 | SSxSS | ||||||||||||
| 1424 | |||||||||||||
| 1427 | |||||||||||||
| 1447 | CD | ||||||||||||
| 1448 | SSxSS |
2.2 In vitro clonal propagation
The seed from all 16 full-sib families was disinfected by immersion in 70% w/w ethanol (ETOH) for 30 sec and in 0.5% sodium hypochlorite for 20 min, followed by 48 h in running tap water and finally four to five rinses in sterile distilled water. Seeds were stratified for 6 weeks in moist cheesecloth at 4°C and were transferred to potting medium (3–1-1peat-vermiculite-perlite) with an additional 400 g of a low rate Osmocote® (18–6-12) per kg of potting medium. Seedlings were transferred to larger pots every 6 months and were sprayed once with Funginex® (Triforine; at a rate of 2 ml per litre). Apical shoot buds, 2–3 cm in length, 1-3 mm in diameter were collected during the spring season (these are referred to as micro-shoots). Collected micro-shoots were rinsed under running tap water for 15 min. Under aseptic conditions, micro-shoots were then surface sterilized by treatment with 70% ETOH for 30 s, then 0.5% NaOCl solution containing 0.5 ml per litre for 1 minute; and then in 5% Tween 20 (Sigma Chemical, St. Louis, MO) stirred for 10 min. After surface sterilization, micro-shoots were rinsed in sterilized water for 5 min separately and established in medium. Cultures were placed at 25°C under 16/8 h photoperiod with irradiance intensity of 350μWcm−2 supplied by cool white fluorescent lamps. Following this disinfection procedure, culture contamination rates were below 10%. Individual micro-shoots (approximately 1 cm long) were sectioned, and each micro-shoot placed in individual 25 × 150 mm test tubes containing approximately 25 ml of medium. Several base media were tested: LS (Linsmaier & Skoog, 1965), MS (Murashige & Skoog, 1962), WPM (Lloyd & McCown, 1981), B5 (Gamborg et al., 1968), GD (Gresshoff & Doy, 1972), and SH (Schenk & Hildebrandt, 1972). All base media were supplemented with 20 g l−1 sucrose, and 5.6 g l−1 agar (Sigma-Aldrich). The pH for various media was adjusted based on the respective protocol before autoclaving. Micro-shoots were maintained on establishment media with no plant growth regulators (PGR) for 5 weeks before subculture on multiplication media (base media with PGR). Following a five-week establishment period, new micro-shoots were sub-cultured into 150 ml sterilized jars (Sigma-Aldrich, St. Louis, MO) containing 25 ml of multiplication media. Micro-shoots were harvested from stock cultures, cut into nodal explants 3–5 cm long and placed vertically into multiplication media containing basal salts, and 5.6 gL−1 agar (Sigma-Aldrich, St. Louis, MO). Based on the explant's growth rate during the establishment phase, they were sub-cultured on WPM- and GD-based media. Various concentrations of PGRs (e.g., 6- benzyl aminopurine (BA), kinetin (KIN), and thidiazuron (TDZ)) were used alone or in combination with different concentrations of indole-3 acetic acid (IAA), indole 3- butyric acid (IBA), or 1–naphthaleneacetic acid (NAA).
2.3 Pathogen culture and inoculation
Five distinct single-spore isolates of C. ribicola (from one single pustule; each from a different site) were collected from different western white pine experimental sites in coastal and interior British Columbia, Canada. For this study, the most aggressive isolate of C. ribicola (based on our preliminary experiments) was chosen from a severely diseased tree in our full-sib family experimental site at Coombs, BC. We used this isolate to infect our Ribes nigrum L. plants (an alternate host of the pathogen). Basidiospores collected from infected leaves of ribes were used for inoculation of micro-shoots.
A suspension solution of basidiospores was prepared based on the methods outlined by Kinloch and Dupper (1996). The resulting suspension solution was twice filtered through two layers of cheesecloth to remove telia and other residues. The spore suspension solution was calibrated with a haemocytometer and adjusted with de-ionized water to obtain a final concentration of 1 × 104 ml−1prior to inoculation; a specific amount of inoculum (100 μl droplet) was applied per needle to avoid any ‘overexposure’ or ‘underexposure’ of plants to the pathogen. Experimental micro-shoots were inoculated with basidiospores suspension solution. Negative control micro-shoots were inoculated with only distilled water. Symptoms were evaluated 3 weeks after inoculation (Figure 1). Symptoms including typical bright yellow spots that form and enlarge on needles were recorded (Kinloch & Dupper, 1996).
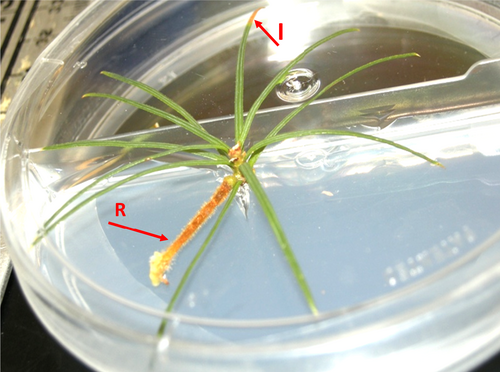
2.4 Disease assessment index
The relative susceptibility of ‘difficult-to-infect’ (DI) full-sib families was tested by controlled inoculations of the experimental micro-shoots in vitro as mentioned above and assessed using a disease assessment index (DAI). Forty seedlings were selected from each family, and five explants (clones) were created for each seedling for a total of 3200 explants (200 explants per family) available for inoculation. For each replication, one explant out of the five was selected from each seedling. A selection of explants was used as negative controls (i.e., negative control explants were treated identically except for the application of distilled water in place of pathogen suspensions).
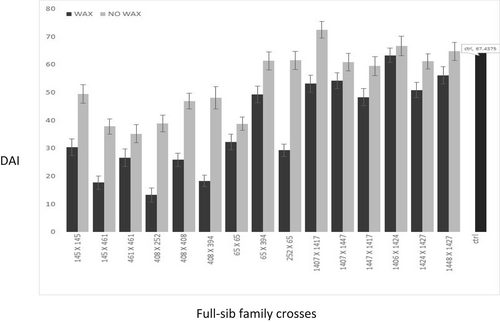
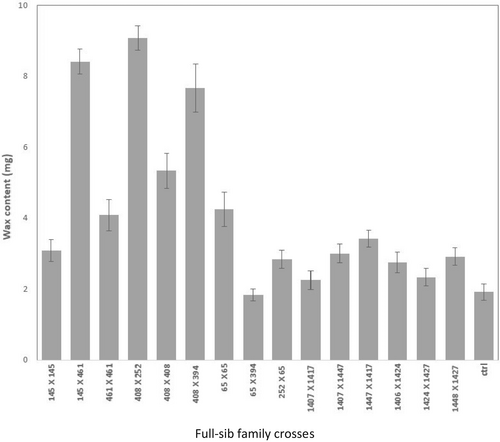
Data were recorded after 8 weeks. One-way analysis of variance was undertaken for the various treatments mentioned above with the ‘aov’ function in R (R Core Team, 2019) and full-sib family means for the various traits are presented, with standard errors, for graphical purposes in Figure 2, Figure 3 and Figure 4.
2.5 Wax content experiment
To test how wax content might affect this resistance mechanism, gravimetric comparisons of epicuticular wax content from needles of 12 seedlings from each of the 16 white pine full-sib families were completed using the protocol of (Smith et al., 2006) with slight modifications. Wax was removed by placing the needles from the explants (1 gr of needle tissue from a few explants per seedling was measured) in pre-weighed 5-ml glass tubes filled with chloroform. After 2 min, the needle tissue was removed, and the tubes were placed in a fume hood for evaporation. After 24 h, the tubes were reweighed, and dry weights were calculated.
2.6 Wax removal experiment
We examined the importance of wax on infection rate by removing wax from 12 seedlings per full-sib family and C. ribicola inoculation was done after explants were treated to remove epicuticular using the Smith's protocol (Smith et al., 2006). Five explants were created for each seedling for a total of 960 explants (60 explants per family) available for inoculation. Control explants were inoculated without removing wax for each explant. Sterile Q-tips were used to swab needle surfaces with a 50% (vol/vol) solution of chloroform (in 95% ETOH). Immediately after brushing the needle surfaces with the Q-tip, the explants were rinsed in water to remove any excess chloroform. After plants were treated, they were sub-cultured into 150 mm jars (Sigma-Aldrich, St. Louis, MO) and held for a week at 15°C until they were inoculated. Inoculation with C. ribicola followed the protocol described by Kinloch and Dupper (1996). After 8 weeks, in vitro grown plantlets were evaluated for symptoms, and the DAI scores were calculated for each family.
2.7 Stomata morphology experiment
To investigate the morphological differences between DI and susceptible control explants, we conducted microscopic studies. Needles from several DI and susceptible families (approximately 12–15 needles per family) were compared using both scanning electron microscopy (SEM) and environmental scanning electron microscopy (ESEM). Stomata were considered occluded if there were no gaps in the wax plug greater than five μm. Spore germination and possible stomatal penetration differences between P. monticola families were observed visually using electron microscopy. Several needles were collected from each family and transferred to ESEM stubs with a thin layer of finger-nail polish to hold samples in place. The samples were studied in each predetermined field of view (five per sample).
2.8 Electron microscopy
To study mechanisms of resistance in more detail, needles from our experimental micro-shoots were prepared for electron microscopy. We collected the needles (from both inoculated and controlled micro-shoots) after 4 weeks. Then needles were cut under fixative (3% gluteraldehyde in 0.05% phosphate buffer) into 1 cm pieces with a razor blade on a sheet of dental wax. Fixation was achieved by placing needle sections in 3% gluteraldehyde in 0.05 M phosphate buffer for 90 min. Samples were then washed in 0.05 M phosphate buffer six times for 15 min each. After the last wash, post-fixation was attained with 2% osmium tetroxide in 0.05% phosphate buffer for 2 h. Following fixation, needle fragments were dehydrated in an ethanol series of 10%, 30%, 50%, 70%, 95%, and 100% for 5 min each followed by ethanol /acetone (1:1) for 5 min. Prior to drying, samples were immersed in 100% acetone for 5 min, followed by 30 min in propylene oxide. Needle fragments were then dried using a critical point dryer (model 28,000; LADD Research Industries) utilizing CO2 as the transitional fluid. Samples were mounted on 25 mm aluminium stubs using double-sided tape and coated with gold/palladium using a Technics Hummer V coater (San Jose) and viewed on a JEOL JSM-35C scanning electron microscope equipped with an Orion 5.26 digital image capture system. After coating, samples were stored in a desiccator. For confirmation of results, samples for the environmental scanning electron microscope (ESEM) study were fixed in FAA (85 ml of 70% ETOH, 10 ml of glacial acetic acid, and 5 ml of 37% formaldehyde) for 24 h and were then transferred to 70% ETOH for 48 h. Samples were cut into 25 mm segments and air-dried for one to 2 min to allow the surface ethanol to evaporate. The sections were then mounted on aluminium stubs using double-sided carbon tape. Stomata were imaged using a Hitachi 3400 N Type II Variable Pressure Scanning Electron Microscope operating at 20 kV and 30 Pa.
3 RESULTS
3.1 In vitro propagation and inoculations
The in vitro screening methods were proven consistent over time and able to differentiate resistance. First, the concentration of basidiospores provided adequate pathogenicity to allow assessment of disease incidence and severity (i.e., displaying needle symptoms (Figure 1)), but did not completely overwhelm the defence mechanism(s) and prevent family differences from being displayed. One-way analysis of variance showed full-sib family differences were highly significant for severity (i.e., df = 15, F (family) values =20.99, Pr <2 e-16). Second, no negative control micro-shoot as well as no positive control micro-shoots developed any symptoms (inoculated with only sterilized water). These observations validate our screening methodology and support its continued use in identifying genetic variation among white pines for this resistance trait.
3.2 Disease assessment index
The DAI was also a reliable measure to quantify differences in family susceptibility to C. ribicola as there were significant differences between the susceptible control family and several DI families (Figure 2). The most resistant family was cross 408 × 252 (DAI = 15.9), and the most susceptible family was the control (DAI = 67.4).
3.3 Variation in wax abundance and its relationship with resistance
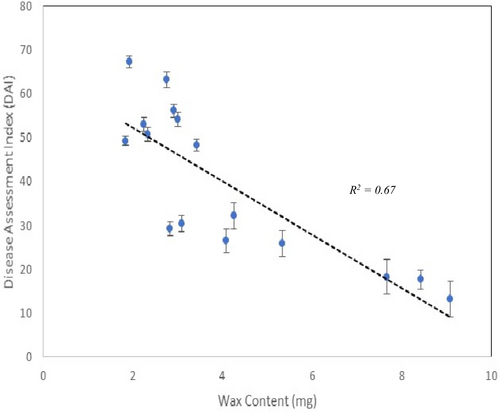
Our results indicated that some of the DI families (i.e., 145 × 461, 408 × 252, 408 × 394) had significantly higher wax content on their needles (means of 8.42 ± 1.20, 9.08 ± 0.77 and 7.66 ± 1.16, respectively) than the control susceptible ones (1.9 ± 0.39) (Figure 3). Moreover, families with higher wax content had lower DAI scores which signifies higher level of resistance against blister rust (Figure 4; R2 = 0.67).
3.4 Variation in stomata occlusion and its relationship with resistance
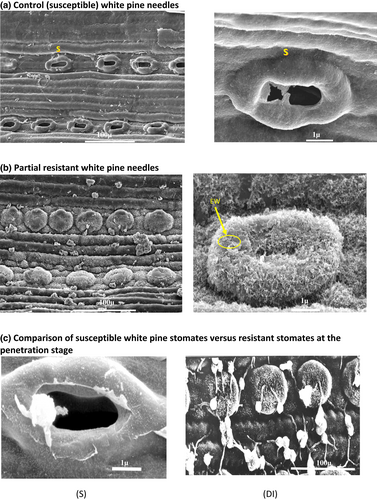
Several detailed SEM observations of epicuticular wax formation indicated that stomata of explants derived from DI families were predominantly occluded with wax in comparison to the susceptible families (Figure 5).
3.5 Impact of wax removal on disease levels
There were significant differences among families in wax content and generally DI families had a higher epicuticular wax (df = 15, F (family) values = 10.75, Pr < 2 e-16). In general, removal of the wax increased DAI in all of the families both DI resistant and susceptible (df = 15, F (family) values =2.4, Pr <0.004) (Figure 2 and Figure 3).
4 DISCUSSION
Trees in the BC white pine screening program that were considered to have better DI phenotype were exposed to blister rust basidiospores and generally showed fewer symptoms of infection (spots) on the needles than control plants. Using in vitro techniques and a quantitative index of infection, we were able to show that DI families exposed to basidiospores under controlled conditions displayed less symptoms than susceptible control families (Figure 2).
The DI partial resistance trait, in the small set of families that we tested, appears to likely prevent development of symptoms by interfering in the infection process on the surface of the needles. However, there may be other factors involved in this class of resistance, and they will require further investigation. Epicuticular wax content was quantified for each family to investigate the relationship between wax content and infection levels based on the DAI. We have shown through two methods that epicuticular wax likely plays a strong role in resistance (Figure 4). Scanning electron micrographs showed differences in the occlusion of stomata by epicuticular wax, which could possibly prevent penetration by blister rust hyphae (Figure 5). However, we did not measure penetration percentages in this study. Removal of epicuticular wax resulted in higher infection levels for all families (Figure 2), and these results suggest that epicuticular wax content, which prevents the penetration of fungal hyphae through the stomata, is one of the first and maybe the primary mechanisms of resistance associated with the DI phenotype in western white pine. We suggest that a role for epicuticular wax content in resistance to C. ribicola in western white pine is also similar to that shown for eastern white pine (Smith et al., 2006).
However, our results are in contrast with results reported by Woo et al. (2001) for a partial resistance mechanism called reduced needle lesion frequency in western white pine. They found no evidence that resistance was related to occlusion by epicuticular wax but made a note about differences in the size and shape of stomata between resistant and susceptible families. We did not measure the effect of shape and size of stomates in our study because of limitation of our resources. However, it could be possible the difference was also related to the families used in the Woo et al. (2001) study which were from Idaho, and we found that the Idaho families had lower wax content than our coastal families. How this resistance mechanism could have evolved in coastal populations is speculative, but could have formed due to higher disease pressure in wetter coastal areas than in drier interior regions.
Challenges in a typical naturally infected field screening program include variation in environmental conditions, inconsistent and uneven inoculation rates, possible spore loading far above normal levels on some trees (over exposure), variation in the genetic material of both host and pathogen, and variation in susceptibility because of tree age and needle type (Hunt & Jensen, 2001; Hunt, 2004b; Patton, 1961; Patton & Riker, 1966). These challenges can be partially overcome by using in vitro techniques. However, we acknowledge using in vitro techniques alone may present a higher level of susceptibility than field trials in screening results (e.g., we may miss some phenological aspects of screening), but they are powerful tools that can be used together with field trials and greenhouse tests to help researchers in characterization of resistance mechanisms.
In the current study, we showed that in vitro screening combined with a quantitative index method for characterizing infection rates can be used to successfully rank families in a way that is consistent with field results. Many other partial resistance mechanisms have been characterized by the many agencies involved in screening species for resistance to white pine blister rust (Yanchuk et al., 1994). Although this technique is not practical to evaluate the large numbers of families required for operational breeding programs, it is an excellent approach to investigate the mechanisms of resistance that underlie field observations for partial resistance traits especially in elite families going into seed orchards and advanced generation breeding.
ACKNOWLEDGEMENTS
The authors would like to thank Drs. Barbara Hawkins, Andy Benowicz, Michael Stoehr, Richard Hunt, and Michael Murray for their constructive comments. Also, we thank members of the University of Victoria microscopy lab especially Mr. Brent Gowen for their help and constructive comments.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.