A key effector, BxSapB2, plays a role in the pathogenicity of the pine wood nematode Bursaphelenchus xylophilus
Qun Zhao and Long-Jiao Hu contributed equally to this work.
Abstract
The pine wood nematode (PWN), Bursaphelenchus xylophilus, causes huge economic losses in pine forests. The plant-parasitic nematodes have a complex life cycle that includes the secretion of effector proteins through a stylet into the host cell to promote parasitism. In this study, SignalP 4.1 and TMHMM 2.0 were used in preliminary screens for candidate effectors and were expressed in Nicotiana benthamiana through the PVX virus expression vector. The yeast signal sequence trap system was used to further study the function of the signal peptide of an effector, BxSapB2. In situ hybridization was conducted to investigate the localization of BxSapB2, followed by RNA interference technology (RNAi) to assess the functions of BxSapB2. The results demonstrate that BxSapB2 is a secreted protein that induces cell death in N. benthamiana and is highly expressed in esophageal gland cells and amphids of B. xylophilus. BxSapB2 was determined to be related to the pathogenicity of B. xylophilus. The results of this work indicate that BxSapB2 plays an important role in the interactions between B. xylophilus and the hosts.
1 INTRODUCTION
Pine wilt disease (PWD), a severe problem in pine forests worldwide, is caused by the pine wood nematode (PWN), Bursaphelenchus xylophilus (Braasch et al., 2001). Bursaphelenchus xylophilus is native to North America (Kanzaki & Futai, 2002). The disease was first discovered in Nanjing, China, in 1982. Based on the latest reports, PWD has spread to many provinces in the south and east of China, including Jiangsu, Zhejiang, Anhui, Fujian, and others. Bursaphelenchus xylophilus damages pine trees and natural landscapes in most areas of China and results in notable economic losses (Huang, Wang, Tian, Zhu, & Ye, 2019; Li, Tan, & Chen,2018; Zhou et al., 2017). Although the genome sequence of B. xylophilus is available (Kikuchi et al., 2011), many details of the mechanisms underlying interactions between this nematode and the host trees have not been elucidated. Plants are hosts of microbial and viral pathogens, as well as multicellular parasites, such as insects, nematodes and parasitic plants. Despite many biological differences between nematodes and other pathogens, many of the principles governing the molecular interactions between these pathogens and their hosts are thought to be similar. Given that plant pathogens often secrete a series of proteins into the host–pathogen interaction to manipulate host cell physiology and ultimately promote infection, functional verification of these secreted proteins is of particular interest for identifying potential virulence factors (Li, Yin, Fan, Xu, & Huang, 2015). Plants have developed an effective two-layered immune system to defend themselves against pathogen attacks (Jones & Dangl, 2006). Initially, membrane-spanning proteins, known as pattern-recognition receptors (PRRs), recognize conserved pathogen-associated molecular patterns (PAMPs). PAMP-triggered immunity (PTI) involves the recognition of PAMPs by hosts. To overcome PTI, host-adapted pathogens employ secreted proteins known as effectors, to promote infection (Van Esse et al.., 2008). Many effectors are delivered to the host cytoplasm (Win et al., 2012) or apoplast (Shabab et al., 2008). In plant-parasitic nematodes (PPNs), a number of effectors are known to promote pathogenesis by inhibiting host defence proteins (Ali et al., 2015; Chen et al., 2015, 2017). ETI is often associated with the development of the hypersensitive response, a type of programmed cell death localized around the infection site and effectively restricting pathogen growth (Heath, 2000).
Effectors play an important role in the host–pathogen interface during infection (Giraldo & Valent, 2013; Haegeman, Bauters, Kyndt, Rahman, & Gheysen, 2013). Plant-parasitic nematodes (PPNs) secrete effector proteins through a hollow stylet either into the cytoplasm of the host cell or into the apoplast to promote parasitic effects (Mitchum et al., 2013). In previous studies, some parasitism genes and plant cell wall-degrading enzymes, regarded as “effectors,” have been characterized from B. xylophilus, such as glycoside hydrolase family 45 (GH45) cellulases, pectate lyases, expansins and β-1,3-endoglucanases (Kikuchi, Jones, Aikawa, Kosaka, & Ogura, 2004; Kikuchi et al., 2009; Kikuch, Shibuya, Aikawa, & Jones, 2006; Kikuchi, Shibuya, & Jones, 2005). Recently, some researchers focused on the study of effectors in B. xylophilus. For example, Espada et al. (2016, 2018) predicted some B. xylophilus candidate effectors and found a promoter motif associated with spatial expression in the esophageal glands of B. xylophilus. Moreover, Hu et al. (2019) identified and characterized a novel effector, BxSapB1, from B. xylophilus. One issue urgently requiring investigation is whether B. xylophilus secretes effectors other than those already known.
Transcriptome data on B. xylophilus provide an opportunity to identify more effectors that are specifically involved in B. xylophilus-host interactions (Tsai et al., 2016). In this work, we used a bioinformatic pipeline for the preliminary identification of candidate effectors and expressed them in Nicotiana benthamiana through the PVX virus expression vector. The effector known as BxSapB2 was targeted initially, with verification of its secretion being achieved through the yeast signal sequence trap system. In addition, we investigated the localization of BxSapB2 in B. xylophilus using in situ hybridization (ISH). RNA interference (RNAi) was used to assess the functions of BxSapB2 during PWN infection of its host. These findings may help to elucidate the related functions of PWN effectors and provide a reference for future research investigating the interactions between B. xylophilus and the host.
2 MATERIALS AND METHODS
2.1 Nematode isolates
The highly virulent AMA3 strain of B. xylophilus was isolated from wood chips of infested Pinus thunbergii in Anhui, China. Nematodes were raised on colonies of Botrytis cinerea on PDA for 7 days at 25℃ before extraction overnight using the Baermann funnel method (Viglierchio & Schmitt, 1983).
2.2 Screening of candidate secreted effector protein and sequence analysis
The transcriptome of B. xylophilus at early stage of infection (2.5 hr post-inoculation) was obtained in previous work (Tsai et al., 2016). Genes shown to be upregulated in the transcriptome library included signal peptides but no transmembrane domains were found. Subsequently, these genes were further screened as candidate effectors and matched to the secretome of B. xylophilus (Shinya et al., 2013). Signal peptide prediction was performed with SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) (Thomas, Søren, von Gunnar, & Henrik, 2011) and transmembrane domain prediction was performed using TMHMM 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) (Sonnhammer, von Heijne, & Krogh, 1998). The putative protein domain of BxSapB2 was predicted by using the Simple Modular Architecture Research Tool (SMART) (Letunic, Doerks, & Bork, 2015). Alignment of the amino acid and nucleotide sequences of BxSapB2 and BxSapB1 was performed using BioEdit with ClustalW multiple alignment (Li, Wang, et al., 2018). Further, BxSapB2 was found in the upregulated gene list from the B. xylophilus transcriptome in the early stages of infection (Hu et al., 2019). In addition, the 1,000 bp upstream of the predicted coding region of BxSapB2 was examined to determine the promoter motif.
2.3 RNA isolation and CDNA synthesis of B. Xylophilus
The total RNA of mixed life-stage nematodes was extracted using Trizol (Invitrogen, Waltham, MA, USA), examined by electrophoresis on a 1% agarose gel and quantified using a spectrophotometer. The TransScript II One-Step gDNA Removal and cDNA Synthesis SuperMix were used, following the manufacturer's instructions (TransGen Biotech), with 1 µg of total RNA to synthesize cDNA.
2.4 Cloning of BxSapB2 and plasmid constructs
Forty-three candidate effectors with high abundance expression were amplified using specific primers (Table S1). Amplified PCR products were confirmed by electrophoresis on 1% agarose gels and purified using the MiniBEST Agarose Gel DNA Extraction Kit Ver. 4.0 (Takara). Subsequently, the PCR products were cloned into the PVX vector (pGR107). Escherichia coli was incubated overnight on Luria Bertani agar containing Kanamycin (final concentration: 50 μg/ml) at 37℃. All plasmids were validated via sequencing by GenScript, Inc..
2.5 Agrobacterium tumefaciens infiltration assays
Constructs were introduced into A. tumefaciens strain GV3101 by electroporation (Jing et al., 2016). The transformed bacterial cells were grown on LBA supplemented with kanamycin and rifampicin (final concentration: 50 μg/ml, respectively). For agroinfiltration assays, recombinant A. tumefaciens strains were grown in a shaking incubator at 200 rpm for 36-48 hr at 28℃. Cells were resuspended in washing buffer (10 mM MgCl2, 10 mM MES and 100 µM AS, pH 5.6) and density was adjusted to an OD600 = 0.4. Bacteria were infiltrated into leaves of 4-week-old N. benthamiana plants through a small incision made with a needleless syringe (Yang et al., 2017). As controls, leaves were infiltrated with A. tumefaciens carrying a Green fluorescence protein (GFP). After 4–5 days, cell death symptoms were photographed and evaluated. Each assay was performed three times.
2.6 Western blotting
Proteins from the agroinfiltrated N. benthamiana leaves lysate were fractionated using SDS-PAGE. Proteins were separated on 12% polyacrylamide gel and transferred to an Immobilon-PSQ polyvinylidene difluoride membrane. The membrane was blocked using PBS (pH 7.4) plus 3% non-fat dry milk (PBSM) for 30 min at room temperature with shaking at 50 rpm and washed with PBST (PBS with 0.1% Tween 20). The anti-HA-tag primary monoclonal antibody (Sigma-Aldrich) was added to PBST (PBST with 3% non-fat dry milk) and incubated at room temperature for 90 min, followed by three washes in PBST. The membrane was incubated with a goat anti-mouse IRDye 800CW (Odyssey, no. 926–32210) secondary antibody (1:10,000) in PBST at RT for 30 min with shaking at 50 rpm. The membrane was washed three times in PBST and once for 5 min in PBS. Ultimately, the membrane was visualized using excitation at 780 and 800 nm (Yu et al., 2012).
2.7 Electrolyte leakage assay
The levels of cell death in tobacco were assayed by measuring ion leakage from leaf discs (Yu et al., 2012). Five leaf discs (9 mm in diam.) were floated on 5 ml distilled water at room temperature for 3 hr before measuring conductivity of the solution with a conductivity meter (Con700) to obtain “value A.” Leaf discs were returned to the solution and boiled in sealed tubes for 25 min. Conductivity was measured to acquire “value B” at room temperature. Ion leakage was expressed as the percentage of total ions, that is (value A/value B) × 100. All assays were repeated three times.
2.8 Yeast signal sequence trap system
The yeast signal trap system was used, through vector pSUC2T7M13ORI (pSUC2), which carries a truncated invertase gene, SUC2, lacking the initiation Met and signal peptide (Jacobs et al., 1997). DNA fragments coding for the signal peptides of BxSapB2 were introduced into pSUC2 using EcoRI and XhoI restriction sites to create in-frame fusions to the invertase. Primer information is listed in Table S1. The YTK12 (negative control) was transformed with 20 ng of each of the pSUC2-derived plasmids using lithium acetate. After this step, yeast was plated on complete minimal plates lacking tryptophan (CMD-W [minus Trp]) plates (0.67% yeast N base without amino acids, 0.075% W dropout supplement, 2% sucrose, 0.1% glucose, 2% agar). Transformed colonies were incubated on fresh CMD-W at 30℃ and transformation confirmed by PCR. Colonies were subcultured to 1% yeast extract, 2% peptone, 2% raffinose, and 2 μg/mL antimycin A (YPRAA) plates (Oh et al., 2009). Invertase activity was detected by the reduction of 2,3,5 triphenyltetrazolium chloride (TTC) to the insoluble red coloured triphenylformazan.
2.9 In situ hybridization
In situ hybridization (ISH) using digoxigenin-labelled probes was performed to determine the spatial expression pattern of candidate effector BxSapB2 in B. xylophilus following published methods (De Boer, Yan, Smant, Davis, & Baum, 1998). For ISH, the DNA fragment used as the probe was amplified from the cDNA clones of BxSapB2 with specific primers (Table S1). Hybridization and detection were performed with the DIG-High Prime DNA Labelling and Detection Starter Kit I (Roche Diagnostics, Mannheim, Germany). Photographs of B. xylophilus were taken with a Zeiss Axio Imager M2 microscope (Zeiss MicroImaging GmbH).
2.10 BxSapB2 interference using double-stranded RNA
BxSapB2 and GFP were amplified using primers with the T7 promoter (Table S1), connected with pMD19-T vector and transformed into E. coli strain JM109. The two plasmids carrying the BxSapB2 fragment and GFP in JM109 were extracted using MiniBEST Plasmid Purification Kit Ver.4.0 according to the manufacturer's instructions (TaKaRa, Japan). The double-stranded RNA (dsRNA) corresponding to BxSapB2 and the negative control GFP were synthesized using the MEGAscript RNAi Kit (Ambion Inc.). The RNAi soaking method was performed (Urwin, Lilley, & Atkinson, 2002). Approximately 10,000 nematodes (a mixture of juveniles and adults) were soaked in dsBxSapB2, dsGFP, and non-dsRNA solutions and incubated at 20℃ at 180 rpm in a shaking incubator for 48 hr. Each treatment had four replicates.
2.11 Detection of silencing efficiency of BxSapB2 by QRT-PCR
The nematodes from the three different treatments described above were thoroughly washed in three changes of ddH2O after soaking. Subsequently, approximately 2,000 nematodes from each treatment were collected to evaluate the silencing efficiency of BxSapB2 by qRT-PCR, using SYBR green master mix (Vazyme, Nanjing, China), following the manufacturer's instructions. The Actin gene of B. xylophilus (GenBank number EU100952) was used as a reference for all reactions. Relative expression levels were determined by ABI Prism 7,500 software (Applied Biosystems) and calculated with the 2 -△△Ct method. Primer information is listed in Table S1.
2.12 Analysis of reproduction of B. xylophilus after RNAi
Ten pairs of female and male nematodes were selected and transferred to B. cinerea on PDA and cultured at 25℃ for 8 days. Three biological replicates were prepared. Subsequently, nematodes were extracted from cultures using the Baermann funnel method and counted.
2.13 Evaluation of virulence of B. xylophilus after RNAi
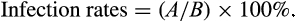
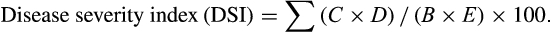
3 RESULTS
3.1 Identification of candidate effectors in Bursaphelenchus xylophilus
Sixty-two candidate effectors upregulated in the transcriptome library of B. xylophilus at 2.5 hr post-inoculation were predicted to include a signal peptide and lack transmembrane domains. Forty-three of these candidate effectors were cloned and transformed into the PVX vector for expression in N. benthamiana (Table S2).
3.2 BxSapB2 induces cell death in N. benthamiana
Phytopathogen effectors often induce a phenotype upon overexpression in planta, reflecting their virulence activities. To investigate the function of the candidate effectors of B. xylophilus, we tested the ability of the candidate effectors to induce cell death in N. benthamiana. Based on transient overexpression in N. benthamiana performed for 43 candidate effectors, one B. xylophilus candidate effector (BXY_0494700.1) induced cell death (Figure 1a). We predicted the domains of this candidate effector using SMART and found that it contained a SapB domain with an e-value of 0.756, whose reference number was IPR008139 in the Interpro database, and the domain was from the 47th - 130th amino acid in this protein. Thus, the protein was a saposin-like protein, and we denoted it as BxSapB2. Its open reading frame (ORF) contained 450 base pairs (bps), encoding a 149 amino acid polypeptide. Consistent with the predicted apoplastic localization, BxSapB2-induced symptoms were completely abrogated when the SP was deleted (PVX-BxSapB2ΔSP-HA; Figure 1a).
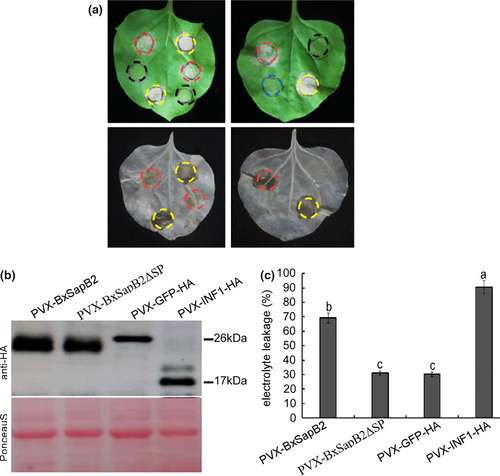
Transiently expressed protein was detected by Western blotting (Figure 1b), and the results indicated that BxSapB2 was normally expressed in N. benthamiana. Ion leakage of the leaves showed consistent results (Figure 1c). Taken together, these results strongly implied that BxSapB2 caused cell death in N. benthamiana when targeted to the apoplast.
In this study, alignment of the nucleotide and amino acid sequences of BxSapB2 and BxSapB1 with BioEdit showed that the nucleic acid and amino acid sequences of the two effectors differed. The ORF of BxSapB2 contained 450 bps, encoding a 149 amino acid polypeptide; the ORF of BxSapB1 contained 435 bps and encoded a 144 amino acid polypeptide. Moreover, no similarities between the nucleic acid sequences of BxSapB2 and BxSapB1 were found. The amino acid sequence of BxSapB2 showed only 21.33% identity to that of BxSapB1. In addition, the position of the SapB domain (characterized by six conserved cysteine residues) was also different: in BxSapB1 SapB was between amino acids 61 to 144 compared to 47 to 130 in BxSapB2 (Figure 2 A-B). Moreover, we also searched the promoter motifs of BxSapB2, BxSapB1, and showed that a STATAWAARS motif in the promoter region of BxSapB2 and one of BxSapB1 was STATAWAASS.

3.3 Signal peptide of BxSapB2 is functional in yeast
To functionally validate the signal peptide predictions of BxSapB2 in yeast, we used a genetic assay based on the requirement of yeast cells for invertase secretion to grow on sucrose or raffinose media (Lee et al., 2006). As a result, the positive control Avrblb2 and BxSapB2-SP strains were able to grow on YPRAA medium (with raffinose instead of sucrose, growing only when invertase was secreted) (Figure 3). The results showed that Avrblb2 and BxSapB2-SP had the same signal peptide activity and could secrete sucrase to the extracellular space after fusion with SUC2. The sucrase degraded raffinose into monosaccharide. The sucrase secreted by Avrblb2 and BxSapB2-SP strains can hydrolyze sucrose to monosaccharide. Invertase secretion was confirmed with an enzymatic activity test based on reduction of TTC to the insoluble red-coloured triphenylformazan. The yeast signal sequence trap system strongly demonstrated that the signal peptide of BxSapB2 was functional in yeast.
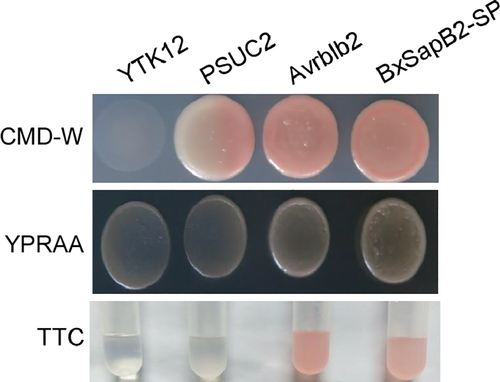
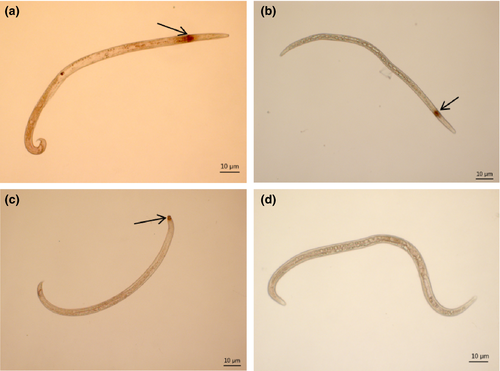
3.4 Localization of BxSapB2 in B. xylophilus by in situ hybridization
Localization of BxSapB2. Using ISH, BxSapB2 was expressed in the esophageal gland cells of most B. xylophilus (Figure 4a,b) and in the amphids of several B. xylophilus (Figure 4c), as indicated by the dark punctate colour. No hybridization signal was observed in B. xylophilus treated with a sense probe (the negative control) (Figure 4d). These results indicated that BxSapB2 was expressed in gland cells and amphids.
3.5 Synthesis of double-stranded RNA and detection of RNAi efficiency
Using RNAi showed that dsBxSapB2 and dsGFP migrated slightly slower than the corresponding linear DNA, which indicated that dsBxSapB2 and dsGFP were successfully synthesized (Figure S1). Quantitative reverse transcription PCR (qRT-PCR) performed to determine the effect of RNAi on the BxSapB2 mRNA levels suggested that the relative expression of BxSapB2 in nematodes soaked in dsBxSapB2 was 0.21, which was significantly lower than that of BxSapB2 in nematodes soaked in dsGFP or non-dsRNA solution (0.96 and 1), respectively. Therefore, the expression of BxSapB2 was effectively silenced by RNAi (Figure 5b).
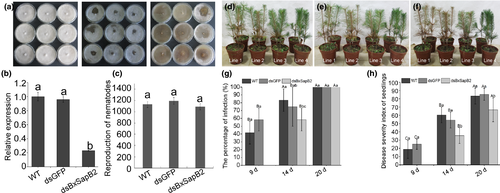
3.6 Effect of BxSapB2 on B. xylophilus reproduction on fungal mats
To test the effect of BxSapB2 on B. xylophilus reproduction, ten pairs of virgin female and male B. xylophilus were selected from dsBxSapB2, dsGFP and non-dsRNA solutions, transferred to cultures of B. cinerea on PDA and incubated at 25°C for 9 days. The nematodes cultured on B. cinerea were removed from dsGFP or non-dsRNA solution for use as controls. The amount of B. cinerea mycelium remaining on the PDA in the three treatments was similar (Figure 5a). After 9 days, the reproduction rates of nematodes in the non-dsRNA solution [wild-type (WT)], dsGFP, dsBxSapB2 were 1133-, 1194- and 1091-fold, respectively (Figure 5c), indicating that B. xylophilus reproduction was not influenced when BxSapB2 was silenced.
3.7 BxSapB2 contributes to virulence during the infection stage
Three-year-old P. thunbergii seedlings were inoculated with dsBxSapB2-treated, dsGFP-treated and WT nematodes with four replicate seedlings per treatment (Figure 5d,f, Figure S2). At nine days post-inoculation, a small number of needles turned brown in seedlings inoculated with nematodes soaked in the dsGFP and non-dsRNA solutions (Figure 5d): infection rates of P. thunbergii were 58.33% and 41.67% and disease severity indices were 25 and 18.75, respectively. At this time, seedlings inoculated with nematodes soaked in dsBxSapB2 showed no symptoms (Figure 5g-h). At 14 days post-inoculation, infection rates of P. thunbergii inoculated with WT nematodes and those soaked in dsGFP and dsBxSapB2 were 83.33%, 75% and 58.33%, respectively. At the same time, the disease severity index of P. thunbergii inoculated with dsBxSapB2-treated nematodes (35.41) was significantly lower than those of seedlings inoculated with WT nematodes (60.41) or dsGFP-treated nematodes (54.16) (Figure 5e,g,h). At 20 days post-inoculation, disease incidence in seedlings inoculated with WT nematodes, dsGFP-treated or dsBxSapB2-treated nematodes all reached 100%, but disease severity on P. thunbergii inoculated with dsBxSapB2-treated nematodes (66.67) was still significantly lower than those of seedlings inoculated with WT nematodes (83.33) and dsGFP-treated nematodes (85.41) (Figure 5f-h). The results indicated that the virulence of B. xylophilus was influenced when BxSapB2 was silenced, which could delay the occurrence of pine wilt disease.
4 DISCUSSION
Similar to plant pathogens, nematodes are presumed to employ effector proteins, which are secreted to alter plant cellular functions enabling successful infection of hosts. The molecular function of nematode effectors is currently the subject of intensive research. A bioinformatics pipeline was used in this study to make preliminary screens of candidate effectors from B. xylophilus and identified one effector designated BxSapB2, which could induce cell death in foliage of N. benthamiana through a virus in planta overexpression system. Currently, the N. benthamiana virus expression vector is widely used to identify effectors from various pathogens and parasites (Li et al., 2015; Ma et al., 2015). For example, effector Avh241 from Phytophthora sojae induces plant cell death (Yu et al., 2012). In this study, this method was used successfully to screen the candidate effectors of B. xylophilus. In addition, expressing candidate effectors in N. benthamiana using other expression vectors can be used to evaluate cell death with effector genes. For example, after 7 candidate effectors of Heterodera avenae were constructed into the pND108 expression vector, the infiltration assay showed induced cell death in N. benthamiana (Chen et al., 2018). Although different expression vectors were used, the effectors were identified by transient expression of proteins.
Overall, few nematode effectors were proven to induce cell death. In general, effectors of plant pathogens can be secreted into the cytoplasm and apoplast of the host (Ali & Bakkeren, 2011). Previous work showed that apoplastic effectors were highly important in pathogen-host interactions (Eves-van den Akker, Lilley, Jones, & Urwin, 2014; Ma et al., 2015). For example, GrEXPB2, an apoplastic effector of Globodera rostochiensis, suppresses host defenses (Ali et al., 2015). In this study, BxSapB2-induced symptoms were completely eliminated when SP was deleted. Moreover, the yeast signal trap system assay showed that the SP of BxSapB2 was functional in yeast. These results suggested that BxSapB2 triggered cell death when secreted into the apoplast in N. benthamiana. The RNAi assay showed that BxSapB2 influenced pathogenicity during the B. xylophilus-pine interaction. These results indicated that BxSapB2 plays a key role in the parasitism of B. xylophilus.
In this article, BxSapB2 was predicted to be a saposin-like protein. Saposins A, B, C and D are small heat-stable glycoproteins derived from a common precursor protein, prosaposin (Yuan et al., 2007). Prosaposin activates several lysosomal hydrolases involved in the metabolism of various sphingolipids. Prosaposin is a multifunctional glycoprotein that is an activator of lysosomal enzymes involved in the hydrolysis pathway of sphingolipids and acts on the nervous and reproductive systems (Guo et al., 2007). In our study, RNAi showed that silencing BxSapB2 could effectively delay the process of pine wilt disease and contribute to B. xylophilus virulence in pine. Further research is necessary to determine whether the clear mechanism of BxSapB2 in regulating plant tissue responses is similar to the one suggested in this present work.
Identification of nematode extracellular secretions is key to understanding the parasitic mechanisms of these organisms. Previous studies have shown that the most important nematode organs producing secretions were the esophageal glands, the hypodermis and the amphids (Haegeman, Mantelin, Jones, & Gheysen, 2012). The esophageal glands of nematodes produce a large number of proteins, including several cell wall-degrading enzymes and pioneer candidate effectors (Kikuch et al., 2006; Espada et al., 2016). These effector proteins contain predicted signal peptides for secretion from gland cells into host cells via the nematode stylet, facilitating feeding, penetration and migration of nematodes in pine tissues. The amphids is also an important secretory organ that is exposed to the environment and can secrete effectors directly into plant tissues (Haegeman et al., 2012). For example, the Meloidogyne incognita effector MAP-1 was expressed in amphids (Semblat, Rosso, Hussey, Abad, & Castagnone- Sereno, 2001). The candidate effector of B. xylophilus putative epoxide hydrolase (BUX.s00298.34) was also expressed in glandular tissues surrounding the anterior sense organs (Espada et al., 2016). In this study, BxSapB2 was highly expressed in the esophageal gland cells of most B. xylophilus examined and was also expressed in the amphids of a few B. xylophilus at the same time. Moreover, based on BLAST analysis, BxSapB2 showed 100% homology to BUX.s01063.105, a protein identified from the B. xylophilus secretome during infection of P. thunbergii (Shinya et al., 2013). Therefore, it is speculated that BxSapB2 could be secreted from esophageal glands and amphids of B. xylophilus at an early stage in the infection process.
Espada et al. (2018) proposed that if a STATAWAARS motif is present in the promoter of the gene containing a signal peptide, the gene was expected to encode an effector protein. In this study, BxSapB2 was shown to have the same promoter motif, indicating that the two studies cross-validated each other.
In our previous study, another saposin-like protein, BxSapB1 was identified and characterized and was also proven to induce cell death in N. benthamiana (Hu et al., 2019). A comparison between the two proteins was conducted in this study. In addition to differences in sequence lengths, the placement of the SapB domain and promoter motifs, there were two further major differences. First, BxSapB2 was upregulated in the transcriptome of B. xylophilus (2.5 hr post-infection) but was not upregulated in the transcriptome of B. xylophilus (6, 12 and 24 hr post-inoculation) (Hu et al., 2019; Tsai et al., 2016). BxSapB1 was upregulated in the two above transcriptomes of B. xylophilus. Second, apart from the esophageal glands, BxSapB2 was also expressed in the amphids of several B. xylophilus. These two major differences in the expression of these effectors indicated that, although both were saposin-like proteins, they might play various roles in the interaction between B. xylophilus and pines.
In future research, more effectors will be identified. Meanwhile, it is crucial to identify the host target of BxSapB2, which may help to elucidate the mechanisms governing the interactions occurring between B. xylophilus and its host.
ACKNOWLEDGEMENTS
This work is supported by the National Key Research and Development Program of China (2018YFD0600203), Jiangsu Provincial Agricultural Science and Technology Innovation Fund (CX (16) 1005), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We are grateful to Dr. De-Wei Li (The Connecticut Agricultural Experiment Station, USA) for polishing the language of the manuscript.




