Pharmacophagy in insects: Ecological and evolutionary perspectives on the non-nutritional use of plant specialized metabolites
Abstract
Herbivorous insects can interact with plants in ways that go beyond nutrition, with plant specialized (secondary) metabolites (PSMs) mediating complex non-nutritional relationships. While PSMs often function as anti-herbivore defenses, many insects have evolved strategies to counteract and even exploit these compounds, using them for purposes such as their own defense against antagonists, enhanced mating success, or self-medication. This review explores pharmacophagy, where insects actively seek and acquire specific PSMs from both food and non-food plants for benefits unrelated to nutrition, across different insect orders such as Orthoptera, Lepidoptera, Hymenoptera, Coleoptera, Diptera, and Neuroptera. Key examples are provided for species taking up PSMs of different compound classes, including pyrrolizidine alkaloids, cardiac glycosides, neo-clerodane diterpenoids, cucurbitacins, raspberry ketone, methyl eugenol, and other metabolites such as ethanol or resin. The insect species demonstrate unique adaptive uses of these non-nutritional plant chemicals. We discuss the intra- and intergenerational transfer of pharmacophagously acquired PSMs among conspecifics and the methods for identifying and testing pharmacophagy, emphasizing the importance of interdisciplinary approaches that combine field observations, behavioral studies, and chemical analyses. The evolutionary pathways leading to pharmacophagy are considered, highlighting selective pressures such as predation, parasitism, and sexual selection. We also address the costs associated with pharmacophagy, including energetic demands and potential toxicity. Extending the discussion to non-insect taxa suggests that pharmacophagy may be a broader ecological phenomenon. By establishing a comprehensive framework for understanding pharmacophagy, we aim to stimulate further research into this intriguing aspect of plant–insect interactions and highlight its potential applications in pest management, conservation, and human health.
PLANT–INSECT INTERACTIONS: THE NON-NUTRITIONAL DIMENSION
Plant–insect interactions are multifaceted, with plant specialized (secondary) metabolites (PSMs) playing a critical role in mediating these interactions. Plants produce a wide array of PSMs (Mason & Singer, 2015) that primarily serve as defenses against various abiotic and biotic harms, including insect herbivory. To counteract these defenses, herbivorous insects have evolved several strategies, such as avoidance, enzymatic degradation, excretion, or a reliance on specific microbiota (Coolen et al., 2022; Genta et al., 2006; Jeckel et al., 2022; Shukla & Beran, 2020). When they are able to overcome plant defenses, herbivorous insects can use plants as a source of nutrients, while the uptake of PSMs is a by-product of feeding, often followed by detoxification in some way. However, in several cases, insects even concentrate these PSMs directly, or after some modification, in certain body parts and sequester them for purposes such as, for example, defense against their own predators. Over the past decades, various studies have demonstrated that potent PSMs are sequestered by a wide variety of insects (Beran & Petschenka, 2022; Opitz & Müller, 2009). This ability has independently evolved across major herbivorous insect lineages and encompasses the uptake of diverse classes of PSMs.
Interestingly, some insect species have been found to explore plants independently of nutrient acquisition, focusing exclusively on obtaining particular PSMs. For example, certain insect species rely on specific PSMs and acquire them through specialized behaviors that extend beyond normal feeding. These sequestered compounds can significantly influence insect fitness, providing benefits, for example, in terms of defense and/or reproduction. When insects actively search for certain PSMs, take them up, and use them for purposes other than their metabolism or simple recognition of food plants, this behavior is called pharmacophagy (Boppré, 1984) (from the Greek “pharmakon”—drug and “phagein”—feeding). Earlier studies have extensively examined specific aspects of PSM utilization, including roles in chemical defense, self-medication, and pheromone synthesis (De Pasqual et al., 2021; Erb & Robert, 2016; Erler et al., 2024; Nishida, 2014; Opitz & Müller, 2009; Stökl & Steiger, 2017). Yet, relatively few studies have differentiated whether these metabolites are obtained from food versus non-food plants, or whether insects seek out these chemicals independently of nutritional needs. Note, however, that insects may not always be specifically attracted to the PSM independently of the food plant, though they may still utilize the PSM when it is present. This review explores representative examples of pharmacophagy of PSMs of different compound classes (Table 1), highlighting key features along with associated benefits and costs. We also discuss methods for identifying and testing pharmacophagy, along with its evolution. Our goal is to establish a framework for understanding how insects explore plants for non-nutritive purposes and to identify key areas for further research. Additionally, we extend the discussion to other animal taxa to demonstrate the broader relevance of pharmacophagy.
| Compound (class) being utilized (either without modification or its derivative) | Plant taxon used for pharmacophagy for the compound (class) | Insect order | Insect species | Insect life stage, sex | Function/effect of compound | Reference |
|---|---|---|---|---|---|---|
| Pyrrolizidine alkaloids (e.g. heliotrine, senecionine and fuchsisenecionine) |
Senecio (Asteraceae), Heliotropium (Boraginaceae), and Crotalaria (Fabaceae) species |
Orthoptera | Zonocerus elegans | Nymphs and adults, both sexes | Potentially chemical defense against predation | Boppré et al. (1984) |
| Pyrrolizidine alkaloids |
Plant species of Apocynaceae, Asteraceae, Boraginaceae, and Fabaceae |
Lepidoptera | Many species of the subfamily Danaini, including Danaus gilippus | Adults, usually males | Chemical defense against predation, sex pheromone, reduce or prevent infection | Boppré (1986); Boppré and Monzón (2023); Dussourd et al. (1989); Lawson et al. (2021) |
| Pyrrolizidine alkaloids (specifically dehydropyrrolizidine, alkaloid monoesters and their n-oxides) | Asteraceae (Eupatorieae) and Boraginaceae | Lepidoptera | Species of the Ithomiine | Adults | Chemical defense against predation, increases mating success | Brown (1984) |
| Pyrrolizidine alkaloids | Senecio longilobus | Lepidoptera | Grammia incorrupta (Grammia geneura) |
Larvae |
Resistance against parasitoids, but reduced growth efficiency | Singer et al. (2004) |
| Cardiac glycosides and aglycones | Asclepias species | Orthoptera | Phymateus leprosus | Juveniles & adults | Potentially chemical defense against predation | Seibt et al. (2000) |
| Cardiac glycosides | Primarily Apocynaceae species | Hemiptera | Milkweed bugs | Juveniles and adults | Chemical defense against predation | Petschenka et al. (2022) |
| Neo-clerodane diterpenoids (“clerodanoids”) | Lamiaceae, e.g. Ajuga reptans and Clerodendrum trichotomum (but insects can also take up clerodanoids from conspecifics via “nibbling”) | Hymenoptera | Athalia rosae | Adults, both sexes | Chemical defense against predation, increases mating success, but reduces lifespan in absence of predator or when in groups with asymmetric clerodanoid access, possibly due to higher agonistic interactions | Amano et al. (1999); Paul et al. (2021); Paul and Müller (2021); Singh et al. (2022); Singh et al. (2024a); Zanchi et al. (2021) |
| Cucurbitacins (triterpenoids) | Cucurbitaceae, e.g. Cucurbita pepo, Cucumis sativus | Coleoptera | Diabrotica undecimpunctata, D. virgifera, other Diabrotica spp. | Adults, both sexes | Chemical defense against predation, survival in the presence of entomopathogenic fungi, mating success | Eben (2022); Gillespie et al. (2003, 2004) |
| Raspberry ketone |
Plants that release raspberry ketone as a fruit scent in raspberry or as a floral fragrance in several orchid species, e.g., Dendrobium annosmum, Bulbophyllum ecornutum and B. macranthum |
Diptera | Bactrocera cucurbitae | Adult males | Chemical defense against predation, courtship and aggregation pheromone | Tan (2000) |
| Methyl eugenol |
Reported from 30 plant families, such as Ocimum basilicum, Persea americana, Mangifera indica, Melaleuca spp. |
Diptera | Bactrocera carambolae | Adults, males | Sex pheromones, increase in mating success | Wee et al. (2007) |
| Ethanol | Rotting fruit | Diptera | Drosophila melanogaster | Larvae | Resistance against endoparasitoids | Milan et al. (2012) |
| Resin | Conifers | Hymenoptera | Formica paralugubris | Adults | Antimicrobial for colony | Castella et al. (2008) |
| Resin | Resin-producing plants | Hymenoptera | Apis mellifera | Adults | Antifungal for colony | Simone-Finstrom and Spivak (2012) |
| Terpenoids | Orchids | Hymenoptera | Tribe Euglossini | Adults, males | Territorial display and courtship, enhances longevity | Cameron (2004); Eltz et al. (1999); Zimmermann et al. (2006) |
| Iridoid precursors | Nepeta cataria, Actinidia polygama | Neuroptera | Chrysopa oculata, observations also for other Chrysopa species | Adults, males | Sex pheromone synthesis | Aldrich et al. (2016); Aldrich and Zhang (2016) |
- a Note that our interpretation of the phenomena as pharmacophagy may differ from the authors' terminology in their studies.
EXAMPLES OF PHARMACOPHAGY
Pharmacophagy occurs across diverse insect taxa. Insects actively seek out specific PSMs from various plant sources to gain chemical defenses, enhanced reproductive success, or self-medication against pathogens and parasites. A broad range of PSMs has been found to be used in that way. Pyrrolizidine alkaloids (PAs) represent one of the most widely sought PSMs in pharmacophagy, playing key roles in chemical defense, reproductive success, and self-medication across multiple insect orders, including Orthoptera and Lepidoptera. In the grasshopper Zonocerus elegans Thunberg (Orthoptera: Pyrgomorphidae), both nymphs and adults are strongly attracted to and consume PAs, also from non-food sources (Boppré et al., 1984). The grasshoppers were observed walking upwind toward PA sources and ingesting the compounds, showing that they can detect and seek out these chemicals from a distance. Notably, these tests were conducted in a resident population of Z. elegans, indicating that the PAs effectively lured the insects away from their food plants. The strong stimulatory effect of PAs on Z. elegans was further demonstrated by the fact that the insects ingested PAs from the ground, which is unusual for this species, as they typically feed on the tips of plants (Boppré et al., 1984).
Adults of Ithomiinae and Danainae butterflies (Lepidoptera: Nymphalidae) exhibit the peculiar behavior of being attracted to PA-containing plants (Pliske, 1975) and ingesting PAs from non-nutritional sources such as withered or damaged plants, particularly from the plant families Asteraceae, Apocynaceae, or Boraginaceae, such as species of Eupatorium and Heliotropium. To take up the compounds, they apply a fluid capable of dissolving PAs with their proboscides and then take up the fluid again. Alternatively, they can scratch fresh leaves and then take up sap exuding from these wounds (Boppré, 1984). The PAs protect Ithomiini butterflies from predators such as spiders (Brown, 1984) and their derivatives can have a sexual function as male pheromones (Schulz et al., 2004). Likewise, in the adults of some danaid species, PA acquisition is mostly male-biased, as they can use the PAs as precursors for pheromone production and transfer PAs to females as a nuptial gift, although this can vary across species. For example, in the queen butterfly, Danaus gilippus Cramer (Lepidoptera: Nymphalidae), males transfer PAs to the female during mating as a nuptial gift (Dussourd et al., 1989; for more details, see Intra- and intergenerational transfer section). In contrast, in the monarch butterfly, Danaus plexippus Linnaeus (Lepidoptera: Nymphalidae), PA pharmacophagy has been documented but occurs to a lesser extent than in other Danainae species (Edgar et al., 1976; Kelley et al., 1987; Stelljes & Seiber, 1990), and likely contributes to chemical defense rather than courtship. In addition, adult monarchs possess cardiac glycosides (cardenolides) sequestered during the larval stage, which provide further chemical protection against predators (Lawson et al., 2021).
An intriguing function of self-medication by PAs can be found in Grammia incorrupta H. Edwards (now Apantesis incorrupta, but previously referred to as Grammia geneura) (Lepidoptera: Erebidae). The caterpillars selectively consume toxic plants rich in PAs for defense against parasitoids such as tachinid flies or wasps (Singer et al., 2004, 2009). Despite the lower nutritional value of PA-containing plants such as Senecio longilobus Benth. (Asteraceae), caterpillars prioritize these over more nutritious food plants, reflecting an evolutionary trade-off between growth and survival. Parasitized caterpillars increase their intake of PAs, which are toxic to developing parasitoids, improving survival against parasitism, but also reducing their own growth efficiency. While parasitized caterpillars benefit from PA ingestion, unparasitized individuals experience decreased survival, illustrating the context-dependent nature of this adaptive change in feeding preferences (Singer et al., 2009). Moreover, pharmacophagous behavior seems to be highly dynamic in G. incorrupta. Individuals with early-stage wasp parasitoids enhance feeding on plants containing antioxidants, while fly-parasitized caterpillars engage more in the feeding of iridoid glycoside-containing plants. PA intake is mostly found in the later time phase of infection (Smilanich et al., 2011). These findings underscore the complexity of pharmacophagy, where non-nutritional PSMs are crucial for survival under ecological pressures.
Beyond PAs, other PSMs are also actively acquired through pharmacophagy. Cardiac glycosides, for example, are pharmacophagously acquired by the African bushhopper, Phymateus leprosus Fabricius (Orthoptera: Pyrgomorphidae). Both juveniles and adults are attracted to and ingest PSMs such as cardiac glycosides and their aglycones from different plant species, including Asclepias species (Asclepiadaceae) (Seibt et al., 2000). The bushhoppers incorporate these chemicals into their secretions. Seibt et al. demonstrated that the insects preferentially fed on filter papers soaked with extracts containing these cardiac glycosides and aglycones, showing a clear attraction to and consumption of these substances. Likewise, milkweed bugs (Hemiptera: Lygaeidae) have evolved specialized associations with certain plants, primarily species of Apocynaceae, from which they sequester cardiac glycosides for protection against predators (Petschenka et al., 2022). Some milkweed bug species have also independently colonized phylogenetically distant plants that convergently produce cardiac glycosides, while others tolerate and sequester colchicine and related alkaloids from Colchicum autumnale L. (Colchicaceae) plants. Although feeding on toxic seeds does not improve growth and can even impair development, sequestration significantly enhances survival by deterring predators such as lacewing larvae and passerine birds. The close association of the milkweed bugs to these PSMs occurring in phylogenetically distant plants, along with their defensive use, may thus also be considered pharmacophagy.
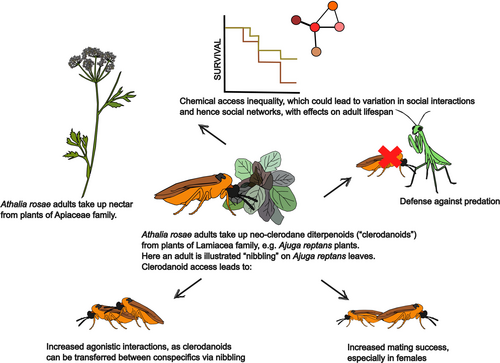
Pharmacophagy of neo-clerodane diterpenoids (“clerodanoids”) has been observed in Athalia sawflies (Hymenoptera: Tenthredinidae). For example, adults of the turnip sawfly, Athalia rosae Linnaeus (Hymenoptera: Tenthredinidae), usually take up nectar of Apiaceae plants; however, they also were found to visit non-food plants of the Lamiaceae family, such as Ajuga reptans L. or Clerodendrum trichotomum Thunb., where they “lick” or “nibble” on the leaves without any visible damage to the plant (Amano et al., 1999; Kawai et al., 1998; Paul & Müller, 2021; Singh et al., 2022) (Figure 1). In that way, the adults acquire clerodanoids from the plants, which they store after slight modification in their body and on the body surface (Brueggemann et al., 2023). These compounds are described as being extremely bitter-tasting (Nishida & Fukami, 1990). Clerodanoids applied on filter paper likewise evoke a pronounced attraction to the adults of different Athalia species (Nishida et al., 1989; Opitz et al., 2012). Insects that have gained clerodanoids are better defended against predators such as praying mantids than insects without access to clerodanoids (Singh et al., 2022). Moreover, clerodanoid-containing adults of A. rosae are more successful in mating—particularly females (Amano et al., 1999)—and engage in more social interactions (Singh et al., 2024a) than those without these compounds.
Similar to clerodanoids, cucurbitacins, a group of triterpenoids, are described as bitter tasting. Rootworm beetles (Diabrotica spp., Coleoptera: Chrysomelidae), such as Diabrotica undecimpunctata howardi Barber and Diabrotica virgifera virgifera LeConte, sequester these PSMs from plants of the Cucurbitaceae family, including Cucurbita pepo L. and Cucumis sativus L. (Eben, 2022; Gillespie et al., 2003, 2004). Adult rootworms, even those that do not feed on cucurbits during their larval stages, actively seek out cucurbitacin-containing plants to acquire these PSMs. Rootworms that have consumed cucurbitacins experience reduced predation, as the compounds make them unpalatable to predators, such as the Chinese praying mantid (Ferguson & Metcalf, 1985; Shapiro & Mauck, 2018; but see also Gould & Massey, 1984).
Notable cases of pharmacophagy of raspberry ketone or methyl eugenol occur in several Diptera. For example, males of the melon fly, Bactrocera cucurbitae Coquillett (Diptera: Tephritidae), are attracted to and consume raspberry ketone from different plant species. This PSM, once ingested, is stored in the flies' rectal glands and utilized as a sex pheromone to attract mates (Shelly, 2010). Furthermore, raspberry ketone and other pheromone compounds serve a defensive function against predators such as the Asian house gecko, Hemidactylus frenatus (Squamata: Gekkonidae) (Tan, 2000). When houseflies were treated with synthetic raspberry ketone and offered to geckos, the geckos exhibited a reduced tendency to consume these flies over time. Males of some other fruit fly species, such as Bactrocera dorsalis Hendel, Bactrocera carambolae Drew & Hancock, and Bactrocera correcta Bezzi (Diptera: Tephritidae), are attracted to and consume methyl eugenol from plant species of the Myrtaceae and Lauraceae (Shelly, 2010; Tan & Nishida, 2012; Wee et al., 2007). Methyl eugenol is metabolized and likewise stored in the rectal glands of the flies as a sex pheromone. Methyl eugenol-fed males release pheromones during courtship that not only attract females but also contribute to aggregation behavior, further amplifying the benefits of pharmacophagy (Shelly, 2010).
Other Diptera, such as Drosophila melanogaster Meigen (Diptera: Drosophilidae), use pharmacophagy of ethanol for self-medication. Larvae of D. melanogaster that are infected by parasitoids seek out food sources containing ethanol, which is produced by yeast fermentation of sugars in decaying fruit. The presence of ethanol reduces oviposition by endoparasitic wasps of the generalist Leptopilina boulardi Barbotin, Carton & Kelner-Pillault (Hymenoptera: Figitidae) and larvae of these wasps show a higher mortality in fruit fly larvae that consumed ethanol (Milan et al., 2012). Since D. melanogaster feed on fungi developing on rotting fruit, they have evolved a high resistance to fermentation products, which may make them highly suited to explore ethanol for self-medication.
In some cases, the target of medication is rather the group or colony, and resins serve as a valuable source for pharmacophagy. For instance, wood ants (Formica paralugubris Seifert, [Hymenoptera: Formicidae]) frequently incorporate large amounts of solidified conifer resin into their nests (Castella et al., 2008). Laboratory experiments have demonstrated that this resin inhibits the growth of bacteria and fungi under conditions simulating their natural environment. The ants display a clear preference for resin over other common building materials such as twigs and stones. This preference varies seasonally, being more pronounced in spring compared to summer, while in autumn, the ants collect both twigs and resin at similar rates. In laboratory conditions, the collection rate of resin versus stones is not influenced by infection with the entomopathogenic fungus Metarhizium anisopliae (Metchnikoff) Sorokin (Hypocreales: Clavicipitaceae), suggesting that resin collection serves a prophylactic rather than a therapeutic function against this pathogen species (Castella et al., 2008).
Honeybees (Apis mellifera Linnaeus [Hymenoptera: Apidae]) also integrate plant-produced resins into their nest structure, which can help lower the overall bacterial load of the colony, thereby reducing the need for individual bees to allocate resources to immune function. When honeybee colonies are exposed to the fungal parasite Ascophaera apis (Maassen ex Claussen) Olive & Spiltoir (Onygenales: Ascosphaeraceae), they intensify their resin foraging activities. Furthermore, colonies that are experimentally provided with additional resin show reduced levels of fungal infection (Simone-Finstrom & Spivak, 2012). Apart from resin, honeybees collect various plant-derived compounds from sources such as nectar, pollen, and propolis, a resinous mixture of plant exudates and bee secretions. These compounds contribute to colony health by preventing infections. PSMs in these foraged materials, such as phenolics and flavonoids from pollen, are instrumental in boosting the bees' immune systems and protecting them from pathogens. Honeybees also use antimicrobial compounds found in propolis for nest sanitation and individual immunity (Erler & Moritz, 2016).
Male orchid bees belonging to the tribe euglossini (Hymenoptera: Apidae) are highly attracted to complex fragrances of orchids (Cameron, 2004; Dressler, 1982). The bees collect these fragrances, store them in pouches in their hind legs, and expose them during courtship display (Zimmermann et al., 2006). The odors attract both males and females, forming leks, and seem to exclusively attract individuals within species, acting thus as pheromone analogues, as shown in the species Eulaema bombiformis Packard (Hymenoptera: Apidae) (Zimmermann et al., 2006). Quite complex mixtures of fragrances can be collected by some euglossini, with up to 105 different compounds, mostly essential oils, found in the fragrance in Euglossa tridentata Moure (Hymenoptera: Apidae) (Eltz et al., 1999). Thus, this peculiar collection behavior is discussed to act as “aroma therapy,” potentially increasing the longevity of the bees.
Finally, also predatory insects have been found to be pharmacophagous on plants. Adults of green lacewings (Chrysopa spp., Neuroptera: Chrysopidae) usually feed on pollen and nectar, but males can also be predaceous. Males of Chrysopa oculata Say (Neuroptera: Chrysopidae) are hypothesized to obtain the aphid pheromone nepetalactol from aphids, while other Chrysopa species are reported to obtain the monoterpenoid neomatatabiol by feeding on plants, such as Actinidia polygama (Siebold & Zucc.) Maxim. (Actinidiaceae) (silver vine) (Aldrich et al., 2016; Aldrich & Zhang, 2016). These insect- or also plant-derived iridoid precursors are sequestered and converted into the lacewing pheromone iridodial, which males use to attract females. When kept in the laboratory without access to such food sources, males of C. oculata could not produce iridodial on their own (Aldrich et al., 2016), highlighting that they need another food source for reproduction. This behavior showcases the significance of pharmacophagy also in predatory insects, particularly in utilizing plant-derived compounds for their reproductive success.
INTRA- AND INTERGENERATIONAL TRANSFER OF PHARMACOPHAGOUSLY ACQUIRED PSMS AMONG CONSPECIFICS
In some species, PSMs that are acquired pharmacophagously can be transferred within the same generation to conspecifics and even to members of the next generation. Male queen butterflies use PAs as precursors for their courtship pheromone danaidone and transfer PAs to females with their spermatophore during mating, which are then incorporated by the females into the eggs (Dussourd et al., 1989). Males that collected more PAs can also produce more pheromones and signal their high quality to the females (Dussourd et al., 1989). By selecting males with high pheromone amounts, females gain fitness benefits since they also get more PAs with their nuptial gifts, which can provide protection against predators. Furthermore, by incorporating PAs into their eggs, females presumably also provide protection to their offspring against parasites and predators (Dussourd et al., 1989). Likewise, in the moth Utetheisa ornatrix Linnaeus (Lepidoptera: Arctiidae), the eggs are endowed with PAs from both parents and these PSMs serve as protection against predators and parasitoids (Bezzerides et al., 2004; Eisner et al., 2000). Females of U. ornatrix can also obtain PAs from males during mating, where they play a role in mating success (Bezzerides et al., 2004) although PAs are usually acquired during the larval stage by both sexes with the diet and retained into adulthood. Interestingly, laboratory-reared U. ornatrix can develop on PA-lacking diets (Eisner & Eisner, 1991), suggesting that while PAs enhance survival through chemical defense, they are not essential for development.
Similarly, cucurbitacin consumption in rootworms has been linked to reproductive benefits, as the compounds are passed on to eggs, providing protection to offspring (Shapiro & Mauck, 2018; Tallamy et al., 2000). Indeed, eggs and larvae from parents that had sequestered a cucurbitacin-rich diet survived exposure to the fungus M. anisopliae, indicating a clear antibiotic benefit (Tallamy et al., 1999). In contrast, when testing the defense of eggs of adults that had fed on cucurbitacin C-containing vs. cucurbitacin C-lacking cucumber, the predation rate by four arthropod predators did not differ significantly (Brusti & Barbercheck, 1992). However, eggs from adults that had fed on a cucurbitacin-containing diet had a significantly faster development and larvae hatched faster under different moisture conditions than eggs from adults fed on a cucurbitacin-lacking cucumber (Brusti & Barbercheck, 1992). Thus, PSMs acquired pharmacophagously may also have important functions with regard to abiotic challenges.
PSMs acquired from plants may furthermore be “stolen” by conspecifics. For example, adult sawflies of the species A. rosae can obtain clerodanoids not only from plants but also from conspecifics, which had prior access to plant clerodanoids, via agonistic interactions, such as “nibbling” or licking, on the body surface of the conspecific (Figure 1) (Paul et al., 2021; Singh et al., 2022). The concentrations in such individuals are usually lower than in the ones that took up clerodanoids directly from the plants (Singh et al., 2022; Singh et al., 2024a), but they can still provide protection against predators and enhance mating success (Singh et al., 2022). Sawflies without direct access to clerodanoids also benefit from reduced predation when in groups with clerodanoid-defended individuals (Singh et al., 2022). Experiments with sawflies in different group compositions revealed that groups, in which all or some members had acquired clerodanoids, exhibited more frequent social interactions compared to those without clerodanoid access (Figure 1) (Singh et al., 2024a). These findings suggest that pharmacophagy may influence not only individual defense but also group dynamics. More research is needed to understand the broader consequences of pharmacophagy on social interactions and the shaping of individual social niches (Kaiser et al., 2024; Singh et al., 2024b).
IDENTIFYING AND TESTING PHARMACOPHAGY
Field observations and behavioral studies are essential for identifying non-nutritional interactions in insects, yet behaviors beyond feeding, egg-laying, or nectar-gathering are often overlooked. For instance, insects resting on plants or dried plant material without feeding may be misinterpreted as idle, when in fact they could be gathering crucial non-nutritional chemicals. To systematically investigate such interactions, researchers should document repeated behaviors, particularly on non-host plants, and track insect movements, contact duration, and revisitation patterns. For instance, A. rosae adults have been reported to congregate on plant species of the Lamiaceae (Amano et al., 1999; Nishida et al., 1989), which are not food plants for the larvae and unsuitable for oviposition. Such observations were also made by a former member of our group in a botanical garden, who found many A. rosae hovering over and sitting on Ajuga reptans plants (Opitz, personal observation), leading to further investigations of this peculiar behavior. Clerodanoids, occurring in these plants and being taken up by A. rosae (see above), or cucurbitacins, occurring in cucumber and attracting Diabrotica spp., are known to have a distinctly bitter taste, at least for humans (Brusti & Barbercheck, 1992; Nishida & Fukami, 1990). Thus, researchers assume that insects may use these compounds for defensive purposes. If insects that typically feed on other plants are observed visiting plants known for active PSMs—such as those with bitter-tasting or antimicrobial properties—such instances could be studied in more detail, as they may suggest pharmacophagy.
Citizen science initiatives and targeted field surveys can also help capture instances of pharmacophagy in diverse locations and understudied habitats. For example, in a garden in Maryland, monarch butterflies were observed gathering PAs from withered Eupatorium serotinum Michx. (Asteraceae) plants by probing damaged parts with their proboscides (Lawson et al., 2021). Similarly, expeditions, naturalist walks, and citizen science efforts have revealed cases of adult butterflies engaging with unusual resources, including fungi and animal remains, suggesting that pharmacophagy from plants, but also uptake from other organisms, may be more widespread than previously assumed (Thomas et al., 2025).
To experimentally test pharmacophagy, key predictions should be addressed: (i) Insects are attracted to and take up the chemicals, (ii) these chemicals confer a specific functional benefit, and (iii) the chemicals are even obtained from non-food plants, though they may occasionally come from food plants as well. For this, chemical analysis and metabolite profiling of plant and insect samples collected after apparent interactions can provide further insights. Analytical platforms such as GC–MS or LC–MS can be used to analyze plant and insect samples for the presence of the same PSMs. By comparing metabolite profiles of insects exposed to specific plants with those of controls that were not exposed, we can test for the uptake of PSMs. However, in several insect species, the plant metabolites are further metabolized upon uptake, with minor or larger changes to the core structure, as found, for example, for clerodanoids in A. rosae (Brueggemann et al., 2023). This needs to be considered when comparing metabolic data of plant and insect samples. Here, markers or isotopic tracers may help to test for uptake and potential modification of PSMs. Labeling PSMs with markers or isotopes may also allow us to track these chemicals within insect bodies, confirming their integration into tissues or glands for potential use in defense or reproduction. Imaging techniques, such as MALDI-imaging, can be used to localize the areas in an insect body where sequestered compounds are stored (Abdalsamee et al., 2014).
Furthermore, choice and preference bioassays in the laboratory provide a controlled environment for testing attraction to and uptake of specific PSMs. By offering insects a choice between different plant materials, containing or lacking the PSMs in question, their attraction, feeding behavior, and effects can be observed, as, for example, performed with rootworms and cucurbitacin (Brusti & Barbercheck, 1992). In that way, we can assess preferences for certain PSMs and score interaction frequencies, further validating field observations. To confirm the role of specific PSMs, they should be purified or synthesized and preference tests should be repeated with these compounds being applied on plant material, filter paper, artificial diet, or other materials that the insects would accept for feeding. Purified or synthetic compounds offered in that way could also be used to monitor changes in insect behavior, physiology, or pheromone production, comparing treated groups with controls to assess specific functional benefits. In such bioassays, the effectiveness of compounds against various predators, parasitoids, or pathogens can be tested. Finally, phylogenetic and evolutionary comparisons can reveal patterns of convergent evolution of pharmacophagy (Zaspel et al., 2014), which we outline in detail in the following section.
EVOLUTION OF PHARMACOPHAGY
Non-nutritional uptake of PSMs by insects may evolve through a combination of selection pressures that favor survival, reproduction, and niche expansion. For example, selection for defense against predation and parasitism may drive the evolution of pharmacophagous behavior, as insects capable of acquiring PSMs from a variety of plants, including non-food plants, often gain a direct survival advantage. For instance, PAs obtained from various non-host plants provide effective chemical defense in multiple insect taxa, such as ithomiine butterflies (Brown, 1984) and G. incorrupta caterpillars (Singer et al., 2004). This defensive strategy may represent an evolutionary shift, where non-food plants become crucial resources for survival rather than nourishment. Moreover, studies suggest that self-medication may be widespread among insects (Erler et al., 2024), potentially acting as a selective force behind pharmacophagous behavior. Insects affected by parasitism or disease may develop preferences for non-food plants containing PSMs with medicinal properties. Consequently, behaviors that enhance health through non-nutritional chemical intake can evolve as adaptive traits in populations under high parasite or pathogen pressures.
Behavioral shifts and adaptive flexibility may be prerequisites for shifts to other host plant sources to evolve. Behavioral plasticity allows insects to adapt existing behaviors to new functions, such as utilizing PSMs for purposes other than feeding. Initial contact with non-food plants may be incidental or linked to shelter-seeking behavior. The evolution of these behaviors likely involves exaptation, where traits originally evolved for herbivory are repurposed for non-nutritional uses. This may even lead to exploitation of conspecifics, as in A. rosae, where adults nibble on conspecifics to acquire defensive compounds from individuals that had acquired these compounds from plants (Paul et al., 2021; Singh et al., 2022; Singh et al., 2024a).
Sexual selection and reproductive benefits associated with the uptake of the PSMs are other strong selective forces, particularly if these PSMs enhance mating success or offspring fitness, as seen in the examples above of the queen butterfly (Dussourd et al., 1989) or fruit flies (Shapiro & Mauck, 2018; Tallamy et al., 2000). The usage of non-nutritional plant chemicals from non-food plants may have arisen from ancestral host plant use and subsequent host plant shifts. If these chemicals confer fitness benefits, such associations are likely to persist. For example, the close relationship between danaines and PAs presumably originated from the exploitation of PA-containing plants as ancestral hosts. Despite shifts from PA-host plants to non-PA hosts, adult danaine butterflies have maintained their PA-pharmacophagous behavior, suggesting that certain PAs continue to play a crucial role in their reproductive strategy (Nishida, 2002).
The oscillation hypothesis proposes that herbivorous insect species alternate between periods of host range expansion, incorporating plant species from multiple families, and phases of specialization on a narrower subset of hosts (Nylin & Janz, 2009). In the genus Athalia, such a pattern may explain a potential host shift from Lamiales to Brassicaceae. Phylogenetic and chemical evidence suggest that the ancestral larval host plants of Athalia were likely Lamiales, containing iridoid glucosides, while many recent species feed as larvae only on Brassicaceae, containing glucosinolates, indicating that host shifts to this plant family have occurred repeatedly within the genus (Opitz et al., 2012). The ability to sequester glucosides potentially facilitated this shift. In line with the predicted host shift, adults of several Athalia species, including Athalia cordata Serville, Athalia circularis Klug, A. rosae, Athalia liberta Klug, and Athalia lugens Klug (Hymenoptera: Tenthredinidae), are stimulated to feed on filter paper treated with clerodendrin B, which only occurs in certain Lamiales, but not in Brassicaceae. Likewise, a convergent evolution of cucurbitacin feeding has been reported in spatially isolated rootworm taxa. In line with the ancestral host hypothesis, a common ancestor is predicted that fed solely on cucurbits, while the majority of rootworm species have undergone host plant shifts but are all still pharmacophagous on cucurbitacins (Gillespie et al., 2003).
There can also be coevolution with niche expansion to new PSMs. Plants evolve novel PSMs, toward which herbivores evolve adaptations, driving a coevolutionary arms race between plants and insects (Agrawal et al., 2009; Ehrlich & Raven, 1964), which can then lead to their co-diversification. This co-diversification may also foster the evolution of pharmacophagy. Moreover, accessing PSMs from non-food sources allows insects to explore new ecological niches, reducing competition and promoting adaptive niche expansion and diversification. We may also expect such niche shifts to occur more readily in species that are more mobile and can sample multiple plants during their lifetime or at least during adulthood, such as lepidopteran flying adults. In contrast, species such as smaller aphids may not be mobile enough to visit a non-food plant to collect new PSMs, although this may depend on the species-characteristic dispersal ability.
Finally, genetic adaptation and physiological changes are important prerequisites to manifest the genetic basis for detoxification, sequestration, or metabolizing PSMs and to be able to detect them (Heidel-Fischer & Vogel, 2015). Over time, selection pressures may favor individuals with genetic mutations that confer tolerance or detoxification capabilities, enabling them to exploit non-food plants for defense or reproductive benefits. Also, taste receptors may need modifications to be stimulated by the PSMs causing pharmacophagous behavior. The loose receptor hypothesis postulates that some gustatory receptors have only loose binding properties and thus allow for novel, potentially deleterious PSMs to trigger feeding (Tallamy et al., 1999). This may thus be a coincidence rather than adaptive in the beginning, but further selection or modification of receptors may occur afterwards if consuming a specific PSM may have a benefit. This path is discussed to have, for example, supported pharmacophagy of cucurbitacins in rootworm beetles (Tallamy et al., 1999). Further research into the genetic, physiological, and ecological mechanisms could illuminate the broader evolutionary framework underlying pharmacophagy and its role in shaping individual niches and insect biodiversity in general.
COSTS OF PHARMACOPHAGY
The uptake of PSMs may become costly in some scenarios. For example, locating and acquiring these compounds can be both energetically demanding, time-consuming, and risky, as it may increase exposure to predators during the search for suitable plants. Additionally, metabolizing these chemicals could further strain the individual's energy resources, especially if the chemicals are toxic in high concentrations. For example, growth and survival costs have been found in G. incorrupta when feeding on PA-containing plants (Singer et al., 2009). Thus, these compounds are likely only taken up when the immune response is not sufficient. Individuals that acquire PSMs through pharmacophagy may exhibit reduced longevity compared to control individuals that do not take up these compounds, even in the absence of antagonists, including pathogens, as observed in A. rosae (Zanchi et al., 2021). Moreover, competition for these valuable PSMs might trigger intraspecific aggression, as also found in A. rosae, where fighting for clerodanoids can interfere with mating (Paul & Müller, 2021). Further costs arise, as adults with clerodanoids paired with individuals lacking these metabolites experience a shortened lifespan, likely due to increased social and agonistic interactions (Singh et al., 2024a).
Finally, beyond individual costs, pharmacophagy may also constrain species by making their persistence dependent on the availability of certain PSMs. For example, in D. gilippus, males depend on pharmacophagy for PA acquisition, which is important for pheromone production and thus mating (Dussourd et al., 1989). This reliance on PA-rich plants means that habitat loss or shifts in plant availability may severely impact reproductive success. In the absence of alternative chemical sources, such specialization may lead to evolutionary dead-ends, underscoring the long-term costs of pharmacophagy.
NON-NUTRITIONAL EXPLORATION OF PLANTS IN NON-INSECT TAXA
Non-insect taxa also engage in non-nutritional interactions with plants, exploring them for benefits beyond food. Indeed, some vertebrates exhibit self-medication behaviors, seeking specific plants to counteract parasites or treat ailments (De Roode et al., 2013). For example, a Sumatran orangutan was observed using a liana (Fibraurea tinctoria Lour. [Menispermaceae]) containing furanoditerpenoids and protoberberine alkaloids to treat a wound (Laumer et al., 2024). Moreover, primates such as gorillas and chimpanzees have been observed swallowing whole leaves, which remain undigested as they pass through the digestive system and are later excreted intact, aiding in parasite removal (Huffman, 2003). For instance, chimpanzees consume leaves from Aspilia plants (Asteraceae), whose trichomes physically attach to intestinal parasites, facilitating their expulsion. Chimpanzees also ingest the bitter pith of Vernonia amygdalina Delile (Asteraceae) to control intestinal nematode infections. African elephants reportedly chew leaves from certain Boraginaceae species to induce labor, a practice also utilized by local human populations for the same purpose (Huffman & Vitazkova, 2007). Sheep infected with nematodes adjust their feeding behavior by increasing their preference for diets rich in bioactive PSMs, likely for self-medication. However, this preference does not necessarily reduce parasite loads or significantly impact health markers (Poli et al., 2018). These behaviors suggest a widespread, adaptive strategy where animals use plants for health benefits, far beyond simple nutrition. However, in some instances, it may be plant morphology (i.e., physical defenses) rather than plant chemicals that provides the advantage. Additionally, in cases involving PSMs, it remains unclear whether these compounds act as phagostimulants. Thus, further research is needed to test whether pharmacophagy, in the strict sense (Boppré, 1984), also occurs in non-insect taxa.
FUTURE DIRECTIONS
Research into pharmacophagy among insects offers exciting opportunities to broaden our understanding of insect ecology and evolution. Future studies should prioritize expanding taxonomic and ecological coverage of pharmacophagy, as current evidence largely focuses on a few well-studied taxa and chemical classes, leaving significant gaps in understanding the prevalence and diversity of these interactions. Moreover, while we here primarily focused and discussed pharmacophagy in terrestrial insects, it may occur in aquatic and marine animals too (Dettner, 2019; Putz & Proksch, 2010), which needs to be further explored. A more detailed examination of the physiological and genetic mechanisms that enable pharmacophagy is also essential. Integrated “omics” approaches combining genomics, transcriptomics, and metabolomics will be instrumental in mapping the fate of PSMs within insect bodies—from uptake and sequestration to potential chemical modifications. Exploring the ecological consequences of pharmacophagy may reveal how these interactions affect community dynamics and plant–insect networks. For instance, by incorporating PSMs, pharmacophagous insects may influence predator–prey interactions, contribute to shaping trophic relationships (Trigo, 2011), and affect overall community resilience, particularly if sequestration of PSMs alters palatability or defensive strategies. Examining whether pharmacophagy affects conspecific and interspecific interactions, such as competition, mate choice, or mutualistic relationships, will provide a deeper understanding of its ecological implications. Moreover, the role of gut microbiota in modulating the detoxification, sequestration, or bioactivation of ingested PSMs remains underexplored (but see, e.g., Genta et al., 2006 and Shukla & Beran, 2020 for detoxification); the gut microbiome may facilitate pharmacophagy by metabolizing otherwise toxic PSMs or enhancing their bioactivity.
Finally, there is potential to apply insights from pharmacophagy research to pest management and conservation. Identifying PSMs that act as attractants for insects could support the development of biologically-based pest management strategies in agriculture. For example, kairomone traps with cucurbitacins are used to control Diabrotica spp. (Arruda-Gatti et al., 2006), which is more environmentally friendly and sustainable than applying synthetic pesticides. PSMs that attract different species may also be used for biodiversity surveys. Moreover, investigating how pharmacophagous behavior impacts insect survival under environmental challenges, such as pathogen presence and climate change, could inform conservation strategies for both beneficial and threatened species. Additionally, humans have long utilized PSMs for various purposes, including medicinal uses (Wink, 2010), highlighting a significant relevance of PSMs for human health, for example, to synthesize antimicrobial or antifungal agents (Reichling, 2010) or as templates for drug development. Collectively, advancing our understanding of pharmacophagy promises to uncover intricate ecological relationships and reveal valuable applications in sustainable pest management, conservation, and even human health, emphasizing the broader relevance of this unique exploration of PSMs.
AUTHOR CONTRIBUTIONS
Pragya Singh: Conceptualization; investigation; writing – original draft; writing – review and editing; visualization. Caroline Müller: Resources; funding acquisition; conceptualization; investigation; writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
We would like to thank Konstancja Girnt for help with collecting literature for this review. This work was funded by the German Research Foundation (DFG) as part of the SFB TRR 212 (NC3), project number 396777467 (granted to CM). Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




