Dietary range varies among Aphis craccivora populations associated with different host plants: Insights into the alfalfa–cowpea aphid system in Spain
Abstract
Agroecosystems are frequently disrupted by aggressive management practices. In the case of alfalfa, regular cutting events during the season cause abrupt resource depletion, often leading to the spillover of insects into nearby areas. In this study, we assessed whether alfalfa populations of the polyphagous aphid Aphis craccivora Koch (Hemiptera: Aphididae) can utilize black locust and vetch, two common plants found near alfalfa fields, as alternative hosts following cutting events. We also determined the ability of these plants to act as A. craccivora reservoirs for the recolonization of alfalfa stands once regrowth occurs. To address these questions, we conducted host specialization trials involving host shifting in three different A. craccivora populations collected from alfalfa, black locust, and vetch. We recorded a comprehensive set of life history parameters using the two-sex life table procedure. The degree of host specialization varied among different A. craccivora populations. The alfalfa-origin population showed relatively good performance when shifted to either black locust or vetch, suggesting these plants may act as refuges for population rebuilding after cutting events, until alfalfa regrowth allows for recolonization. In contrast, populations originating from black locust or vetch performed very poorly on alfalfa, suggesting the colonization of alfalfa fields by these populations is unlikely. The mechanisms underlying host specialization in different A. craccivora populations and the observed differences in dietary diversity are discussed. This study provides valuable insights into the ecology of this insect in the most significant Spanish alfalfa-producing region.
INTRODUCTION
Most agroecosystems are disrupted by intensive management regimes, including aggressive practices such as tilling, insecticide application, and harvesting and cutting. This has a severe impact on the arthropod communities inhabiting such unstable systems (Gallé et al., 2018; Rusch et al., 2010), whose movement is expected to follow resource availability (González et al., 2016).
Alfalfa (Medicago sativa L.) is one of the most important legume forage crops (Guo et al., 2022; Latif et al., 2023), with a global cultivated area of ~30 million ha (Delgado & Lloveras, 2020). Although primarily used as fodder for livestock, alfalfa cultivation is also valued for its positive effects on soil quality (Lloveras & Delgado, 2020; Osterholz et al., 2019). In Spain, it is a traditional component of crop rotations, covering ~266 000 ha, which represents 20% of the total area devoted to this crop in Europe (Delgado & Lloveras, 2020). The main production area is concentrated along the Ebro Basin (north-east Iberian Peninsula), where small crop stands are interspersed with scattered non-cultivated patches (older fallows, natural habitats, margins, forests repopulated by Pinus halepensis [Mill], irrigation canals, roads, and small urban areas). In this region, alfalfa stands typically persist in fields for 3–5 years and require low inputs of phytosanitary products compared to other crops (Lloveras & Delgado, 2020). This makes alfalfa a more stable habitat, serving as a reservoir for numerous insect species (Núñez, 2002; Pons et al., 2005, 2009, 2011; Pons & Núñez, 2020). However, alfalfa management involves regular cutting events during the growing season (usually 5–6 cuttings from April to September, at intervals of 30–40 days), resulting in significant habitat disruption due to abrupt resource depletion. Cutting and harvesting thus force the surviving insects to spill over into surrounding areas, such as field margins, adjacent arable fields, or nearby natural/semi-natural areas (Blitzer et al., 2012; Madeira & Pons, 2015; Rand et al., 2006), where they seek alternative food sources. Alfalfa stands are recolonized by insects from adjacent crops or natural habitats following regrowth (di Lascio et al., 2016; Madeira et al., 2019; Madeira & Pons, 2015).
The cowpea aphid (Aphis craccivora Koch) (Hemiptera: Aphididae) is one of three main aphid species inhabiting Spanish alfalfa stands and is the most polyphagous. It is a cosmopolitan species that attacks a wide range of host plants, targeting more than 300 species within the family Fabaceae, as well as several members of at least 70 other families (Holman, 2009). Aphis craccivora is anholocyclic throughout most of its range (Blackman, 2024) and is an important vector for more than 50 plant viruses (Stoetzel & Miller, 2001; Zhaozhi et al., 2017). Its phenology in the alfalfa stands of the Ebro Basin is well-defined, where it remains present throughout the growing season, occasionally reaching high population densities that can compromise forage quality (Meseguer et al., 2021; Pons et al., 2005, 2009; Pons & Núñez, 2020). However, its significant polyphagy raises two main questions. First, during alfalfa cutting, can alate morphs migrate to nearby areas to find alternative hosts where the populations increase until alfalfa regrowth and recolonization? Second, can individuals from fully developed populations on other host plants in the surroundings colonize alfalfa fields?
Black locust (Robinia pseudoacacia L.) and vetch (Vicia sativa L.) are common elements in the agricultural landscapes of the Ebro Basin. Both species belong to the family Fabaceae and are host plants for A. craccivora (Blackman, 2024). Black locust can be found growing spontaneously on field margins and as an ornamental tree in street alignments, parks, and gardens of nearby urban areas, whereas vetch is an annual crop cultivated as fodder for cattle or used for green manure (Lloveras et al., 2004). Due to their proximity to alfalfa fields, both plants could serve not only as alternative hosts for alfalfa-origin A. craccivora populations following cutting events but also as potential sources of A. craccivora populations fully developed on those species for the subsequent colonization of alfalfa fields.
Host fidelity and specialization studies could help to unravel the ecological processes underlying the alfalfa–cowpea aphid system. Genetic screening has revealed the presence of different host races or host-associated biotypes in several aphid species (Ferrari et al., 2011; Shufran et al., 2000; Vanlerberghe-Masutti & Chavigny, 1998). The genetic variations and diverse facultative symbiont associations among various A. craccivora populations worldwide suggest the existence of different biotypes within this species (Brady et al., 2013; Helmi & Sharaf, 2016). However, there is little empirical evidence supporting host specialization in European populations. The use of life tables in host fidelity trials can fill this knowledge gap by providing standard sets of life history data that allow the performance of insect populations to be assessed on different hosts (Ruggle & Gutierrez, 1995). Accordingly, we used life table data in this study to assess (i) the fitness of an alfalfa-origin A. craccivora population when switched to two potential alternative hosts (black locust or vetch) and (ii) the fitness of populations from black locust and vetch when switched to alfalfa. Specifically, we asked whether alfalfa-origin populations can use these common alternative hosts as refuges following alfalfa cutting events and whether alfalfa colonization by black locust- or vetch-origin A. craccivora populations can occur. We propose that cowpea aphid populations show host fidelity, leading to better performance on their original host plants. Nonetheless, we expect them to be able to develop on the alternative hosts.
MATERIALS AND METHODS
Host plants
Alfalfa (Aragon ecotype) seeds were obtained from a local farmer (41°46′50.8″N, 0°31′12.7″ E), whereas black locust and vetch seeds were purchased from CANTUESO Natural Seeds S.L., Córdoba, Spain (ref. SC00365 and SC02376, respectively).
Plant cultivation
We sowed 5–7 seeds per host plant in plastic pots. For vetch and black locust, we used 0.5-L circular pots (8 cm diameter × 10 cm), and for alfalfa we used 0.2-L tubular pots (4.6 cm diameter × 12 cm) to provide optimal conditions for the taproot system. The plants were grown in soil with drainage and air capacity suitable for young plants (Traysubstrat, Klasmann-Deilmann, Geeste, Germany) under controlled conditions in a greenhouse (20–25°C and a L16:D8 photoperiod under Grolux fluorescent tubes). Pots were placed on cultivation tables with automatic flood irrigation to maintain the appropriate soil moisture. Plants were kept under these conditions until they reached the required stage of development (alfalfa plants 15–20 cm high after 2–3 cuttings, fully-developed black locust plants ~15 cm high, and vetch plants 15–20 cm high with secondary shoots). The density was reduced to two host plants per pot by pulling out the remaining plants before conducting the trials.
Aphid rearing
In 2021, three populations of A. craccivora were collected from alfalfa, black locust and vetch, respectively. One population was collected from a black locust street alignment in the city of Lleida, Spain (41°37′43.043″ N, 0°37′47.816″ E), whereas specimens from both alfalfa and vetch crops were collected from different fields in the Almenar agricultural area of Lleida, Spain (41°46′47.252″ N, 0°31′15.363″ E and 41°46′25.532″ N, 0°33′9.367″ E, respectively). The specimens were brought to the laboratory and colonies were established on their original host plants for 6 months (~20 generations) before conducting the trials. The aphids were kept in BugDorm-1 nylon net rearing cages (30 × 30 × 30 cm; MegaView Science, Taichung, Taiwan) in a growth chamber at 22 ± 1°C, 75% ± 5% r. h. and a L16:D8 photoperiod. Aphid populations were provided with a new plant twice per week, and the old ones were removed.
Experimental setup of the host fidelity study
Life table data were collected using a group rearing design (Chang et al., 2016). Each experimental unit consisted of one growing pot (prepared as described above). Ten winged females were placed in each pot and allowed to produce nymphs overnight (~15 h). Females were removed the day after, and the number of newborn aphids was standardized to 10 by gently removing any remaining nymphs with a fine brush. Each pot was isolated by a transparent methacrylate tube (15 cm diameter × 25 cm) to facilitate handling and prevent aphid escape. The number of aphids and their instars were recorded daily. Molting to a new instar was determined by the presence of exuviae and a considerable increase in body size and siphuncle length. A handmade white paper disk placed around the plant stems facilitated the collection of exuviae. Upon reaching adulthood, aphid weights were recorded during the first 24 h using a Sartorius Entris II BCE precision balance (±0.1 mg). Additionally, their progeny was recorded (number of newborn aphids) and removed daily until their death. The aphids were transferred to a new pot every week with young host plants prepared as described above due to practical considerations, such as ease of manipulation and space constraints.
To assess the host fidelity of the three different A. craccivora populations, the experiment was divided into three main sets: (1) aphids from the alfalfa-origin population were switched to the alternative hosts black locust and vetch (three treatments: alfalfa control, alfalfa → black locust, and alfalfa → vetch); (2) aphids from the black locust-origin population were switched to alfalfa (two treatments: black locust control and black locust → alfalfa); and (3) aphids from the vetch-origin population were switched to alfalfa (two treatments: vetch control and vetch → alfalfa). Each treatment consisted of eight replicates. All tests were performed at 22 ± 1°C, 75% ± 5% r. h. with a L16:D8 photoperiod under fluorescent lamps.
Data analysis
The life table data were collected and analyzed according to the age-stage, two-sex life table procedure (Chi, 1988; Chi & Hsi, 1985) using TWOSEX-MSChart (v. 11/21/2023) (Chi, 2024a). This approach considers stage differentiation, variable developmental rates among individuals, and both sexes (Chi et al., 2020), allowing for an accurate description of the development, survival, and reproduction of both two-sex and parthenogenetic populations (Huang & Chi, 2012; Tuan et al., 2016). To calculate the different life table parameters, group-reared life table data were converted into individual-based life tables (Chang et al., 2016). The different estimated life table parameters and their corresponding formulae are presented in Table 1. We also determined the age of 0.5 lx (time (d) when only 50% of the initial cohort survives), pre-adult developmental time, adult longevity, and total longevity (from birth to death), only including individuals that reached adulthood. The pre-reproductive period (days from birth to onset of first reproduction), total fecundity (total number of newborn aphids produced per female during its lifetime), and the reproductive days (the number of days in which females produce offspring) were also estimated. To increase robustness, standard errors of the different life table parameters were estimated using a bootstrap technique with 100 000 iterations (Polat-Akköprü et al., 2015). Within each experimental set, parameters were then compared between treatments using a paired bootstrap test (Chi, 2024a; Mou et al., 2015). The program TIMING-MSChart (v. 12/15/2023) (Chi, 1990, 2024b) was used to project the growth of the different populations over 60 days.
| Life-table parameter | Formula | Definition |
|---|---|---|
| x (day) | – | Age class of individuals from stage N1 |
| Age-stage-specific survival rate | Probability of survival for a newborn nymph to age x and stage j (x = age; j = stage; nxj = number of individuals which survived to age x and stage j; n01 = initial number of newborn nymphs with which the experiment was started) | |
| Age-specific survivorship | Simplified version of Sxj with no stage differentiation (k = number of life stages). Probability of survival for a newborn nymph to age x | |
| Age-stage specific fecundity | The fecundity of an individual at age x and stage j (Nx = number of nymphs produced at age x) | |
| Age-specific fecundity | Daily number of offspring produced per individual of age x | |
| Age-specific net maternity | Daily number of offspring produced per individual of age x when considering the survival rate | |
| Gross reproductive rate | Mean number of offspring produced per individual during its lifespan, ignoring the survival rate (α—β = reproductive period, the time period between the first [α] and last [β] reproduction) | |
| Net reproductive rate | Mean number of offspring produced per individual during its lifespan, considering the survival rate | |
| Intrinsic rate of increase (r) | The population instantaneous growth rate as time tends toward infinity and the population approaches stable age-stage distribution | |
| Finite rate of increase | Rate at which the population increases from 1 day to the next as it approaches stable age-stage distribution | |
| Mean generation time | Time that a population needs to increase by R0-fold as it approaches stable age-stage distribution and increase rate |
To compare adult aphid weights, data normality and homoscedasticity were assessed using Shapiro–Wilk and Levene's tests, respectively. Nonparametric tests were applied due to the absence of a normal distribution. Within the alfalfa → alternative hosts experimental set, weights were compared between treatments using a Kruskal–Wallis test. Then, pairwise Wilcoxon rank sum tests with Holm correction were run for multiple comparisons. Within the two remaining experimental sets (black locust → alfalfa and vetch → alfalfa), weights were compared using a Mann–Whitney test. The statistical analysis was conducted using R v.4.0.3. (R Development Core Team, 2022).
RESULTS
Life table parameters when host switching
Alfalfa-origin aphid population—Survival rate, developmental time, and longevity
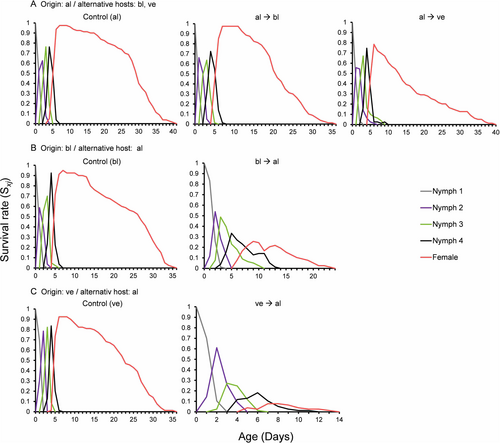
The alfalfa-origin aphid population successfully completed all developmental stages when switched to both alternative hosts. The age-stage-specific survival rates (Sxj) for different instars are shown in Figure 1A. Overall, the pre-adult survival rates of the initial cohorts were high on both the original and alternative host plants; still, significant differences were found, with aphids reared on vetch showing the lowest pre-adult survival (Table 2). The age-specific survival rate (lx) decreased more slowly when aphids were reared on alfalfa (Figure 2A), reaching the 50% survival rate significantly later than on either of the alternative hosts, with vetch showing the shorter period (Table 2). Pre-adult development was rapid on all three host plants, although it took significantly longer on black locust than alfalfa (Table 2). The adult and total longevity of the alfalfa-origin population were also affected by host switching. Aphids reared on their original host presented the longest lifespan and duration of the adult stage. In contrast, when the alfalfa-origin population was switched to an alternative host, both adult and total longevity decreased significantly: by 7 days on black locust and 10 days on vetch (Table 2). None of the initial newborn nymphs developed as alates.
| Pre-adult survival (%) | Age of 0.5 lx (d)† | Pre-adult development (d) | Adult longevity (d) | Total longevity (d) | |
|---|---|---|---|---|---|
| Origin: al/alternative hosts: bl, ve | |||||
| Control (al) | 97.5 ± 1.7 a | 30 ± 0.57 a | 5.5 ± 0.07 a | 23.04 ± 0.74 a | 28.54 ± 0.79 a |
| al → bl | 98.7 ± 1.2 a | 21 ± 0.85 b | 5.72 ± 0.08 b | 16.23 ± 0.62 b | 21.95 ± 0.67 b |
| al → ve | 86.1 ± 3.9 b | 13 ± 1.27 c | 5.74 ± 0.14 ab | 12.21 ± 1.10 c | 17.94 ± 1.15 c |
| Origin: bl/alternative host: al | |||||
| Control (bl) | 95 ± 2.4 a | 27 ± 1.71 a | 5.29 ± 0.06 a | 19.39 ± 0.88 a | 24.68 ± 0.87 a |
| bl → al | 41 ± 5.6 b | 7 ± 1.62 b | 8.88 ± 0.44 b | 5.91 ± 0.58 b | 14.78 ± 0.74 b |
| Origin: ve/alternative host: al | |||||
| Control (ve) | 93.7 ± 2.7 a | 23 ± 1.37 a | 5.22 ± 0.05 a | 17.84 ± 0.79 a | 23.05 ± 0.81 a |
| ve → al | 13 ± 3.8 b | 4 ± 0.70 b | 7 ± 0.53 b | 3 ± 0.50 b | 10 ± 0.72 b |
- Abbreviations: al, alfalfa; bl, black locust; d, days; ve, vetch.
- † Within each experimental set, different letters in the same column indicate significant differences between host plants at α < 0.05. Standard errors were estimated by 100 000 bootstrap resampling.
Alfalfa-origin aphid population—Reproduction
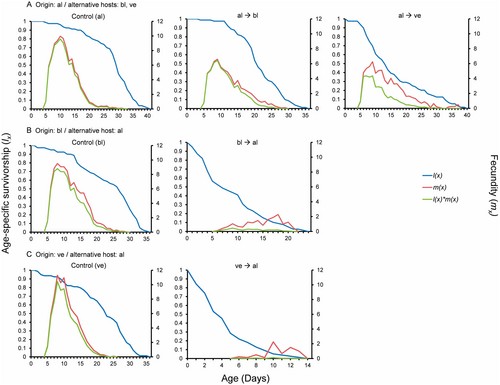
Host switching had varied effects on the reproduction parameters of the alfalfa-origin aphid population. Age-specific fecundity (mx) and age-specific net maternity (lxmx) are shown in Figure 2A. The pre-reproductive period was similar across all three host plants. However, when reared on their original host, females produced on average 32.57 and 47.04 more nymphs during their lifespan than when reared on black locust and vetch, respectively. Moreover, they reproduced for a significantly greater number of days on alfalfa compared to either of the alternative hosts. Vetch was the least suitable alternative host plant for the reproduction of the alfalfa-origin population (Table 3). Peak fecundity was observed during Days 9 and 10 in all three host plants (Figure 2A).
| Pre-reproductive period (d)† | Total fecundity (offspring female−1) | Reproductive days (d) | |
|---|---|---|---|
| Origin: al/alternative hosts: bl, ve | |||
| Control (al) | 5.59 ± 0.06 a | 90.86 ± 1.50 a | 19.19 ± 0.59 a |
| al → bl | 5.73 ± 0.08 a | 58.29 ± 1.74 b | 15.08 ± 0.49 b |
| al → ve | 5.79 ± 0.15 a | 43.85 ± 2.92 c | 10.87 ± 0.86 c |
| Origin: bl/alternative host: al | |||
| Control (bl) | 5.34 ± 0.06 a | 87.78 ± 3.33 a | 16.26 ± 0.67 a |
| bl → al | 8.97 ± 0.42 b | 7.88 ± 0.97 b | 5.45 ± 0.58 b |
| Origin: ve/alternative host: al | |||
| Control (ve) | 5.23 ± 0.05 a | 80.57 ± 2.40 a | 14.34 ± 0.50 a |
| ve → al | 7.71 ± 0.53 b | 2.7 ± 1.44 b | 2.43 ± 0.67 b |
- Abbreviations: al, alfalfa; bl, black locust; d, days; ve, vetch.
- † Within each experimental set, different letters in the same column indicate significant differences between host plants at p < 0.05. Standard errors were estimated by 100 000 bootstrap resampling.
Alfalfa-origin aphid population—Population parameters
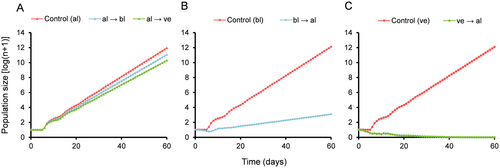
Host switching affected the population parameters of the alfalfa-origin aphid population. The estimated intrinsic rate of increase (r) and finite rate of increase (λ) indicated rapid growth of the aphid population on both the original and alternative hosts. However, aphids reared on alfalfa showed significantly higher growth rates, whereas those reared on vetch showed the lowest (Table 4). Gross (GRR) and net (R0) reproductive rates on alfalfa exceeded those on either of the alternative host plants by more than 23 and 30 nymphs per female, respectively (Table 4). The aphid population reared on alfalfa showed a significantly longer mean generation time (T) compared to the population reared on vetch, with no significant differences between alternative hosts (Table 4). All this resulted in slight differences in the projected population size over 60 days, with the population reared on alfalfa showing the most pronounced growth, followed by those reared on black locust and finally vetch (Figure 3A). All three population growth curves on a logarithmic scale approach linearity after ~30 days (Figure 3A), suggesting that the alfalfa-origin aphid population, regardless of the host, tends toward a stable age-stage distribution when it has access to unlimited resources.
| Intrinsic rate of increase r (d−1)† | Finite rate of increase λ (d−1) | Gross reproductive rate (GRR) | Net reproductive rate R0 | Mean generation time T (d) | |
|---|---|---|---|---|---|
| Origin: al/alternative hosts: bl, ve | |||||
| Control (al) | 0.44 ± 0.004 a | 1.55 ± 0.01 a | 95.01 ± 0.62 a | 88.59 ± 2.16 a | 10.2 ± 0.09 a |
| al → bl | 0.4 ± 0.004 b | 1.5 ± 0.01 b | 66 ± 1.84 b | 57.56 ± 1.87 b | 10.04 ± 0.10 ab |
| al → ve | 0.37 ± 0.01 c | 1.45 ± 0.01 c | 71.94 ± 2.51 b | 37.75 ± 3.04 c | 9.72 ± 0.15 b |
| Origin: bl/alternative host: al | |||||
| Control (bl) | 0.45 ± 0.01 a | 1.56 ± 0.01 a | 97.37 ± 2.08 a | 83.39 ± 3.81 a | 9.93 ± 0.07 b |
| bl → al | 0.09 ± 0.01 b | 1.1 ± 0.02 b | 16.65 ± 1.22 b | 3.23 ± 0.59 b | 12.61 ± 0.53 a |
| Origin: ve/alternative host: al | |||||
| Control (ve) | 0.44 ± 0.01 a | 1.56 ± 0.01 a | 88.39 ± 0.89 a | 75.47 ± 3.15 a | 9.72 ± 0.07 a |
| ve → al | −0.1 ± 0.08 b | 0.9 ± 0.06 b | 6.44 ± 3.16 b | 0.35 ± 0.21 b | 10.39 ± 1.01 a |
- Abbreviations: al, alfalfa; bl, black locust; d, days; ve, vetch.
- † Within each experimental set, different letters in the same column indicate significant differences between host plants at p < 0.05. Standard errors were estimated by 100 000 bootstrap resampling.
Alfalfa-origin aphid population—Adult weight
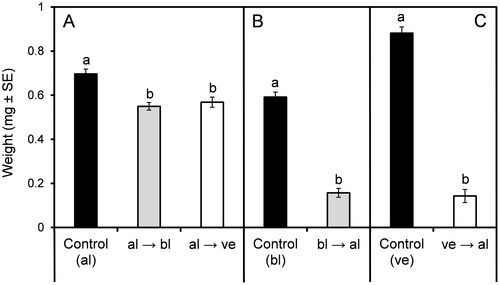
Aphids reared on their original host plant, alfalfa, weighed significantly more than those reared on either of the alternative hosts, but no differences in weight were recorded between aphids reared on black locust and vetch (Figure 4A).
Black locust-origin aphid population—Survival rate, developmental time, and longevity
The black locust-origin population was able to complete all developmental stages when switched to alfalfa as the alternative host. The age-stage-specific survival rates (Sxj) for different instars are shown in Figure 1B. The pre-adult survival rates of the initial cohorts differed significantly between the original and alternative host, dropping by half following the switch (Table 2). Similarly, the age-specific survival rate (lx) decreased sharply when aphids were reared on alfalfa (Figure 2B), reaching the 50% survival rate much sooner than when reared on black locust (Table 2). Significant differences in pre-adult development were also observed, lasting 3.6 days longer on alfalfa (Table 2). In this case, we observed a substantial overlap between the nymphal and adult stages due to the variable duration of the development of particular stages within cohorts (Figure 1B). Host switching also affected the adult and total longevity of the black locust-origin population, which showed a significantly shorter lifespan when reared on alfalfa. The difference was more evident in terms of adult longevity, which was ~14 days shorter on the alternative host (Table 2). None of the initial newborn nymphs developed as alates.
Black locust-origin aphid population—Reproduction
Reproduction parameters of the black locust-origin population were significantly affected by the switch to alfalfa. Age-specific fecundity (mx) and age-specific net maternity (lxmx) are shown in Figure 2B. The pre-reproductive period was notably longer on alfalfa, where females exhibited significantly lower fecundity and fewer reproductive days compared to those reared on their original host, differing by 79.9 nymphs per female and 10.81 days, respectively (Table 3). Peak fecundity was observed on Day 8 on black locust plants, but was significantly later on alfalfa plants (Figure 2B). Alfalfa proved to be an unsuitable alternative host plant for the reproduction of the black locust-origin population.
Black locust-origin aphid population—Population parameters
We observed a significant impact on the population parameters of the black locust-origin population following the switch to alfalfa. The estimated values of the intrinsic (r) and finite (λ) rates of increase differed significantly between the original and alternative hosts, indicating slower growth on alfalfa (Table 4). The estimated gross (GRR) and net (R0) reproductive rates were also much lower on alfalfa than on black locust, with a mean generation time (T) 2.98 days shorter (Table 4). All this caused notable differences in the predicted course of population growth, with the population reared on alfalfa showing very slow growth compared to the population maintained on the original host, black locust (Figure 3B). Both population growth curves approach linearity after ~30 days (Figure 3B), suggesting that the black locust-origin population, whether reared on its original host or on alfalfa, would probably move toward a stable age-stage distribution when presented with unlimited resources.
Black locust-origin aphid population—Adult weight
Host switching had a strong impact on adult weights, with aphids reared on the alternative host weighing almost 75% less than those reared on black locust plants (Figure 4B).
Vetch-origin aphid population—Survival rate, developmental time, and longevity
The vetch-origin aphid population was able to complete all developmental stages when switched to alfalfa as the alternative host. The age-stage-specific survival rates (Sxj) for different instars are shown in Figure 1C. The pre-adult survival rates significantly differed between the original and alternative hosts, being nine times lower on alfalfa. Similarly, a sharper decrease in the age-specific survival rate (lx) was observed when aphids were reared on the alternative host, where the 50% survival rate was reached much sooner than on vetch plants (Table 2). Pre-adult development was significantly extended on alfalfa, requiring 1.8 more days to reach adulthood than vetch (Table 2). As with the black locust-origin population, there was a substantial overlap between the nymphal and adult stages when the aphids were reared on alfalfa due to the variable duration of the development of particular stages within cohorts (Figure 1C). Host switching significantly affected the adult and total longevity of the vetch-origin population, resulting in very low values when the aphids were reared on alfalfa (Table 2). None of the initial newborn nymphs developed as alates.
Vetch-origin aphid population—Reproduction
The reproduction parameters of the vetch-origin population were significantly affected by the switch to alfalfa. Age-specific fecundity (mx) and age-specific net maternity (lxmx) are shown in Figure 2C. The pre-reproductive period was significantly longer on alfalfa than the original host. Additionally, total fecundity and the number of reproductive days were very low on alfalfa, where females barely produced any offspring (Table 3). On vetch, fecundity peaked between Days 8 and 10 (Figure 2C). Alfalfa proved to be an unsuitable alternative host plant for the reproduction of the vetch-origin population.
Vetch-origin aphid population—Population parameters
We observed significant impact on the population parameters of the vetch-origin population when switched to alfalfa. Although the population increased rapidly on its original host, both the intrinsic (r) and finite (λ) rates of increase indicated population decline on alfalfa (Table 4). Moreover, we observed significant differences in reproductive rates between hosts, with the population switched to alfalfa showing a net reproductive rate (R0) close to 0 (Table 4). There was no difference in mean generation time (T) between the original and alternative host plants (Table 4). Overall, the original host was much more suitable for aphid development and reproduction than alfalfa, resulting in a prominent difference in the predicted course of population growth over 60 days. The population shifted to alfalfa approaches collapse over time, whereas that reared on vetch increases rapidly (Figure 3C). The population growth curve of the vetch aphid population reared on its original host reaches near linearity around day 30 (Figure 3C), suggesting that it would probably approach a stable age-stage distribution when presented with unlimited resources.
Vetch-origin aphid population—Adult weight
Host switching significantly affected the weight of adult aphids, with those reared on vetch achieving an average weight six times higher than those reared on alfalfa (Figure 4C).
DISCUSSION
Agricultural landscapes are frequently disrupted by agronomic practices, which may lead to insect spillover into adjacent habitats in search of refuge and alternative food sources (Rand et al., 2006; Thorbek & Bilde, 2004). In this context, trials assessing host fidelity in phytophagous insects are valuable because they can help identify potential alternative hosts in the surroundings, enhancing our understanding of their ecology within these anthropic systems.
Overall, we found that the life history parameters of A. craccivora were significantly influenced by host switching. However, the degree of host specialization varied among different populations. The alfalfa-origin population appeared to be more generalist. Although it performed better on its original host, it showed good performance on both black locust and vetch, with acceptable life history parameters that led to a significant increase in the population over time. The aphids switching to black locust performed better than those switching to vetch, with higher pre-adult survival rates, adult and overall longevity, and fecundity, as well as more favorable population parameters. These findings suggest that, following multiple alfalfa cuttings throughout the season, surviving A. craccivora can use adjacent vetch crops or black locust in field margins to develop their populations until alfalfa regrowth allows recolonization. Previous studies have documented the spillover of different insect taxa to neighboring crops followed by recolonization after the cutting and harvesting of alfalfa stands (di Lascio et al., 2016; Madeira et al., 2019; Madeira & Pons, 2015). Nevertheless, predators such as Nabis provencalis Remane (Heteroptera: Nabidae) and Orius niger Wolff (Heteroptera: Anthocoridae) remained in the alfalfa fields following these disturbances (Madeira et al., 2019). Further investigation is required to determine which scenario applies to A. craccivora because the rebuilding of populations from survivors remaining in managed fields should also be considered. Additionally, it seems essential to explore whether nearby urban areas, where black locust has been planted in street alignments, parks, and gardens, could serve as refuges for A. craccivora populations migrating from alfalfa.
Based on our results, the colonization of alfalfa by A. craccivora populations originating from black locust or vetch seems improbable because their performance was poor on this alternative host. All estimated parameters were significantly lower on alfalfa compared to their respective original hosts, which reduced population growth to minimal levels or even caused population decline. The poor suitability of alfalfa as a host, coupled with the unstable abiotic stress factors and predation/parasitism pressure that aphid populations experience under field conditions, suggests population collapse is likely if A. craccivora populations originating from black locust or vetch were to colonize alfalfa. These results highlight the greater degree of host specialization in these two A. craccivora populations compared to that originating from alfalfa.
Polyphagous species often include host races or host-specialized populations with a narrow host range, which suggests an ongoing process of speciation by adaptation to distinct host plants (Carletto et al., 2009; Margaritopoulos et al., 2005; Via et al., 2000). Various ecological factors can drive host plant specialization, with their significance varying across systems. First, host plants serve as the sole food resource and habitat for their associated aphids (Peccoud et al., 2010). Consequently, structural and chemical differences between host plants can select for specific characteristics in the insects that attack them, such as feeding mechanisms, digestive physiology, or morphological traits (Ma & Liu, 2020; Shih et al., 2023). In this context, populations of A. craccivora feeding on black locust feature a significantly larger ultimate rostral segment than conspecifics associated with other host plants, suggesting this is an adaptation that allows the penetration of thick tree bark to facilitate the feeding process (Mehrparvar et al., 2012). However, adaptations to a specific environment usually involve a trade-off against performance in other environments (Caillaud & Via, 2000), which may explain the poor performance of the black locust-origin population when shifted to alfalfa. The impact of this morphological adaptation on the fitness of aphids transferred to other host plants, and the underlying mechanisms, requires further investigation.
Another important factor contributing to host specialization is the tendency of aphids to mate where they develop, therefore limiting gene flow between populations associated with different hosts (Caillaud & Via, 2000; Feder, 1998; Peccoud et al., 2010; Via, 1999). Nevertheless, this pattern does not broadly apply to A. craccivora because this species is anholocyclic throughout most of its range (Blackman, 2024). Other mechanisms probably contribute to host specialization in asexual lineages. Loxdale (2009) suggested that, due to the high reproductive rate of aphids, numerous mutations can arise during parthenogenesis, leading to widespread genetic variation among different clonal lineages. Many mutations are deleterious or (particularly if they occur in noncoding regions) neutral in effect, whereas others may affect key genes, providing adaptive characteristics and becoming fixed in a specific biotype or lineage (Shih et al., 2023). This suggests that genetic differences between populations of A. craccivora associated with different hosts (Coeur d'acier et al., 2007) may reflect genetic variation that arises during parthenogenesis, which is then subject to selection by the prevalent ecological conditions. Different mitochondrial cytochrome c oxidase subunit I (COI) haplotypes have been reported when comparing alfalfa and black locust-origin A. craccivora populations (Brady et al., 2013). However, the analysis of aphids from the single vetch-origin population included in the same study revealed only haplotypes associated with alfalfa and black locust populations, suggesting they were vagrant individuals established on this crop. This is consistent with findings in the closely related species fava bean (Vicia faba L.), where aphid populations also featured alfalfa and black locust-associated haplotypes (Brady et al., 2013). Fava bean is an acceptable host for different clones of A. craccivora (Ferrari et al., 2008; Wagner et al., 2015), as well as the pea aphid Acyrthosiphon pisum Harris (Ferrari et al., 2008). Additional genetic studies involving a representative number of vetch-origin populations are therefore needed to determine whether a specific haplotype is associated with this plant or if its close relationship with fava bean makes it a universal host for A. craccivora, which would explain the good performance of our alfalfa-origin population when shifted to this host.
Finally, heritable symbiotic bacteria found in herbivorous insects may influence dietary breadth either by facilitating the utilization of additional plant species or promoting host specialization by enhancing performance on some plants while diminishing it on others (Shih et al., 2023; Tsuchida et al., 2004). Aphis craccivora populations on black locust are strongly associated with the facultative symbiont Arsenophonus (Brady et al., 2013), which enhances aphid performance on this host probably by compensating for the low quantities of plant-derived vitamin B (Hansen, 2018; Wagner et al., 2015). However, Wagner et al. (2015) also found that Arsenophonus decreases fitness on alfalfa, potentially explaining the poor performance observed in our study when the black locust-origin population was shifted to alfalfa. The relatively good performance of the alfalfa-origin population when shifted to black locust may also be a consequence of infection with Arsenophonus. A comprehensive study of worldwide A. craccivora populations revealed a unique population from alfalfa naturally infected with a variant Arsenophonus strain (Brady et al., 2013), and this population was found in Lleida (Spain), the same province where we collected our insects. Our findings suggest that our alfalfa-origin population could also be infected, thereby explaining its good performance when shifted to black locust. In a host switching experiment, Wagner et al. (2015) reported the poor performance of alfalfa-origin clones on black locust, but transinfection with Arsenophonus increased their fitness on this host and broadened dietary breadth, making the aphids more generalized. This aligns with our results and supports the hypothesis that our alfalfa-origin population is infected with Arsenophonus. The associated endosymbiont diversity may also explain the performance differences of alfalfa and vetch-origin populations when shifting between those two hosts. However, more research involving these host-associated populations is required to fully understand our results. Pea aphids infected with Regiella performed slightly better on vetch than uninfected conspecifics (Tsuchida et al., 2004), which could also explain the relatively good performance of our alfalfa-origin population when shifted to this host. Regarding the poor performance of our vetch-origin population on alfalfa, our hypothesis points again to Arsenophonus, given the high prevalence of this symbiont in two A. craccivora populations collected from the closely related species V. faba (Brady et al., 2013).
Our study provides valuable insights into the ecology of A. craccivora in the most significant Spanish alfalfa-producing region. The application of life tables in host fidelity studies provided comprehensive life history data, enabling the thorough assessment of host suitability. Alfalfa-origin population achieved successful development on both black locust and vetch, indicating the potential role of these plants as alternative hosts for population rebuilding following alfalfa cutting events. Conversely, the colonization of alfalfa by populations originating from black locust or vetch appears unlikely, given the poor performance observed following the switch to this host. Our findings also suggest the alfalfa-origin population has a greater dietary range, and that alfalfa clones therefore constitute a distinct and more generalist biotype than black locust and vetch clones. It is important to note that this study focused on a single population from each host plant. Future research expanding the number of populations examined for each host would strengthen our findings and allow for broader extrapolations to other populations worldwide. Additionally, screening the symbiont diversity among the A. craccivora populations from alfalfa, black locust, and vetch will enhance our results and provide deeper insights into the A. craccivora system in the Ebro Valley. Given the broad host range of the cowpea aphid complex, further studies are also needed to identify other potential hosts in the Ebro Valley region that may serve not only as receptors for alfalfa-origin populations but also as potential sources.
AUTHOR CONTRIBUTIONS
Roberto Meseguer: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing – original draft. Alexandre Levi-Mourao: Investigation; methodology; writing – review and editing. Xavier Pons: Conceptualization; funding acquisition; methodology; resources; supervision; validation; writing – review and editing.
ACKNOWLEDGEMENTS
We thank Oriol Cervelló and Dr. Addy García for support with laboratory tests. Special thanks are due to Prof. Dr. Hsin Chi for his help and advice during life table data analysis. We also thank Dr. Richard M. Twyman for English language editing. This project received funding from the Ministerio de Ciencia, Innovación y Universidades of the Spanish Government (project AGL2017-84127-R). R.M. acknowledges an FPI grant provided by the Ministerio de Ciencia e Innovación of the Spanish Government, and A.L-M. was funded by a Jade plus grant from the Universitat de Lleida.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest, financial or otherwise, that might potentially bias this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




