Footprints of the lady beetles Cryptolaemus montrouzieri and Tenuisvalvae notata affect their foraging behavior and predation rate
Abstract
The lady beetles Tenuisvalvae notata (Mulsant) and Cryptolaemus montrouzieri Mulsant (both Coleoptera: Coccinellidae), are used in biological control of mealybugs in many countries, including Brazil. As individuals of these species walk on plant surfaces, they leave chemical footprints that can affect their interactions with conspecifics, as well as other coccinellid species in the same environment. Therefore, this study aimed to evaluate the effects of T. notata and C. montrouzieri footprints on both their own and each other's foraging behavior and predatory potential. Arena bioassays were conducted to evaluate the effect of the footprints on conspecifics and heterospecifics. In addition, the chemical profiles of these footprints were analyzed by gas chromatography, flame ionization detection, and mass spectrometry (GC-FID and GC–MS). For this, extracts of footprints were obtained from a glass Petri dish where 20 adults (males or females) were allowed to walk for 24 h. Behavioral bioassays showed that both species can recognize each other's footprints. Tenuisvalvae notata females avoided areas treated with footprints of heterospecific females, whereas C. montrouzieri females were arrested on areas with footprints of T. notata females. Tenuisvalvae notata adults did not change their predation rate when exposed to the footprints of heterospecifics, but second instars had a higher predation rate on areas treated with heterospecific footprints. Second instar C. montrouzieri also had an increase in predation when exposed to footprints of heterospecifics, and females captured significantly more prey when exposed to footprints of conspecifics, whereas males' predation rate decreased when exposed to footprints of both conspecifics and heterospecifics. The chemical analyses showed that the hexane extracts of footprints were composed mainly of linear hydrocarbons, saturated and non-saturated, from C20 to C33. The composition (qualitative and quantitative) of footprint extracts is species- and sex-specific. This is the first report on the effects of footprints on the behavior of these species.
INTRODUCTION
The family Coccinellidae (Coleoptera) is a large group of beetles distributed worldwide, commonly known as ladybugs, ladybirds, or lady beetles (Krinsky, 2019). Among the described species of coccinellids, about 90% are carnivores, which make them important biocontrol agents of insects, such as aphids, mealybugs, psyllids, insect eggs, and small caterpillars, in multiple crops (Hemptinne & Dixon, 1997; Scopel & Roza-Gomes, 2011; Triplehorn & Johnson, 2011; Hodek et al., 2012; Sarwar, 2016).
In classical biological control, an exotic natural enemy species is introduced/imported to control an exotic pest species (DeBach, 1964). In this context, the lady beetle known as the mealybug destroyer, Cryptolaemus montrouzieri Mulsant, which is native to Australia, was introduced in Brazil to control Planococcus citri (Risso) in citrus orchards (Sanches & Carvalho, 2010), and later used to control the hibiscus mealybug Maconellicoccus hirsutus (Green) (Culik et al., 2013; Lima & Santos, 2018). This species has also been introduced in more than 40 countries in both temperate and tropical regions to control mealybugs (Kairo et al., 2013). Another lady beetle species used in classical mealybug control programs is Tenuisvalvae notata (Mulsant) (= Hyperaspis notata). It is native to South America, and was introduced in Africa in the late 1980s to control the cassava mealybug, Phenacoccus manihoti Matille-Ferrero (Chakupurakal et al., 1994). In Brazil, T. notata has been found in the north, northeast, midwest, and southeast regions preying upon various mealybug species (Dreyer et al., 1997; Barbosa et al., 2014; Peronti et al., 2016; Ferreira et al., 2020).
In agroecosystems, multiple lady beetle species, both indigenous and introduced, may cooccur, especially if they have similar thermal requirements and geographical distributions, as is estimated for T. notata and C. montrouzieri (Ferreira et al., 2021). Moreover, these cooccurring species may compete for the same primary food resource (mealybugs) or just for space and supplementary food resources, such as pollen and nectar. It is known that many ecological interactions among lady beetle species occur mostly by chemical communication, and that emission and perception of semiochemicals are important for foraging, mating, finding refuges and oviposition sites, and defense against predators (Vilela & Pallini, 2002; Zarbin et al., 2009; Pettersson, 2012).
Hydrocarbons have been found in volatiles, footprints, and cuticular lipids of lady beetles (Kosaki & Yamaoka, 1996; Al Abassi et al., 1998; Pattanayak et al., 2014). According to Pattanayak et al. (2015), the amount and composition of those chemical compounds are affected by the nutrition and habitat of the lady beetles and can play a role in species recognition (Hemptinne & Dixon, 2000), aggregation (Wheeler & Cardé, 2013), mating behavior (Fassotte et al., 2016), and oviposition (Mishra et al., 2013). These compounds may also reduce intraguild predation in lady beetles (Katsanis et al., 2017).
Coccinellids leave footprints on the plant surface as they walk around. These footprints are perceived by prey and other natural enemies, and may cause them to flee the area, thus, interfering with the biological control of pests at that site (Ruzicka, 2001; Magro et al., 2007; Mishra et al., 2013; Ninkovic et al., 2013). Previous studies have shown that footprints of the lady beetle Coccinella septempunctata L., Cycloneda limbifer Casey, and Semiadalia undecimnotata (Schneider) reduced the oviposition of conspecifics, and decreased the interspecific competition among them (Ruzicka, 2001). Insect parasitoids may also use lady beetle footprints to avoid parasitizing a host that is more vulnerable to predation by lady beetles (Nakashima et al., 2004).
Tenuisvalvae notata and C. montrouzieri share the same environment and the same food source and, therefore, may compete by sharing the same prey (Peronti et al., 2016). Thus, unraveling whether these two species of lady beetles recognize heterospecific footprints and how this recognition alters their behavior and predation rate could provide information for biocontrol programs. Here we evaluate (1) whether T. notata and C. montrouzieri use their footprints to recognize conspecifics, as well as heterospecifics, and the influence of the footprints on their predation rate, and (2) the chemistry of footprint extracts of both species to determine the major hydrocarbons present. We hypothesized that the chemical profile of the footprints is species- and sex-specific and influences the pattern of locomotion and predation rate.
MATERIALS AND METHODS
The insects used in the bioassays were obtained from the colonies maintained in the Insect Behavior Laboratory, at the Universidade Federal Rural de Pernambuco (UFRPE) (−8.017070°S, −34.944362°W). The conditions for insect culture maintenance and execution of bioassays were 25 ± 2 °C, 60 ± 10% r.h., and L12:D12 photoperiod.
Prey
The colony of Ferrisia dasylirii (Cockerell) (Hemiptera: Pseudococcidae) was reared on pumpkins, Cucurbita moschata (Duch.) var. jacarezinho (Cucurbitaceae), in the initial maturation stage, obtained from the local market ‘Centro de Abastecimento Alimentar’ (CEASA), Recife-PE, Brazil. After being rinsed and dried, pumpkins were placed in plastic trays (30 × 45 × 4 cm) lined with a paper towel and infested on the petiole with gravid mealybugs, originating from the stock colony.
When the pumpkin was completely infested with mealybugs, a clean pumpkin was placed on top of it, allowing free movement of the nymphs and adults to the new pumpkin. The average time to complete infestation of a pumpkin was about 30 days, after which time the infested pumpkins were used in lady beetle colonies.
Predators
Colonies of T. notata and C. montrouzieri were kept apart in the laboratory, but under the same environmental conditions as the prey. Adults of each predator species were placed in acrylic boxes (40 × 25 × 20 cm), with circular lateral openings, covered with a fine mesh to allow ventilation. The bottom of the boxes was lined with a paper towel, upon which one infested pumpkin was offered to the predators, following Barbosa et al. (2014). Lady beetles were allowed to feed and mate freely in the rearing boxes. New infested pumpkins were offered to predators every 20 days, as prey were consumed from the previously offered pumpkins. Eggs and larvae of the predators were kept in the same rearing cages as the adults.
Walking behavior of lady beetles exposed to footprints of conspecifics and heterospecifics
Behavioral bioassays were conducted at UFRPE. Virgin adults of T. notata and C. montrouzieri, 5–10 days old, were used in this test to investigate possible effects of footprints on walking behavior of the lady beetles. Petri dishes (9 cm diameter) were used as test arenas. Prior to tests, the base and lid of the Petri dish were covered with 9-cm-diameter filter paper discs (weight 80 g m−2; Celab, Recife, Brazil), and a group of six adult lady beetles (same sex and species) was released in the arena and allowed to walk freely for 24 h to obtain the footprints. To reduce the deposition of excrements, the lady beetles were starved for 24 h before and during the collection of footprints. After this time, insects and filter paper were collected with the help of metal tweezers. Next, the filter paper containing the footprints was cut in half, obtaining two parts per disc. These treated papers were used to prepare the test arenas, which were composed of clean 9-cm-diameter Petri dishes. Each test arena received one half of the filter paper containing the footprints and a clean filter paper half (control). The wall of the Petri dish was treated with vaseline to prevent the lady beetles climbing to the lid. After that, one lady beetle adult (male or female, of either species) was released in the center of the arena, allowed 5 min acclimation, and then its behavior was recorded for 10 min using ViewPoint 288 (ViewPoint Life Sciences, Montreal, Canada). Recorded parameters were: walking distance, walking time, walking speed, and the number of stops. Tested treatments were as follows: (1) males exposed to footprints of conspecific males vs. control (untreated area); (2) males exposed to footprints of conspecific females vs. control; (3) males exposed to footprints of heterospecific males vs. control; (4) males exposed to footprints of heterospecific females vs. control; (5) females exposed to footprints of conspecific females vs. control; (6) females exposed to footprints of heterospecific females vs. control; (7) females exposed to footprints of conspecific males vs. control, and (8) females exposed to footprints of heterospecific males vs. control. There were 40 replicates for each treatment combination, and in each replicate a new arena was used. The position of treatments was alternated between trials to avoid any bias in the response of the lady beetles.
Effect of footprints on predation rate of lady beetles
In this bioassay, we investigated the predation rate of larval and adult T. notata and C. montrouzieri upon F. dasylirii. Prey was offered to lady beetles as follows: six mealybug nymphs were offered to one first- or second-instar lady beetle, or six adult mealybugs were offered to one third or fourth instar and one adult of the lady beetle. This number of mealybugs provided daily to T. notata and C. montrouzieri was determined based on previous tests and studies, which showed that they consumed an average of less than five mealybugs per day (Grafoor et al., 2011; Barbosa et al., 2014). The effect of footprints on predation rate was measured in a glass Petri dish arena (5.5 cm diameter). Prior to the predation experiment, two adult lady beetles (C. montrouzieri or T. notata) were placed in each Petri dish and allowed to walk around freely inside the arena for 24 h to leave their footprints. Meanwhile, the first- and second-instar lady beetles were starved for 2 h, and older larvae and adults were starved for 24 h before the test (adapted from Sengonca et al., 1995) in order to equalize hunger level and induce predation across the various treatments during the assay (Chong & Oetting, 2007; Finlay-Doney & Walter, 2012; Barbosa et al., 2014).
Predation rate upon mealybugs was measured according to the following treatments: (1) first to fourth instars of T. notata on heterospecific footprints; (2) first to fourth instars of T. notata on conspecific footprints; (3) first to fourth instars of C. montrouzieri on heterospecific footprints; (4) first to fourth instars of C. montrouzieri on conspecific footprints; (5) adults (male or female) of C. montrouzieri on heterospecific footprints; (6) adults (male or female) of T. notata on heterospecific footprints; (7) adults (male or female) of T. notata on conspecific footprints; (8) adults (male or female) of C. montrouzieri on heterospecific footprints; (9) and (10) first to fourth instars and adults of T. notata on a ‘clean’ Petri dish, without footprints of other individuals (control); and (11) and (12) first to fourth instars and adults of C. montrouzieri on a ‘clean’ Petri dish (control). Each treatment had 20 replicates. The number of mealybugs consumed was measured after 24 h of exposure.
Collection of footprints
Glass Petri dishes (5.5 cm diameter) were used to collect the footprints of the lady beetles. The pre-experiment cleaning procedure was as follows: Petri dishes were washed with water and acetone and heated at 180 °C for 12 h in a convection oven (Ethik, Vargem Grande Paulista, SP, Brazil). Twenty adults, 5–10 days old, of either lady beetle species, of the same sex, were placed in each clean Petri dish. Insects were allowed to walk around freely for 24 h. After that, adults were removed, and the Petri dishes were washed with 2 mL of distilled hexane PA (high level of purity) for 1 min. Footprint extracts were concentrated to 100 μL under a nitrogen flow and stored in a freezer at −20 °C until further use in chemical analyses. Each treatment (species and sex seperately) had six replicates.
Chemical analyses
For quantitative analysis, all footprint extracts were analyzed by a 7890A gas chromatography coupled to a flame ionization detector (GC-FID; Agilent Technologies, Santa Clara, CA, USA), equipped with a DB-5MS column (0.25 mm inner diameter × 30 m, 0.25 μm film; J&W Scientific, Folsom, CA, USA). The temperature of the detector was adjusted at 270 °C. The oven was programmed to 50 °C for 2 min, then to 280 °C at 5 °C per min, followed by an increase of 10 °C per min to 280 °C (held for 20 min). Aliquots of 2 μL of each sample were injected using the splitless mode, with the inlet at 300 °C, and helium as the carrier gas. Data were collected using GC OpenLabsoftware (Agilent). As an internal standard (IS), each extract sample received 1 μL of 16-hexadecanolide (in distilled hexane) to a final concentration of 0.01 mg mL−1. The quantification was done comparing the area of the IS with the areas of all compounds in the chromatogram profile. The response factor for each compound was considered 1.0.
For qualitative analysis, selected footprint extracts were analyzed using a QP 2010 quadruple mass spectrometer (MS; Shimadzu, Tokyo, Japan) equipped with a DB-5MS column (0.32 mm ID × 60 m, 1.0 μm film; Supelco, Bellefonte, PA, USA), a splitless injector, and helium as the carrier gas. Ionization was by electron impact (70 eV, source temperature at 230 °C). The injector was at 250 °C using the same temperature programme as in GC-FID analysis. Data were collected with GC-solution software (v.2.42; Shimadzu). Identifications were made by comparison of spectra with mass spectral library databases (NIST, 2008), use of retention indices (RIs), and were confirmed by overlap of the footprint extracts with authentic standards. The RIs were calculated by comparison to the retention times of a series of linear hydrocarbon alkanes (C8–C40) analysed with the same temperature program.
The identification of compounds without available authentic standards was based on a comparison with spectra, their fragmentation pattern, and retention index, published at Database of Pheromones and Semiochemicals (Pherobase) and National Institute of Standards and Technology (NIST): Chemistry WebBook websites.
Statistical analysis
Data for walking distance, walking speed, and the number of stops were subjected to a multivariate ANOVA (MANOVA), using the Proc GLM of SAS (SAS Institute, 2002). Walking time in areas of the arena treated and untreated with footprints was subjected to a χ2 test using the Proc FREQ (SAS Institute, 2002). Predation data were subjected to the Shapiro Wilk normality test. As they were not normally distributed, they were subjected to general linear model (GLM) analysis with a Poisson error distribution. Due to the overdispersion of data, they were subjected to a Quasipoisson analysis, followed by a contrast analysis to separate the means. These analyses were performed using R v.4.0.5 (R Core Team, 2011).
Amounts of chemical compounds identified in footprints of lady beetles were subjected to the Shapiro Wilk normality test to check for ANOVA assumptions. As the amounts of compounds present in T. notata footprints were normally distributed, they were subjected to GLM with normal error distribution (Gaussian). In contrast, data of C. montrouzieri were not normally distributed and were subjected to GLM with Gamma error distribution. In addition, the total amount of compounds present in lady beetle footprints were not normally distributed and were √(x + 0.5) transformed to assume a normal distribution. Next, the total amount of compounds was subjected to factorial ANOVA, with species and sex as factors.
The chemical composition similarity between the footprints of T. notata and C. montrouzieri males and females was analyzed by principal component analysis (PCA). The dissimilarity was tested by a permutation ANOVA (PERMANOVA) with 999 permutations, based on the index of similarity of Bray-Curtis, using the R vegan package (R Core Team, 2011).
RESULTS
Walking behavior of lady beetles exposed to footprints of conspecifics and heterospecifics
There was no significant effect of footprints on the walking distance, walking speed, number of stops, and walking time when C. montrouzieri were exposed to footprints of conspecifics (Table 1, Figure 1). Likewise, there were no significant changes in the walking behavior parameters of C. montrouzieri males exposed to footprints of male and female T. notata (Table 1, Figure 1). In contrast, C. montrouzieri females made more stops in areas treated with T. notata male's footprints (F1,79 = 5.53, P = 0.02), increased the walking distance (F1,79 = 7.19, P < 0.01; Table 1), and spent more time in areas treated with footprints of T. notata females (χ2 = 4.5, d.f. = 1, P = 0.03; Figure 1).
| C. montrouzieri female | C. montrouzieri male | |||||||
|---|---|---|---|---|---|---|---|---|
| Footprint by | WD (cm) | WS (cm/s) | No. stops | WD (cm) | WS (cm/s) | No. stops | ||
| Conspecific | Female | Treatment | 75.35 ± 9.78 | 0.48 ± 0.03 | 252.85 ± 33.11 | 82.73 ± 9.60 | 0.46 ± 0.03 | 283.77 ± 35.67 |
| Control | 87.60 ± 9.63 | 0.48 ± 0.03 | 314.05 ± 38.41 | 90.31 ± 10.94 | 0.49 ± 0.03 | 285.12 ± 40.19 | ||
| F1,79 | 0.80 | 0.01 | 1.46 | 0.27 | 0.44 | 0.01 | ||
| P | 0.37 | 0.92 | 0.23 | 0.60 | 0.50 | 0.98 | ||
| Male | Treatment | 89.73 ± 8.46 | 0.45 ± 0.02 | 297.70 ± 30.13 | 95.27 ± 10.09 | 0.47 ± 0.02 | 259.20 ± 29.33 | |
| Control | 83.36 ± 10.16 | 0.49 ± 0.03 | 266 ± 26.73 | 84.54 ± 8.93 | 0.53 ± 0.03 | 232.82 ± 30.83 | ||
| F1,79 | 0.23 | 1.23 | 0.62 | 0.63 | 1.91 | 0.38 | ||
| P | 0.63 | 0.27 | 0.43 | 0.42 | 0.10 | 0.53 | ||
| Heterospecific | Female | Treatment | 121.17 ± 8.43 | 0.54 ± 0.03 | 321.65 ± 29.51 | 105.79 ± 11.92 | 0.58 ± 0.04 | 253.82 ± 43.52 |
| Control | 92.57 ± 6.52 | 0.57 ± 0.03 | 247.40 ± 26.44 | 164.62 ± 38.03 | 0.59 ± 0.07 | 236.05 ± 39.53 | ||
| F1,79 | 7.19 | 0.38 | 3.51 | 2.18 | 0.03 | 0.09 | ||
| P | < 0.01 | 0.53 | 0.06 | 0.14 | 0.8 | 0.76 | ||
| Male | Treatment | 100.57 ± 7.76 | 0.46 ± 0.01 | 354.32 ± 37.53 | 104.47 ± 14.40 | 0.54 ± 0.03 | 236.52 ± 39.61 | |
| Control | 86.69 ± 7.77 | 0.49 ± 0.02 | 242.25 ± 29.37 | 100.03 ± 9.09 | 0.52 ± 0.03 | 198.35 ± 27.81 | ||
| F1,79 | 1.60 | 1.01 | 5.53 | 0.07 | 0.28 | 0.62 | ||
| P | 0.21 | 0.31 | 0.02 | 0.79 | 0.50 | 0.43 | ||
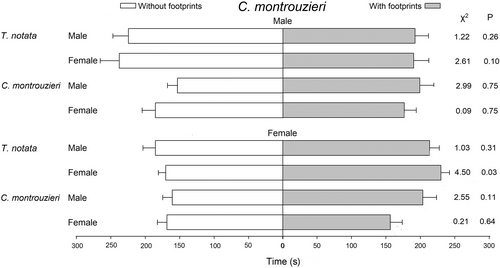
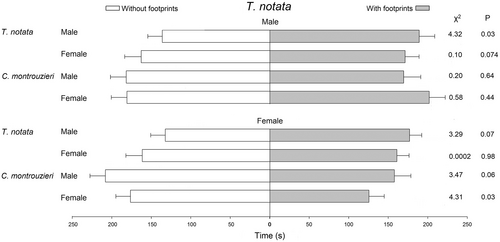
Tenuisvalvae notata males (F1,79 = 4.15, P = 0.04) and females (F1,79 = 9.26, P < 0.01) made more stops in areas treated with footprints of conspecific males (Table 2). Also, T. notata males spent more time in areas with footprints of conspecific males (χ2 = 4.32, d.f. = 1, P = 0.03; Figure 2). However, T. notata males and females did not show any significant change in their walking behavior when exposed to footprints of conspecific females. Tenuisvalvae notata males did not show any significant change in their walking behavior when exposed to footprints of C. montrouzieri males or females. In contrast, T. notata females made fewer stops (F1,79 = 8.92, P < 0.01; Table 2) and spent less time in areas treated with footprints of C. montrouzieri females (χ2 = 4.31, d.f. = 1, P = 0.03; Figure 2). In addition, T. notata females reduced the walking distance (F1,79 = 4.60, P = 0.03) and the number of stops (F1,79 = 8.02, P < 0.01) in areas treated with footprints of C. montrouzieri males.
| T. notata female | T. notata male | |||||||
|---|---|---|---|---|---|---|---|---|
| Footprint by | WD (cm) | WS (cm/s) | No. stops | WD (cm) | WS (cm/s) | No. stops | ||
| Conspecific | Female | Treatment | 69.89 ± 7.15 | 0.45 ± 0.02 | 347.82 ± 38.68 | 90.06 ± 15.59 | 0.47 ± 0.03 | 317.72 ± 36.85 |
| Control | 62.24 ± 9.63 | 0.46 ± 0.02 | 254.15 ± 31.26 | 75.42 ± 8.75 | 0.46 ± 0.04 | 258.07 ± 40.63 | ||
| F1,79 | 0.64 | 0.03 | 3.55 | 0.67 | 0.01 | 1.18 | ||
| P | 0.42 | 0.86 | 0.06 | 0.41 | 0.92 | 0.28 | ||
| Male | Treatment | 77.47 ± 7.44 | 0.42 ± 0.01 | 365.32 ± 33.26 | 77.91 ± 8.37 | 0.41 ± 0.02 | 367.57 ± 41.57 | |
| Control | 60.68 ± 7.74 | 0.44 ± 0.03 | 221.47 ± 33.58 | 58.56 ± 7.56 | 0.38 ± 0.03 | 253.82 ± 37.28 | ||
| F1,79 | 2.44 | 0.27 | 9.26 | 2.94 | 0.67 | 4.15 | ||
| P | 0.12 | 0.60 | < 0.01 | 0.09 | 0.41 | 0.04 | ||
| Heterospecific | Female | Treatment | 56.56 ± 8.46 | 0.39 ± 0.03 | 201.05 ± 31.29 | 98.44 ± 14.47 | 0.47 ± 0.04 | 276.15 ± 32.95 |
| Control | 75.22 ± 8.01 | 0.45 ± 0.03 | 345 ± 36.65 | 85.05 ± 8.97 | 0.52 ± 0.03 | 253.65 ± 33.22 | ||
| F1,79 | 2.56 | 1.45 | 8.92 | 0.62 | 0.94 | 0.23 | ||
| P | 0.11 | 0.23 | < 0.01 | 0.43 | 0.30 | 0.63 | ||
| Male | Treatment | 72.91 ± 7.78 | 0.55 ± 0.07 | 213 ± 30.82 | 85.88 ± 10.62 | 0.48 ± 0.03 | 218.52 ± 34.28 | |
| Control | 103.84 ± 12.14 | 0.53 ± 0.04 | 356.37 ± 40.15 | 99.09 ± 17.41 | 0.51 ± 0.04 | 260.50 ± 31.94 | ||
| F1,79 | 4.60 | 0.04 | 8.02 | 0.42 | 0.31 | 0.8 | ||
| P | 0.03 | 0.83 | < 0.01 | 0.51 | 0.58 | 0.37 | ||
Effect of footprints on predation rate of lady beetles
For second instars there was a higher predation rate on areas treated with footprints of heterospecifics (F2,59 = 11.02, P < 0.01). For all other treatments there was no effect of footprints of conspecific or heterospecific lady beetles on the predation rate of T. notata larvae. Moreover, there was no effect of footprints on the predation rate of adult T. notata females and males (Table 3).
| Stage | Footprint source | Focal species | |
|---|---|---|---|
| T. notata | C. montrouzieri | ||
| LI | Control | 0.80 ± 0.11 | 1.25 ± 0.14 |
| Conspecific | 0.70 ± 0.10 | 1.35 ± 0.13 | |
| Heterospecific | 1.0 ± 0.10 | 1.55 ± 0.11 | |
| F2,57 | 1.90 | 1.33 | |
| P | 0.15 | 0.27 | |
| LII | Control | 1.80 ± 0.13b | 2.70 ± 0.19b |
| Conspecific | 1.95 ± 0.15b | 2.65 ± 0.18b | |
| Heterospecific | 2.8 ± 0.18a | 3.40 ± 0.15a | |
| F2,57 | 11.02 | 5.33 | |
| P | < 0.01 | < 0.01 | |
| LIII | Control | 2.05 ± 0.19 | 2.60 ± 0.16 |
| Conspecific | 2.50 ± 0.11 | 2.65 ± 0.13 | |
| Heterospecific | 2.20 ± 0.09 | 3.10 ± 0.19 | |
| F2,57 | 2.45 | 2.75 | |
| P | 0.09 | 0.07 | |
| LIV | Control | 2.75 ± 0.16 | 3.85 ± 0.18 |
| Conspecific | 3.15 ± 0.15 | 4.10 ± 0.16 | |
| Heterospecific | 3.05 ± 0.19 | 4.15 ± 0.15 | |
| F2,57 | 1.49 | 0.95 | |
| P | 0.23 | 0.39 | |
| F | Control | 1.25 ± 0.16 | 1.75 ± 0.14b |
| Conspecific | 1.65 ± 0.16 | 2.55 ± 0.15a | |
| Heterospecific | 1.70 ± 0.14 | 1.50 ± 0.18b | |
| F2,57 | 2.13 | 10.36 | |
| P | 0.12 | < 0.01 | |
| M | Control | 1.15 ± 0.10 | 2.50 ± 0.15a |
| Conspecific | 1.10 ± 0.12 | 1.95 ± 0.15b | |
| Heterospecific | 0.80 ± 0.13 | 1.85 ± 0.13b | |
| F2,57 | 2.29 | 5.52 | |
| P | 0.11 | < 0.01 | |
- Means within a stage and within a focal species followed by a different letter are significantly different (contrast analysis: P < 0.05).
A similar result was observed for the predation rate of C. montrouzieri immatures exposed to heterospecific and conspecific footprints; only second instars (F2,59 = 5.33, P < 0.01) had a significant increase in predation when exposed to footprints of heterospecifics (Table 3). All other instars of C. montrouzieri showed no effect of the heterospecific or conspecific footprints on their predation rate (Table 3).
Among adult C. montrouzieri, females captured more prey when exposed to footprints of conspecifics (F2,59 = 10.36), whereas males' predation rate was reduced when exposed to footprints of either conspecifics or heterospecifics (F2,59 = 5.52, both P < 0.01; Table 3).
Chemical profile of lady beetle footprints
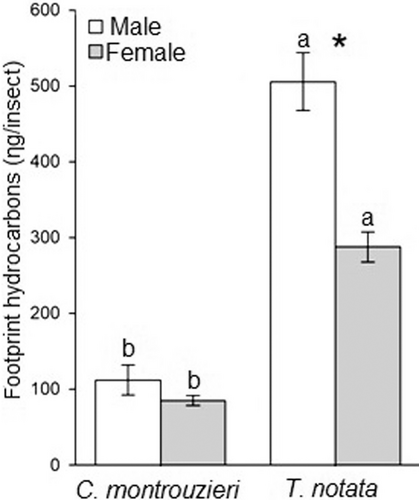
Twenty-four hydrocarbons were identified in the hexane extracts of footprints of male and female C. montrouzieri and 18 hydrocarbons were identified in male and female T. notata (Table 4). The total amount of hydrocarbons present in footprint extracts differed between both lady beetle species (F3,23 = 187.33, P < 0.01) and sexes (F3,23 = 21.48, P < 0.01; Figure 3). There was no difference when comparing the total amount of hydrocarbons obtained from male and female footprint extracts of C. montrouzieri (F1,11 = 1.40, P = 0.26; Figure 3), whereas a higher amount of total hydrocarbons was quantified from T. notata male compared to female conspecific footprint extracts (F1,11 = 28.08; P < 0.01; Figure 3). Footprint extracts of T. notata males and females contained a higher amount of hydrocarbons compared to males (F1,11 = 90.88) and females (F1,11 = 121.83, both P < 0.01) of C. montrouzieri (Figure 3).
| Compounds | RI | Tenuisvalvae notata | Cryptolaemus montrouzieri | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | F1,11 | P | Male | Female | χ2 (d.f. = 1) | P | |||
| Hydrocarbons | Tetradecane* | 1400 | 33.7 ± 1.81 | 23.5 ± 1.11 | 22.99 | < 0.01 | 13.6 ± 4.38 | 26.5 ± 1.88 | 4.01 | 0.04 |
| Pentadecane* | 1500 | 10.9 ± 0.55 | 7.4 ± 0.38 | 25.77 | < 0.01 | 4.4 ± 1.41 | 8.4 ± 0.58 | 3.82 | 0.05 | |
| Hexadecane* | 1600 | 1.7 ± 0.09 | 1.4 ± 0.19 | 2.91 | 0.11 | 0.8 ± 0.27 | 1.4 ± 0.11 | 2.69 | 0.10 | |
| Heptadecane* | 1702 | - | - | 0.9 ± 0.26 | 1.0 ± 0.23 | 0.10 | 0.74 | |||
| 2-Heneicosene | 2021 | - | - | 0.2 ± 0.20 | 0.3 ± 0.19 | 0.24 | 0.62 | |||
| Docosane* | 2200 | - | - | 0.03 ± 0.03 | 0.3 ± 0.05 | 3.70 | 0.05 | |||
| 2-Tricosene | 2224 | - | - | 0.9 ± 0.56 | 0.7 ± 0.55 | 0.03 | 0.86 | |||
| 3-Tricosene | 2230 | - | - | - | 0.7 ± 0.22 | - | ||||
| 7-Tricosene | 2279 | - | - | - | 0.1 ± 0.04 | - | ||||
| Tricosane* | 2299 | - | - | 5.6 ± 0.82 | 3.3 ± 0.56 | 5.59 | 0.01 | |||
| Tetracosane* | 2400 | - | - | 0.9 ± 0.42 | 0.2 ± 0.01 | 7.61 | < 0.01 | |||
| 9-Pentacosene | 2492 | - | - | 0.3 ± 0.26 | - | - | ||||
| Pentacosane* | 2500 | 85.2 ± 4.71 | 42.9 ± 3.34 | 53.52 | < 0.01 | 1.7 ± 0.22 | 1.8 ± 0.33 | 0.04 | 0.82 | |
| Hexacosane* | 2600 | 3.8 ± 0.48 | 4.8 ± 0.97 | 0.76 | 0.40 | 0.4 ± 0.08 | 0.5 ± 0.06 | 0.13 | 0.71 | |
| 11-Heptacosene | 2670 | - | - | 1.9 ± 0.38 | 0.5 ± 0.08 | 26.47 | < 0.01 | |||
| 7-Heptacosene | 2683 | 6.8 ± 0.69 | 4.2 ± 0.68 | 7.07 | 0.02 | 0.8 ± 0.21 | 0.3 ± 0.02 | 15.41 | < 0.01 | |
| Heptacosane* | 2700 | 45.9 ± 3.13 | 20.9 ± 1.53 | 51.25 | < 0.01 | 0.9 ± 0.15 | 0.5 ± 0.07 | 8.46 | < 0.01 | |
| (Z)-13 Docosenoamide | 2771 | - | - | 0.9 ± 0.20 | 0.2 ± 0.05 | 20.86 | < 0.01 | |||
| 9-Nonacosene | 2866 | 3.1 ± 0.30 | 1.3 ± 0.13 | 29.00 | < 0.01 | - | - | |||
| 11-Nonacosene | 2871 | - | - | 38.4 ± 9.03 | 10.8 ± 1.57 | 19.78 | < 0.01 | |||
| 7-Nonacosene | 2885 | 105.2 ± 10.23 | 60.2 ± 5.46 | 15.01 | < 0.01 | - | - | |||
| Nonacosane* | 2900 | 9.1 ± 0.66 | 4.7 ± 0.32 | 36.29 | < 0.01 | 2.1 ± 0.34 | 1.2 ± 0.23 | 4.84 | 0.02 | |
| Triacontene | 2960 | 2.0 ± 0.22 | 1.1 ± 0.12 | 11.74 | < 0.01 | - | - | |||
| 11-Triacontene | 2970 | - | - | 2.3 ± 0.50 | 1.5 ± 0.18 | 2.96 | 0.08 | |||
| Hentriacontadiene | 3051 | 16.3 ± 1.36 | 9.9 ± 1.32 | 11.15 | < 0.01 | - | - | |||
| 11-Hentriacontene | 3066 | 71.9 ± 6.32 | 43.3 ± 3.46 | 15.80 | < 0.01 | - | - | |||
| 9-Hentriacontene | 3071 | - | - | 18.0 ± 3.59 | 13.9 ± 2.08 | 1.06 | 0.30 | |||
| 7-Hentriacontene | 3085 | 13.1 ± 1.36 | 7.3 ± 0.85 | 13.13 | < 0.01 | 13.8 ± 2.97 | 6.7 ± 1.01 | 7.38 | < 0.01 | |
| Hentriacontene | 3100 | - | - | 0.4 ± 0.14 | 0.5 ± 0.12 | 0.04 | 0.83 | |||
| Tritriacontene | 3252 | 59.2 ± 6.05 | 30.9 ± 4.87 | 13.33 | < 0.01 | - | - | |||
| 9-Tritriacontene | 3263 | 19.0 ± 2.35 | 8.6 ± 0.99 | 16.27 | < 0.01 | - | - | |||
| Pentatriacontene | 3451 | 9.4 ± 2.77 | 8.3 ± 1.61 | 3.09 | 0.10 | - | - | |||
| Ester | Methyl hexadecanoate | 1910 | 5.9 ± 3.29 | 1.3 ± 0.11 | 1.99 | 0.18 | 2.8 ± 0.86 | 1.5 ± 0.55 | 1.67 | 0.19 |
- a Identified by authentic standards.
When analyzing the compounds individually for C. montrouzieri, the hydrocarbon tetradecane was present in higher amounts in female footprint extracts Table 4), and 3-tricosene and 7-tricosene were detected only in these extracts. The following compounds were present in higher levels in male footprint extracts: tricosane, tetracosane, 11-heptacosene, 7-heptacosene, heptacosane, (Z)-13 docosenoamide, 11-nonacosene, nonacosane, and 7-hentriacontene; 9-pentacosene was found only in male extracts (Table 4). There was no significant difference between sexes of C. montrouzieri in the amount of the following compounds: pentadecane, hexadecane, heptadecane, 2-heneicosene, docosane, 2-tricosene, pentacosane, hexacosane, 11-triacontene, 9-hentriacontene, and hentriacontene (Table 4).
There was no qualitative difference between the hydrocarbon profiles between sexes of T. notata, but there was a significant difference in the quantities of most compounds. For T. notata the following compounds were detected in higher amounts in male footprint extracts: tetradecane, pentadecane, pentacosane, 7-heptacosene, heptacosane, 9-nonacosene, 7-nonacosene, nonacosane, triacontene, hentriacontadiene, 11-hentriacontene, 7-hentriacontene, tritriacontene, and 9-tritriacontene (Table 4). There was no significant difference in the amount of hexadecane, hexacosane, and pentatriacontene (Table 4).
When we compared the composition and amount of compounds present in the footprint extracts between species, nine hydrocarbon compounds were found in both species, but in higher amounts in T. notata footprint extracts. Those compounds were: hexadecane (χ2 = 8.73, d.f. = 3, P = 0.03), tetradecane (χ2 = 14.37), pentadecane (χ2 = 14.47), pentacosane (χ2 = 638.30), hexacosane (χ2 = 153.57), heptacosane (χ2 = 738.16), nonacosane (χ2 = 125.21), 7-heptacosene (χ2 = 187.71), and 7-hentriacontene (χ2 = 18.17, all d.f. = 3, P < 0.01). Cryptolaemus montrouzieri produced smaller quantities of hydrocarbons overall, but had a higher diversity of compounds, with 15 hydrocarbons identified exclusively in its footprint extracts, such as saturated and unsaturated hydrocarbons between C21 and C24, whereas in T. notata footprints were composed of hydrocarbons of higher molecular weights, both saturated and unsaturated C33 and C35 (Table 4).
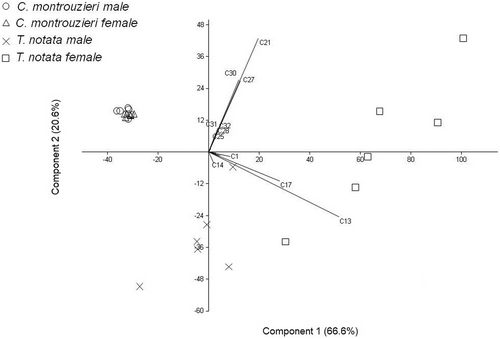
The principal component analysis (PC1 = 66.6% and PC2 = 20.6%) grouped the chemical profiles of footprint extracts of males and females of both ladybeetle species according to the composition of individual hydrocarbons (F3,23 = 57.14, P < 0.01; Figure 4). For C. montrouzieri, males and females grouped together, indicating that they produce a similar chemical profile of hydrocarbons in their footprints (Figure 4). However, T. notata extracts were more spread out and males and females of this species did not group together. Pentacosane (C13), heptacosane (C17), 7-nonacosene (C21), and tritriacontene (C30) are responsible for the separation of males and females of T. notata (Figure 4).
DISCUSSION
The biological control of mealybugs is an important strategy to manage these pests in many crops worldwide. The coccidophagous lady beetles C. montrouzieri and T. notata have been used to control mealybugs in Brazil and other countries in both tropical and temperate climates (Chakupurakal et al., 1994; Maes et al., 2015). These lady beetle species may occur in the same area, as they have similar food preferences and thermal requirements (Ferreira et al., 2020), and in cases of prey scarcity, they may compete for food and have negative interactions such as cannibalism and intraguild predation (Oliveira et al., 2022). To avoid this competition, these lady beetles can use the chemical cues released in the footprints of possible competitors to avoid territory that is already occupied. This influence of chemical cues from lady beetle footprints on the behavior of other insect species has already been demonstrated (Ruzicka, 2001; Mishra et al., 2013; Ninkovic et al., 2013; Patel et al., 2020) and induced avoidance in some possible competitors. It is important to recognize that in the field food availability is not always constant, and there might be days (or patches) where the predator may find food in abundance, as studied here, or situations where food is in shortage, which in turn can alter the outcome of the interactions with prey and competitors. Therefore, further studies may consider food variability in more natural settings as a follow-up question regarding lady beetle footprints' effects on predator–prey and, predator–predator interactions.
The behavioral studies described herein showed that C. montrouzieri did not alter its walking behavior when exposed to footprints of conspecific individuals. This behavioral response, together with the similarity in the amount of hydrocarbons identified in the male and female extracts, indicates that this species may not use footprints to detect the presence of a conspecifics. In contrast, T. notata males and females stopped more often when they detected footprints of conspecific males. Previous studies of the sexual behavior of T. notata showed that females engage in male courtship and try to mate with males (Santos et al., 2017). Therefore, it is possible that the divergence in the chemical profile of male footprints is important for the T. notata female to recognize and detect the presence of potential mates in the area, whereas males can use these footprints to avoid competition. The hydrocarbons 7-nonacosene, pentacosane, heptacosane, and tritriacontene stood out in the separation of male T. notata extracts. Performing behavioral experiments with blends containing these hydrocarbons could help to clarify their role in these interactions.
In the heterospecific interactions, C. montrouzieri females altered their walking behavior when exposed to footprints of T. notata females. Previous studies have shown that C. montrouzieri is capable of detecting the chemical trails of other species (Finlay-Doney & Walter, 2012; Urbina et al., 2018). The possible attraction of C. montrouzieri to footprints of T. notata, as identified in this work, may be related to its capacity to locate the competitor in the same area, and might indicate possible negative effects of the exotic species C. montrouzieri on the indigenous T. notata. Oliveira et al. (2022) showed that when food is scarce, C. montrouzieri behaves as an intraguild predator on T. notata immature stages. In contrast, T. notata spent less time on areas treated with the footprints of C. montrouzieri, suggesting that T. notata, an inferior competitor compared to C. montrouzieri, would tend to avoid such areas to avoid predation.
It is expected that in asymmetric interactions between competitors, the least aggressive species would use chemical clues from heterospecific individuals to avoid unfavorable situations such as competition for food and predation upon immature stages. This avoidance behavior is present in other interactions with lady beetles. For instance, the aphid parasitoid Aphidius ervi Haliday avoids foraging in the same area where the lady beetle C. septempunctata is, or with footprints of this predator (Nakashima et al., 2004). Also, Ugine et al. (2018) found that Coccinella novemnotata Herbst spent more time foraging on plants with conspecific individuals than on plants with C. septempunctata, suggesting that they can recognize visual and/or olfactory cues left by conspecific and heterospecific individuals, and use these cues to avoid occupying the same micro-habitat as heterospecific individuals.
Tenuisvalvae notata adults did not alter their predation rate on mealybugs when exposed to conspecific or heterospecific lady beetles' footprints. This result suggests that even though T. notata adults can recognize the chemical cues left by C. montrouzieri, it does not alter their prey consumption. Instead, to reduce chances of possible competition or intraguild predation, they reduce the time they spend in areas treated with footprints of C. montrouzieri, as we found in the walking behavior bioassay. Prey consumption may not have changed due to limited space in the Petri dish. Further experiments with an escape option may be developed in the future to address this gap. In contrast, although female C. montrouzieri increased the amount of time they spent and the number of stops in areas treated with female T. notata footprints, their predation rate increased only in areas with conspecific footprints. Males, which did not alter foraging behavior when exposed to conspecific and heterospecific footprints, reduced predation when exposed to conspecific and heterospecific footprints.
The ability of C. montrouzieri to recognize conspecific and heterospecific semiochemicals has been demonstrated before (Merlin et al., 1996; Finlay-Doney & Walter, 2012; Urbina et al., 2018). Therefore, even under conditions of food abundance such as those tested in this study, the ability to recognize the presence of a competitor via semiochemicals left on the substrate may result in a reduction in male C. montrouzieri predation of preferred prey (scale insects). Cryptolaemus montrouzieri is described as a superior competitor because it feeds on more prey and generally acts as an intraguild predator when in contact with other natural enemies (Chong & Oetting, 2007; Mustu et al., 2008; Dinesh & Venkatesha, 2014; Oliveira et al., 2022). However, by perceiving tracks of competitors in the area, males will be induced to feed less, engaging instead in behaviors that help them to avoid competition. On the other hand, semiochemicals of conspecifics could also indicate the presence of more food, inducing females to increase their predation rate.
Although some compounds are similar, the chemical profiles of C. montrouzieri and T. notata footprints revealed species-specific blends of footprint hydrocarbons and quantitative differences within species, as expected. Cryptolaemus montrouzieri produces a higher diversity of hydrocarbons than T. notata, and the latter produces higher amounts of hydrocarbon compounds. In addition, there were differences between the hydrocarbons identified in footprints of females and males of C. montrouzieri. However, the hydrocarbons in the footprints of C. montrouzieri males and females were more similar than those of T. notata males and females. Another interesting difference is that C. montrouzieri produced hydrocarbons of lower molecular weight, such as C21 and C24 (saturated and unsaturated), whereas T. notata produced compounds of higher molecular weight, such as C33 and C35. In both species, the major compound has 29 carbon atoms; in C. montrouzieri the compound is 11-nonacosene, and in T. notata it is 7-nonacosene. Further studies could evaluate whether these compounds function in species recognition through footprints left on the plants by conspecifics or their prey. Previous studies have identified that the hydrocarbons of footprints have adhesive functions and are correlated with cuticular hydrocarbons (Kosaki & Yamaoka, 1996; Geiselhardt et al., 2011). These hydrocarbons are specific to each species and sex (Nakashima et al., 2006; Magro et al., 2007; Pattanayak et al., 2014). Therefore, the variation in composition of hydrocarbons present in the footprints of lady beetles is important for species and sex recognition.
The alkanes pentacosane, heptacosane, and nonacosane, found in the footprints of C. montrouzieri and T. notata, were also identified in the footprints of other coccinellids, such as C. septempunctata and Harmonia axyridis Pallas (Kosaki & Yamaoka, 1996; Nakashima et al., 2004; Durieux et al., 2012). The similarity of the chemical profiles of footprints of congeneric coccinellid larvae has already been detected (Magro et al., 2007; Pattanayak et al., 2015) and suggests that, in addition to determining behavioral traits, the chemical footprint profile can be used for chemotaxonomic purposes.
In conclusion, the chemical profile of hydrocarbons extracted from the footprints of T. notata and C. montrouzieri are different, and their behavioral responses suggest that the specific blend of hydrocarbons in their footprints is important for species recognition and decision-making regarding dispersal and predation. Behavioral responses of these predators can vary from avoidance of areas with footprints of potential competitors, as observed in T. notata, or arrestment, as observed in C. montrouzieri, which may lead to changes in preferred prey consumption and intraguild predation on competitors. This is the first report of the effects of footprints of T. notata and C. montrouzieri on predator walking behavior and predation of mealybugs. Further studies are necessary to elucidate the relative importance of blends or specific compounds in the chemical communication of – and between – these species. These results may contribute to pest management decisions in the biological control of mealybugs with these lady beetle species.
AUTHOR CONTRIBUTIONS
Jennifer Oberger Ferreira: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (equal); resources (lead); software (lead); supervision (equal); validation (lead); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Christian Sherley Araújo Silva-Torres: Conceptualization (equal); formal analysis (equal); funding acquisition (supporting); methodology (supporting); project administration (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal). Enggel Beatriz Silva do Carmo: Data curation (supporting); formal analysis (supporting); methodology (supporting); project administration (supporting). Gessica Santos: Data curation (supporting); investigation (supporting); methodology (supporting); project administration (supporting). Raul Laumann: Data curation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). Miguel Borges: Data curation (supporting); methodology (supporting); project administration (supporting); writing – review and editing (supporting). Maria Carolina Blassioli-Moraes: Conceptualization (equal); data curation (supporting); formal analysis (equal); methodology (equal); project administration (supporting); resources (equal); software (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
The present research was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES Foundation, granted to J.O. Ferreira through the CAPES/PROEX. Thanks to Dr. Robert W. Matthews (UGA - Emeritus Faculty) for editing and reviewing a first version of this manuscript and Jaimie Kenney (UC Riverside) for editing a later version. The authors of this work have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




