Using biological invasions to improve plant defense theory
Abstract
Theory to explain how plants defend themselves against herbivorous insects is rich, but can be difficult to test. Biological invasions provide unique opportunities to test and improve upon plant defense theory, as plants experience predictable shifts in insect herbivory after introduction to a new range. Here, we use an invasion to evaluate the power of three cornerstone hypotheses to predict the evolution of defense against herbivorous insects. These three hypotheses represent increasing refinements of classic plant-insect theory regarding optimal defense, and each rests on the same three assumptions: that introduced plant populations escape natural enemies, that insect herbivory reduces plant fitness, and that putative defenses decrease herbivory. These assumptions remain untested in most invasions, which likely contributes to conflicting support for many plant defense hypotheses. We provide evidence that these assumptions are met in common mullein, Verbascum thapsus L. (Scrophulariaceae), which we propose can thus be used as a model system to test plant defense theory. We find that the hypothesis that integrates predictions of within-plant optimal defense theory and the evolutionary dilemma model (i.e., the ‘shifting defense allocation’ hypothesis) provides strong insights into both invasion and evolution of plant defense. Specifically, we show that introduced populations that escape important specialist herbivores increase the concentration of secondary compounds in high-quality tissue in particular, in this case protecting valuable young leaves from generalist herbivores that dominate in the introduced range. We would not have detected this shift without exploring within-plant defense allocation across native and introduced populations, a task rarely undertaken when assessing evolutionary change in plant defenses. This finding provides broad insight into how native and introduced plant populations alike may respond to shifts in herbivore pressure. We close by highlighting future directions of inquiry using introduced plant populations to develop and test plant defense theory more generally.
INTRODUCTION
Plant-insect interactions, including herbivory, shape the functioning of terrestrial ecosystems worldwide (Yang & Gratton, 2014; Cardoso et al., 2020). From crop yield reduction by agricultural pests (Poveda et al., 2008; Mitchell et al., 2016; Bisht et al., 2019) to boom-bust cycles of beetles that can restructure entire forests (Brockerhoff & Liebhold, 2017; Goodsman et al., 2017; Fernandez-Conradi et al., 2021), insects have profound effects on individual plants, plant populations, and communities. How plants defend themselves against attack by a diverse and unrelenting array of herbivores therefore remains an active area of research with far-reaching ecological and evolutionary consequences.
Plant invasions, in which a subset of non-native species become ecologically and/or economically problematic when introduced to a new range, provide a unique opportunity to better understand variation in defense investment among plant populations. Invasions represent unparalleled natural experiments in which the selection pressures to which plants are exposed quickly and vastly shift, with introduced plants often escaping many of their natural enemies, including herbivorous insects. Furthermore, introduced populations can rapidly adapt to their new environmental conditions (Reznick & Ghalambor, 2001), suggesting that reduced herbivory has the potential to quickly drive differences in defense investment between a plant's native and introduced ranges. This sets the stage to explore how plants invest in defense under different attack scenarios, and whether defense allocation in these real-world systems aligns with theory on plant defenses.
A key line of defenses against insect herbivores is the secondary compounds they produce (Rosenthal & Berenbaum, 1992; Mithöfer & Boland, 2012; Richards et al., 2015). Understanding and predicting how plants invest in and allocate these secondary compounds has been a main focus in both basic and applied research on plant-insect interactions. Optimal defense theory (McKey, 1974; Rhoades, 1979; Coley et al., 1985) represents a cornerstone of literature in this field. This theory posits that selection will lead to defenses being most concentrated in plant species, populations, or tissues that experience the most herbivory. When plants or specific tissues experience less herbivory they should evolve to be defended less strongly, which is ‘optimal’ in that the resources needed to produce defensive compounds are concentrated where they are needed most. Tests of optimal defense typically focus on the distribution of defenses within plants (McKey, 1974; Stamp, 2003; Kaplan et al., 2008; McCall & Fordyce, 2010; Tsunoda & van Dam, 2017; Wolinska & Berens, 2019) and among species (Feeny, 1975; Coley et al., 1985), in part because relative rates of herbivory differ in fairly consistent ways at those scales. However, rigorous tests of optimal defense at the within-plant and among-species scales can be difficult, as processes other than adaptation to herbivory also drive variation in defense.
Within plants, source-sink dynamics may drive defense to track allocation of photosynthates, causing young tissues to have particularly high concentrations of defense (Kozlowski, 1992; Honkanen et al., 1999; Meldau et al., 2012; Ferrieri et al., 2015). Defenses may also become diluted as tissues expand, such that old leaves have lower concentrations of defenses than young leaves simply because they are larger and the same amount of defense is now spread across more biomass (Wallace & Eigenbrode, 2002; Brunt et al., 2006). Among species, historical and biogeographical contingencies may also drive variation in defense. For example, climate, resource availability, and UV radiation also influence defense, and may do so differently among species with different phylogeographic histories (Koricheva, 2002; Bidart-Bouzat & Imeh-Nathaniel, 2008; Endara & Coley, 2011; Abdala-Roberts et al., 2018). Thus, disentangling other drivers from adaptation to herbivory can be difficult. Adding population-level tests of optimal defense could improve our confidence in the robustness of patterns that support optimal defense, as many of these drivers that can obscure adaptation to herbivory are better standardized within a species. However, because herbivory is notoriously variable over time and space among populations within a species (e.g., Root & Cappuccino, 1992), the population remains an understudied scale of inquiry in the context of optimal defense (e.g., Hahn & Maron, 2016; Hahn et al., 2019; López-Goldar & Agrawal, 2021).
Research comparing native and introduced populations, which often differ in the herbivory they experience, has begun to fill this gap (reviewed in Doorduin & Vrieling, 2011; Felker-Quinn et al. 2013). However, findings can still be case-specific and conflicting. For example, introduced species can evolve higher or lower investment in defense allocation, or show no change at all (Doorduin & Vrieling, 2011; Felker-Quinn et al., 2013). We suggest that such conflicting findings occur because testing the assumptions underlying theoretical predictions is not always done, given how challenging and time-consuming it can be. However, if these assumptions remain untested, it becomes difficult to disentangle whether conflicting findings indicate true biological differences among study systems, the need to refine theory, or instead that the assumptions underlying theoretical predictions are simply not met.
Here, we present common mullein, Verbascum thapsus L. (Scrophulariaceae), as a useful model for testing plant defense theory in ways that can improve understanding of optimal defense and the evolution of plant defenses in native and introduced plants alike. To this end, we first outline three major hypotheses that build upon optimal defense theory to predict how plants will allocate defenses within an invasion context. We then highlight why V. thapsus is a useful model species for testing these hypotheses, in part by providing clear support for the assumptions that underlie the predictions of these hypotheses. Specifically, we use the support for these assumptions as an empirical foundation to examine the validity of each of these hypotheses in turn, emphasizing how V. thapsus can provide new insights into both invasion and plant defense theory. Finally, we close by highlighting future directions of inquiry in V. thapsus and in other systems, and for using biological invasions in general to advance and test plant-insect theory.
Theoretical predictions of defense evolution in invasive plant populations
Optimal defense theory has given rise to many hypotheses concerning how diverse abiotic and biotic factors may drive the evolution of plant investment and allocation in defense against herbivory (Coley et al., 1985; Stamp, 2003; Mitchell et al., 2006; Massad et al., 2011). Three hypotheses stand out, however, that focus specifically on changes in herbivore pressure as the main driver of shifts in plant defense between a species' native and introduced range.
The first of these hypotheses is the ‘evolution of increased competitive ability’ (or EICA) hypothesis (Blossey & Nötzold, 1995). This hypothesis built upon optimal defense theory and posited that reduced herbivory in a plant's introduced range would drive evolution of decreased defense against herbivory (which in turn could allow increased investment in growth and reproduction, helping explain why plants are typically larger, taller, and produce more seed in their introduced compared to their native range; Parker et al., 2013). Blossey & Nötzold (1995) did not explicitly distinguish among types of herbivores, but simply noted the general pattern of escape from insect herbivores in introduced populations, and thus predicted the evolution of a general reduction in defenses against them.
Nearly a decade later, Müller-Schärer et al. (2004) built upon the EICA hypothesis to incorporate the fact that many introduced plants escape only their specialist herbivores, and still experience herbivory by generalists (Figure 1). They proposed that this shift in herbivore composition would lead to an increase in defensive compounds (in stark contrast to the decrease predicted by EICA). Specifically, they emphasized the differences between qualitative defenses (i.e., toxic or distasteful chemicals such as alkaloids or glucosinolates) and quantitative defenses (i.e., digestibility-reducing compounds, including tannins, lignins, and mechanical structures such as trichomes) (Feeny, 1976; Rhoades & Cates, 1976; Fox, 1981). Qualitative defenses deter generalists at even low doses (Loeuille & Hauzy, 2018) and can incur relatively low physiological costs (Strauss et al., 2002; Peñuelas et al., 2010; but see Neilson et al., 2013), but also act as attractants or feeding stimulants to co-evolved specialists. Quantitative defenses deter both generalist and specialist herbivores but are also physiologically costly to produce and maintain (Strauss et al., 2002; Müller-Schärer et al., 2004). In a plant's native range, selection should drive plants to invest in both types of defense, as both generalists and specialists are abundant (i.e., evolutionary dilemma model; van der Meijden, 1996). Müller-Schärer et al. (2004) proposed that in the introduced range, where generalists are more abundant than specialists, selection should drive plants to invest more in physiologically low-cost qualitative defenses that are both effective against generalists and no longer at risk of incurring an ecological cost by attracting specialists. Concomitantly, these authors also suggested that quantitative defenses (effective against both specialists and generalists) would decrease (Müller-Schärer et al., 2004). This has come to be called the ‘shifting defense’ hypothesis (Doorduin & Vrieling, 2011). In summary, plants should be better defended against generalists, but less well defended against specialists, in the introduced range relative to the native range (Figure 1).
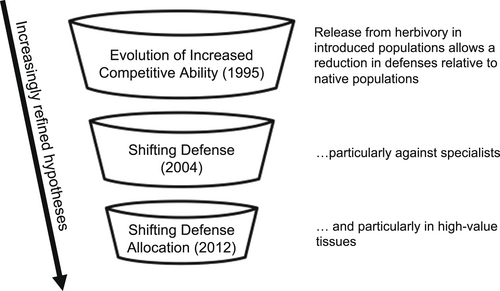
Alba et al. (2012) further refined the predictions of Müller-Schärer et al. (2004) by integrating the predictions of optimal defense that focus on within-plant allocation of defense. Specifically, optimal defense predicts that plants should most highly defend tissues that are most valuable to plant fitness and at highest risk of attack if undefended (McKey, 1974; Rhoades & Cates, 1976). Young leaves, for example, typically bring in more energy for future growth and reproduction than old leaves (McCall & Fordyce, 2010) and – if undefended – typically attract more herbivores than old leaves given their high nitrogen content (Mattson, 1980; Kursar & Coley, 1991). Thus, one prediction of optimal defense is that young leaves should be better defended than old leaves (van Dam et al., 1995; Iwasa et al., 1996; Barto & Cipollini, 2005). Alba et al. (2012) therefore predicted that adaptive shifts in defense in introduced plant populations should be more pronounced for high-value tissues (young leaves) than low-value tissues (old leaves) (Figure 1), and emphasized that this pattern may be overlooked if differences in defense are only investigated at the whole-plant scale. In other words, young leaves should be especially better defended than old leaves against generalists in their introduced range than in their native range. An additional consequence of this is that young leaves on introduced plants may also be more attractive to specialists than young leaves on native plants, as the secondary compounds that deter generalists can stimulate feeding and oviposition of specialists (Figure 1). Here, we call this the ‘shifting defense allocation’ hypothesis.
These three hypotheses represent increasingly powerful refinements of optimal defense theory, in that they incorporate increasing biological realism in ways that should lead to more accurate predictions of the evolution of plant defenses. These hypotheses build upon each other, and thus rest on the same three assumptions, that (1) introduced plant populations escape their natural enemies, (2) insect herbivory reduces plant fitness, and (3) compounds or structures considered to be defenses – i.e., putative defenses – decrease the prevalence or impact of generalist insect herbivory. Here, we evaluate which of these three hypotheses best predicts defense allocation using V. thapsus as a model system by reviewing the research that we and others have done on both native and introduced populations of this plant. We first emphasize the need to directly test the three underlying assumptions of these hypotheses in general. We then outline evidence specifically from the V. thapsus system that addresses the three assumptions, and then that addresses the hypotheses regarding shifts in defense between the native and introduced ranges. In evaluating these hypotheses, we can more rigorously evaluate optimal defense theory.
Verbascum thapsus as a model for testing plant defense hypotheses
Verbascum thapsus is a particularly good plant for evaluating the predictions of these three hypotheses. First, V. thapsus has been in its introduced range long enough to adapt to the new environment. The species is native to Eurasia, and was introduced to North America starting in the 1600s by western European colonizers (Gross & Werner, 1978), which has been corroborated by recent population genetic studies (Gaskin et al., 2021). Its short, often biennial, generation time and 400+ years history in the introduced range therefore provides approximately 200 generations for adaptive evolution to occur. Evolution in the context of introductions and invasions is known for how rapid it can be despite potential genetic bottlenecks (Dlugosch & Parker, 2008), and experimental studies show that even a few generations are adequate (Hufbauer et al., 2015). Additionally, V. thapsus has well-understood chemical and physical defenses. The iridoid glycosides aucubin and catalpol are cyclopentanoid monoterpene-derived compounds with a demonstrated role in chemical defense (other compounds have been isolated from V. thapsus, but are not well studied for their ecological role; Warashina et al., 1991; Hussain et al., 2009). Iridoids are present in more than 50 plant families and are particularly well-studied in the Plantaginaceae (Bowers, 1991; Dobler et al., 2011), including Plantago spp., for which they have been shown to deter generalists and attract specialists (Bowers & Puttick, 1988; Bowers, 1991; Nieminen et al., 2003; Reudler et al., 2011). Trichomes are a main physical defense (Woodman & Fernandes, 1991). As a monocarpic biennial (or rarely annual or triennial) forb (Gross & Werner, 1978; Reinartz, 1984), the growth form (rosette and then flowering stalk) of V. thapsus makes determining the relative age of leaves straightforward, which is crucial to evaluating within-plant predictions of optimal defense. Verbascum thapsus also does not have closely related native congeners in its introduced range, and thus may experience particularly strong enemy escape (Mack, 1996; Parker & Gilbert, 2004). Most importantly, we and others studying this system have assessed all three assumptions underlying the three models in both controlled experiments and observational fieldwork (see below).
Support for basic model assumptions
Despite predicting different evolutionary outcomes, these three hypotheses – EICA, shifting defense, and shifting defense allocation – share three basic assumptions: introduced plant populations escape their natural enemies, insect herbivory reduces plant fitness, and putative defenses decrease the prevalence or impact of generalist insect herbivory. Before evaluating which hypothesis most accurately predicts the evolution of defenses, we first explain the importance of explicitly testing each assumption and then provide evidence that each assumption is met by V. thapsus.
Assumption 1 - Introduced plant populations escape their natural enemies
This assumption is important to test, as moving from one range to another does not guarantee that plants will experience enemy escape, or that enemy escape will be long-term. Plants often experience less herbivory in their introduced ranges than in their native ranges (Wolfe, 2002; Genton et al., 2005; Ebeling et al., 2008; Bajwa et al., 2016), but native insects in a plant's introduced range can start to include that plant in their diet (Hawkes, 2007; Pearse & Altermatt, 2013), especially if the plant is phylogenetically similar to native plant species in its introduced range (Mack, 1996; Parker & Gilbert, 2004). Introduced plants are also more likely to reassociate with old enemies over time, either inadvertently or purposefully as biological control agents. Thus, the degree to which introduced plants experience enemy escape likely declines over time, as they become widespread in their new range (Hawkes et al., 2010; Schultheis et al., 2015).
For V. thapsus, a broad geographic survey illustrates strong, but not complete, enemy release in introduced populations (Alba et al., 2012). Specifically, Alba & Hufbauer (2012) found both herbivore abundance and species richness were higher for V. thapsus in its native range than in its introduced range after surveying 21 locations in the native range and 30 locations in the introduced range. In line with previous literature (Gross & Werner, 1978), most herbivore groups (Lepidoptera, weevils, snails, leafhoppers, and aphids) were more prevalent in the native range than in the introduced range, whereas only two (thrips and grasshoppers) were more prevalent in the introduced range. The main thrips species is Haplothrips verbasci (Osborn) (Thysanoptera: Phlaeothripidae), a specialist Verbascum feeder that was likely inadvertently introduced into North America. The lower diversity and abundance of herbivores in the introduced range was associated with less damage by herbivores as well.
Assumption 2 - Insect herbivory reduces plant fitness
This assumption is important to test, as escape from herbivory will only shift allocation of defenses if herbivory impacts fitness. The biocontrol literature provides many examples of single species of specialist insects reducing the performance of invasive plants – e.g., Hydrilla verticillata (L.f.) Royle (Doyle et al., 2007), Lantana camara L. (Simelane, 2010), Melaleuca quinquenervia (Cav.) S.T. Blake (Franks et al., 2006; Tipping et al., 2009; Rayamajhi et al., 2010), Mimosa pigra L. (Paynter & Hennecke, 2001), Persicaria perfoliata (L.) H. Gross (Smith & Hough-Goldstein, 2014), Schinus terebinthifolia G. Raddi (Prade et al., 2016); Tamarix spp. (Pattison et al., 2011), Trapa natans L. (Ding et al., 2006), and Triadica sebifera (L.) Small (Wang et al., 2012b). Yet how plant fitness responds to the diverse array of specialist and generalist herbivores they encounter in the field often remains less well understood.
Two studies support that insect herbivory strongly reduces fitness of V. thapsus: one in a natural population in the field in the introduced range (Wilbur et al., 2013) and one in field common gardens, with one common garden each in the native and introduced range (Endriss et al., 2018). In both studies, plants were sprayed with an insecticide approximately every 3 weeks to protect them from naturally occurring herbivores. Control plants were sprayed with water. In both studies, V. thapsus grew faster to a larger size when protected from insect herbivory. In the naturally occurring field population (Wilbur et al., 2013), protection from herbivory over the course of the plants' 2-year life span nearly doubled its seed production. These experiments took place in the introduced range where herbivory is low (Wilbur et al., 2013), or in the introduced and native ranges in particularly low herbivory years and locations (Endriss et al., 2018). These studies may thus provide a conservative estimate of the effects of herbivory. In the native range, herbivores are generally more abundant (Alba & Hufbauer, 2012), and so the fitness consequences of herbivory are likely to be even stronger. Escape from those herbivores in the introduced range therefore likely represents a substantial release from selection by herbivorous insects.
Assumption 3 - Putative defenses decrease the prevalence or impact of generalist insect herbivory
For enemy escape – and release from the top-down pressure that enemies impose – to lead to a shift in defenses, those defenses need to reduce herbivory (Agrawal, 1998; Hanley et al., 2007; Schuman et al., 2012). Yet many structures and compounds considered to be defenses against herbivores also have other functions (Strauss & Agrawal, 1999; Hättenschwiler & Vitousek, 2000; Hanley et al., 2007; Erb & Kliebenstein, 2020), so it is important to document their effectiveness as defenses (Karban, 2020). Trichomes in particular are known to protect plants from UV radiation and from water loss (Woodman & Fernandes, 1991; Hanley et al., 2007; Bickford, 2016). Secondary chemical compounds (secondary metabolites) are often known as defenses against insect herbivores, but also have roles in plant–plant communication, plant-pathogen, and plant-microbiome interactions (Pang et al., 2021). Secondary metabolites can also have very different effects on different types of insects – the same compound that effectively deters generalists may attract or act as a feeding stimulant to specialists (Bowers, 1991; Agrawal et al., 1999; Bruce, 2014).
For V. thapsus, both trichomes and iridoid glycosides, particularly catalpol, increase resistance against generalist herbivory. For example, trichomes serve as a potent defense against larvae of a generalist, leaf-feeding lepidopteran, Trichoplusia ni (Hübner) (Noctuidae) – when Alba et al. (2014) shaved trichomes off of one half of young, V. thapsus leaves taken from close to the rosette center, larval T. ni ate 6× as much tissue from the shaved portion than from the intact portion of the leaves.
Further, two studies support that generalist insect herbivory decreases as iridoid glycosides increase: one from field populations (Alba et al., 2013) and one from feeding trials in the laboratory (Alba et al., 2014). The field data represent 50 plants, 10 from each of five populations in the introduced range. Between-plant variation in total iridoid glycoside content was high, and was comprised largely of the iridoid glycoside catalpol (Alba et al., 2013), which has been shown as a major driver of specialist and generalist herbivory of other plant species, especially Plantago lanceolata L. (Bowers & Puttick, 1988; Nieminen et al., 2003; Wurst et al., 2008; Reudler et al., 2011). However, young leaves consistently had higher iridoid glycoside content than old leaves, and were also fed upon less than old leaves. This may suggest that the iridoids served as defenses, but young leaves also are more densely covered in trichomes and may also vary in other classes of defense. Thus, more compelling is that after taking site-level variation into account, within young leaves and (separately) within old leaves, leaf chewing damage – which is only by generalists in the introduced range – significantly decreased as iridoid glycoside content increased. This strongly supports that investment in iridoid glycosides protects plants from generalist herbivory in the field. Building upon this, Alba et al. (2014) measured iridoid glycosides on greenhouse-grown plants, and exposed them to herbivory by T. ni larvae experimentally. They found that percent catalpol strongly reduced leaf area eaten under these controlled conditions.
In summary, both trichomes and iridoid glycosides, particularly catalpol, serve as defenses against herbivory by generalist insect herbivores. In the native range, larvae of the specialist lepidopteran Cucullia verbasci L. (Noctuidae) may respond to these compounds as attractants or feeding stimulants, as they feed heavily on the young leaves, which contain the highest levels of iridoid glycosides (Alba et al., 2012).
Evidence for and against major plant defense hypotheses
Given the strong support for the underlying assumptions of the three plant defense hypotheses, V. thapsus can serve as a model for evaluating which hypothesis most accurately predicts evolution of plant defense in this system. Here, we summarize the support (or lack thereof) in this system for each of these hypotheses. We note that the directional evolutionary shifts predicted by these hypotheses are expected to hold true across various plant growth forms (e.g., annuals vs. perennials, rosette vs. bolting stages). However, as we discuss later in the context of future research directions, the magnitude of these evolutionary shifts may be mediated by ontogeny and by diverse abiotic and biotic factors, such as resource availability (Coley et al., 1985; Coley, 1987; Endara & Coley, 2011).
Lack of support for defense predictions from the EICA hypothesis
The evolution of increased competitive ability hypothesis predicts lower overall defense in introduced populations relative to native ones (Figure 1). However, leaves of introduced populations of V. thapsus did not have lower iridoid glycoside content, less toughness, or shorter trichomes than leaves of native populations when grown in a greenhouse (Alba et al., 2011). Furthermore, herbivory in a field common garden showed no greater palatability of introduced plants relative to native ones (Endriss et al., 2018). Finally, feeding trials actually showed decreased preference for leaves of greenhouse-grown introduced populations as compared to leaves of greenhouse-grown native populations, suggesting the evolution of increased defense against a generalist herbivore (Kumschick et al., 2013). This lack of evidence for decreased investment in defense does not appear to be driven by a lack of genetic variation, as Alba et al. (2013, 2014) found considerable among- and within-population variation in levels of investment and allocation to different classes of defense. In addition to the predictions regarding defense, EICA predicts introduced plant populations will grow faster or to larger size than native populations. Three studies support this (Alba et al., 2011; Kumschick et al., 2013; Endriss et al., 2018), but this evolution of increased growth does not appear to be driven by decreased investment in defense, as is predicted by EICA.
Mixed support for the shifting defense hypothesis
The shifting defense hypothesis predicts that shifts in herbivore composition should select for increased defense against generalists and decreased defense against specialists after plants are introduced to a new range (Figure 1). However, leaves collected from greenhouse-grown V. thapsus did not differ in trichomes or iridoid glycosides between introduced and native genotypes (Alba et al., 2011). In contrast, feeding trials showed that old leaves were less preferred by the generalist T. ni when harvested from introduced genotypes than from native genotypes of greenhouse-grown V. thapsus (Kumschick et al., 2013), providing partial support for the shifting defense hypothesis.
Partial support for the shifting defense allocation hypothesis
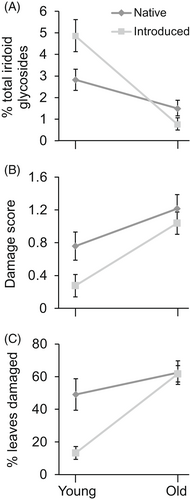
The shifting defense allocation hypothesis predicts that plant populations should exhibit higher levels of qualitative defenses in high-value tissues after introduction to a new range, which should translate into high-value tissue of introduced genotypes being better defended against generalists than high-value tissue of native genotypes (Alba et al., 2012). This shift in levels of qualitative defense should also result in high-value tissue of introduced genotypes being more attractive to specialists than high-value tissue of native populations. In field populations, Alba et al. (2012) found higher iridoid glycoside concentrations in young leaves than in old leaves of V. thapsus, and, importantly, this difference was markedly more pronounced in the introduced range, where specialist leaf chewers that might be attracted to the iridoids were absent (Figure 2A). The within-plant distribution of defense allocation was tracked by patterns of herbivory, with less herbivory of young leaves than of old leaves, again, especially for V. thapsus growing in the introduced range (Figure 2B,C).
Feeding trials conducted by Fettig (2016) suggest these between-range differences in within-plant allocation of defense are genetically based. Fettig (2016) grew native and introduced genotypes of V. thapsus in a common garden and evaluated preference of the specialist weevil Rhinusa tetra (Fabricius) using paired leaf discs from young and old leaves of the same plant. The weevil preferred young leaves over old leaves of introduced genotypes of V. thapsus, but had no leaf-age preference for native genotypes of V. thapsus. This pattern is consistent with high levels of iridoid glycosides observed in the field for young leaves of introduced populations of V. thapsus (Alba et al., 2012), as iridoid glycosides often attract or act as feeding stimulants to their coevolved specialists (Bowers & Puttick, 1988; Bowers, 1991; Nieminen et al., 2003; Reudler et al., 2011).
When Fettig (2016) conducted similar leaf choice experiments with generalist T. ni larvae, generalists consumed more old than young leaf tissue, for both native and introduced genotypes of V. thapsus. However, when she used potted V. thapsus plants to conduct no-choice feeding trials on leaves of the same age (from the center of the plant, so neither young nor old), T. ni consumed more leaf tissue on native than on introduced plants. Thus, plants may be less well defended against generalists in their native range than in their introduced range.
In summary, these studies provide growing support for the evolution of increased defense against generalists in young leaves of introduced genotypes as compared to native genotypes of V. thapsus. Further, we have provided strong evidence for the evolution of attractiveness to specialists in young leaves of introduced genotypes relative to native genotypes of V. thapsus, the second main outcome predicted by the shifting defense allocation hypothesis.
Biological invasions as models for testing plant defense theory
Our main finding is that the shifting defense allocation hypothesis best predicts defenses in V. thapsus. This finding is particularly robust given our full assessment of the three assumptions that underlie theoretical predictions of defense evolution. Support for the shifting defense allocation hypothesis is essentially support for optimal defense theory, and emphasizes how integrating within-plant and across-population scales reveals evolutionary change that might otherwise go undetected. Following the approach we use here for V. thapsus, other well-studied biological invasions could also be used to evaluate the underlying assumptions and theoretical predictions of these cornerstone hypotheses. Such endeavors would produce a better understanding of which patterns of plant defense are species-specific vs. which emerge as general patterns across systems.
Chinese tallow tree, T. sebifera (Euphorbiaceae), for example, is a particularly well-studied species that is already becoming a model for testing plant defense theory (e.g., Lankau et al., 2004; Rogers & Siemann, 2004, 2005; Huang et al., 2010; Wang et al., 2011; Carrillo et al., 2012; Carrillo & Siemann, 2016; Siemann et al., 2017; Li et al., 2020). Further, evidence is available in this system to address the assumptions underlying theoretical predictions of plant defense evolution. First, enemy escape is well documented in the introduced ranges (Siemann & Rogers, 2003; Hartley et al., 2004; Yang et al., 2013), especially escape from specialists (Hartley et al., 2010; Wheeler & Ding, 2014). The strength of enemy release may decline the longer T. sebifera populations have been established in their introduced ranges (Siemann et al., 2006; Hartley et al., 2010), but there are established differences in herbivore abundance and diversity across three ranges. Specifically, plant populations experience low levels of herbivory (mostly by generalists) in the southern USA, high levels of herbivory (mostly by generalists) in the introduced range in Hawaii, USA, and high levels of herbivory (by both specialists and generalists) in the native range in Asia (Siemann et al., 2017). The documented differences between three, not just two, regions represent a unique opportunity to disentangle how shifts in herbivore composition vs. herbivore abundance drive the evolution of plant defense.
Second, insect herbivory reduces plant fitness for native and introduced genotypes of T. sebifera. However, an important nuance is that herbivory may only reduce T. sebifera's fitness under certain environmental conditions (e.g., Yang et al., 2015), with some studies unable to detect effects of simulated herbivory (Rogers & Siemann, 2002) or low levels of generalist, ambient herbivory (Rogers & Siemann, 2003, 2005) on the performance of introduced genotypes of T. sebifera. This may be in part because the plant has evolved higher tolerance to herbivory in its introduced range in the southern USA than in its native range in Asia (Zou et al., 2008), which illustrates another dimension of how plants respond to herbivory.
Third, putative defenses likely decrease the prevalence or incidence of generalist herbivory. Wang et al. (2012a), for example, showed that increased levels of flavonoids resulted in lower biomass of two generalist lepidopteran herbivores, Grammodes geometrica (Fabricius) (Erebidae) and Cnidocampa flavescens Walker (Limacodidae).
Interestingly, Wang et al. (2012a) also demonstrated that introduced genotypes of T. sebifera had higher flavonoids (deterrents to generalists) and lower tannins (high-cost deterrents to specialists and generalists) than native genotypes of T. sebifera grown in a common garden, especially in high-value tissues (young leaves) following induction. Further, lower concentrations of tannins – again, especially in young leaves following induction – also resulted in higher biomass of a specialist herbivore, Gadirtha inexacta Walker (Lepidoptera: Nolidae) (Wang et al., 2012a). Complementary field surveys of herbivore damage and defense levels of young leaves in T. sebifera's native and introduced ranges (Xiao et al., 2020a,b) further revealed a complex interplay between trade-offs in allocation to flavonoids and tannins that suggest both biotic (herbivore type) and abiotic (e.g., latitudinal gradients in solar radiation) environments drive defense. Common-garden studies that investigate whether these trade-offs are genetically based would be especially powerful. Although not designed to specifically test the predictions of the shifting defense allocation hypothesis, studies in T. sebifera demonstrate biologically important shifts in defense allocation within high-value tissues (e.g., young leaves).
Together, the findings from V. thapsus and T. sebifera suggest that designing studies that take into account within-plant variation in defense allocation is crucial to revealing biologically meaningful patterns associated with plant-insect interactions. Indeed, studies by us and colleagues often showed that support for genetically based, between-range differences in defense are evident when taking within-plant variation into account, but are obscured when evaluated using average levels of defense per plant (Alba et al., 2011, 2012; Kumschick et al., 2013). We note that other systems with a strong foundation for these types of investigations include, but are not limited to, Ambrosia artemisiifolia L. (MacKay & Kotanen, 2008; Fukano & Yahara, 2012; Wan et al., 2018; van Boheemen et al., 2019), Pastinaca sativa L. (Berenbaum et al., 1986; Zangerl & Nitao, 1998; Zangerl et al., 1997; Zangerl & Berenbaum, 2003, 2005), Plantago spp. (Bowers & Stamp, 1993; Adler et al., 1995; Harvey et al., 2005; Barton, 2007; Penczykowski & Sieg, 2021), and Solidago spp. (Meyer et al., 2005; Hull-Sanders et al., 2007, 2009; Heath et al., 2014; Sakata, 2022).
Future directions of inquiry using biological invasions to test plant defense theory
Verbascum thapsus, and other well-studied species like it, remain ripe for future tests of plant defense theory. Above, we emphasize that accounting for tissue value (leaf age) can improve predictions of defense evolution. Here, we highlight five other opportunities for improving our understanding of plant-insect interactions, particularly plant defenses against herbivorous insects.
First, explore the consequences of variation in herbivory and tissue value over the course of a season or a plant's life cycle. It is clear that defenses vary over time and across ontogeny (Boege & Marquis, 2005; Barton & Koricheva, 2010; Barton & Boege, 2017), yet current tests of optimal defense often implicitly assume that a tissue's value and risk of attack are static by measuring defense and/or herbivory at a single point in time (e.g., Alba et al., 2012; McCall & Fordyce, 2010; Kooyers et al., 2017; but see Diezel et al., 2011; Heath et al., 2014). Measuring seasonal or ontogenetic variation in defense and herbivory within an invasion context, where populations experience marked differences in herbivory, may reveal new insights into adaptations associated with optimal timing of defense. For example, optimal defense should track the relative vulnerability of different life-history stages as well as herbivore seasonality (which might shift upon introduction to a new range; e.g., Wainwright et al., 2012).
Second, a more complete understanding of plant defense requires integrating belowground defenses into tests of plant defense theory. Above- and below-ground defenses may respond differently to shifts in herbivore pressure after introduction to a new range (Huang et al., 2012). Yet we still know very little about the prevalence and impact of root feeders, especially across populations of invasive plants (Blossey & Hunt-Joshi, 2003; Nunes & Kotanen, 2018). Verbascum thapsus represents an opportunity to address this knowledge gap, as there is a dramatic release from belowground herbivory in the mountain west region of the USA compared to part of the native range in France (SB Endriss, unpubl. data). More extensive surveys of above- and below-ground herbivory in both the native range and introduced range of V. thapsus could therefore be particularly enlightening, especially when paired with common-garden studies that investigate evolutionary differences in belowground defenses between native and introduced genotypes of V. thapsus.
Third, continue to advance research on optimal defense within an invasion context to improve plant defense theory, such as by better incorporating both induced defenses (i.e., those produced or mobilized in response to environmental cues) and constitutive defenses (i.e., those always present) into these investigations. Studies of optimal defense within an invasion context often measure the sum of induced and constitutive defenses, meaning that how induced defenses evolve within the context of plant invasions remains less well understood (Orians & Ward, 2010; Liu et al., 2020). Exploration of between-range differences in induced and constitutive defenses in V. thapsus would help inform theory as well as clarify previous findings. As described earlier, Fettig (2016) was unable to detect between-range differences in defense against a generalist in a feeding choice trial with clipped leaves, but found higher defense of native genotypes than introduced genotypes of V. thapsus when the same generalist was caged on an intact plant (on leaves of similar age to those used in the first experiment). Genetically based between-range differences in defense may therefore be driven by induced rather than constitutive responses in this system, as defenses produced elsewhere in the plant can only be mobilized to leaves if those leaves are still attached to the plant.
Fourth, explicitly testing the three assumptions underlying common plant defense theories – that introduced plant populations escape their natural enemies, that insect herbivory reduces plant fitness, and that putative defenses decrease the prevalence or impact of generalist insect herbivory – is critical for understanding the general applicability of these hypotheses across diverse plant taxa. We therefore need to test whether these assumptions hold true across species, but also within species, such as across resource gradients. For example, one of the best-supported theories regarding the evolution of plant defenses is the ‘resource availability’ hypothesis (RAH), which predicts that defense investment is inversely correlated to relative growth rate (Coley et al., 1985; Coley, 1987). Specifically, fast-growing species adapted to high-resource environments can regrow tissue quickly, and can therefore afford to lose tissue rather than investing in defending that tissue against herbivory. In contrast, slow-growing species have long-lived tissues and thus invest more strongly in defending those tissues. Historically, RAH has been used to explain variation across plant species (e.g., Endara & Coley, 2011). However, increasing evidence supports that the evolutionary consequences of enemy escape may change along environmental or resource availability gradients within species (e.g., Colautti et al., 2009; Woods et al., 2012; Hahn & Maron, 2016; Hahn et al., 2021), such as by mediating a trade-off between constitutive and induced defense (López-Goldar et al., 2020).
Finally, evaluating the evolutionary consequences of reassociations between introduced plant populations and their specialist herbivores represents another opportunity to use plant invasions as experimental systems to better understand plant-insect interactions. Indeed, using biocontrol introductions to advance basic knowledge on herbivore-driven adaptation has been repeatedly suggested in the peer-reviewed literature (e.g., Müller-Schärer et al., 2020) and has already produced valuable tests of EICA (Sun & Roderick, 2018) and the shifting defense hypothesis (Fukano & Yahara, 2012), as well as deeper insights into the evolution of defense across resource gradients (Hahn & Maron, 2016) and into herbivore-driven selection for flowering phenology (Fukano et al., 2013). Specifically, when introduced plant populations become reassociated with their coevolved specialist herbivores through inadvertent introductions or intentional biological control, we would expect defenses to evolve to become more similar to plant populations in the native range (Rapo et al., 2010; Wheeler & Schaffner, 2013; Wan et al., 2019). A small but growing literature base uses this approach to test the shifting defense hypothesis (as reviewed by Wheeler & Schaffner, 2015; Wan et al., 2018), and could be extended to test the shifting defense allocation hypothesis. Further, increasing evidence suggests that introduced plant populations may also evolve new climatic niches (Sotka et al., 2017; van Boheemen et al., 2019), with consequences for how climate may mediate other abiotic and biotic drivers of defense evolution differently between plants' native and introduced ranges. Reassociations between introduced plant populations and their specialist herbivores therefore provide valuable opportunities to test the shifting defense allocation hypothesis across resource gradients, which may allow for additional refinements of this hypothesis moving forward. Thus, if (or more likely when) co-evolved specialists increasingly reassociate with V. thapsus or other species of well-studied invasive plants over time, we will be well poised to better understand how plant defense evolves not just in response to decreased herbivory, but also increased herbivory by specialists – a feat that is especially difficult to accomplish in real-time. Thus, the benefits of introduced plants as models for testing plant defense evolution will only increase moving forward.
AUTHOR CONTRIBUTIONS
Stacy B Endriss: Conceptualization (equal); supervision (lead); visualization (lead); writing – original draft (equal); writing – review and editing (lead). Christina Alba: Conceptualization (equal); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Ruth RA Hufbauer: Conceptualization (equal); visualization (supporting); writing – original draft (equal); writing – review and editing (supporting).
ACKNOWLEDGMENTS
The authors thank all their collaborators working on the Verbascum thapsus system for their contributions. RAH thanks the organizers of SIP17 for inspiring this review, and acknowledges support of the United States Department of Agriculture National Institute of Food and Agriculture (USDA NIFA) Hatch project 1012868. We also thank the two reviewers for improving this manuscript through their thoughtful feedback. Part of this study was presented at the 17th International Symposium on Insect-Plant Relationships (SIP17, 25–30 July 2021, Leiden, The Netherlands).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this mini-review.




