Effects of biodiversity in agricultural landscapes on the protective microbiome of insects – a review
Abstract
Symbiotic bacteria in herbivorous insects can have strong beneficial impacts on their host's survival, including conferring resistance to natural enemies such as parasitoid wasps or pathogens, while also imposing energetic costs on the host, resulting in cost-benefit trade-offs. Whether these trade-offs favour the hosting of symbionts depends on the growth environment of the herbivore. Long-term experimental grassland studies have shown that increasing plant species richness leads to an increased diversity of associated herbivores and their natural enemies. Such a change in natural enemy diversity, related to changes in plant diversity, could also drive changes in the community of symbionts hosted by the herbivorous insects. Aphids are one model system for studying symbionts in insects, and effects of host-plant species and diversity on aphid-symbiont interactions have been documented. Yet, we still understand little of the mechanisms underlying such effects. We review the current state of knowledge of how biodiversity can impact aphid-symbiont communities and the underlying drivers. Then, we discuss this in the framework of sustainable agriculture, where increased plant biodiversity, in the form of wildflower strips, is used to recruit natural enemies to crop fields for their pest control services. Although aphid symbionts have the potential to reduce biological control effectiveness through conferring protection for the host insect, we discuss how increasing plant and natural enemy biodiversity can mitigate these effects and identify future research opportunities. Understanding how to promote beneficial interactions in ecological systems can help in the development of more sustainable agricultural management strategies.
Introduction
Insects are a dominant component of biodiversity in terrestrial ecosystems, driving important ecosystem functions (e.g., pollination, herbivory, and pest control) thereby affecting nutrient cycling (Yang & Gratton, 2014). Plant-feeding herbivorous insects form the second trophic level in food webs, and can cause important feedback effects such as changes in plant community composition and diversity (Brown & Gange, 1999), and increase the speed of nutrient cycling (Belovsky & Slade, 2000; Nitschke et al., 2015). At higher trophic levels, carnivores, omnivores, and parasitoids can control herbivore communities by regulating their density and composition together with pathogens (bacteria, fungi). The total of these antagonists is often referred to as ‘natural enemies’ (Van Driesche et al., 2008). It is increasingly recognised that the microorganisms associated with an individual (its microbiome) can influence a host's biology and modify their response to interactions with other species, or the abiotic environment (Bordenstein & Theis, 2015). Such effects have been well studied in plants where beneficial microbes can boost plant resistance to pathogens, herbivores, and adverse soil conditions (Vandenkoornhuyse et al., 2015). More recently, bacterial symbionts hosted by insect herbivores have been identified as additional important components of ecosystems that can mediate trophic interactions (McLean et al., 2016; Simon et al., 2017). These bacterial symbionts are highly specialised and can have diverse ecological and evolutionary effects on their hosts, for example, by providing essential nutrients or resistance to natural enemies (Moran et al., 2008; Feldhaar, 2011; Ferrari & Vavre, 2011; Berendsen et al., 2012).
Aphid-symbiont interactions
Aphids and their highly specialised bacterial symbionts are a model system for studying the microbiome of insects; with aphid microbiome work focused on the roles of specialised endosymbionts rather than the impact of gut microbiota that is often studied in other insects (Engel & Moran, 2013). The diversity of the aphid microbiome is surprisingly low (Sugio et al., 2015), with one obligate (primary), and nine common facultative (secondary) bacterial symbionts that have been identified from screening in many aphid species across the world (Zytynska & Weisser, 2016). Despite this low diversity, these bacterial symbionts can have strong effects on aphid survival. The main obligate symbiont (Buchnera aphidicola Munson, Baumann & Kinsey) is found in almost all aphid species providing the aphid with essential amino acids (Douglas, 1992). Aphid facultative symbionts have wide-ranging beneficial effects but hosting a symbiont can also have an associated cost due to nutritional demands of the symbiont leading to reduced longevity and fecundity in the aphid (reviewed by Oliver et al., 2014). The extent of these costs is also likely context-dependent on host-plant quality, temperature, or related to which other symbionts are co-hosted by the aphid, and underlies the cost-benefit trade-off in hosting these potentially protective symbionts (Kwiatkowski & Vorburger, 2012). Therefore, not all aphids will host all available symbionts in a population, leading them to be an interesting subject for studying community-level effects of plant-insect-microbe interactions. Aphid symbionts are predominantly vertically transmitted from mother to offspring, but there is also evidence of horizontal transfer of symbionts among aphids during sexual reproduction (Moran & Dunbar, 2006), by parasitoids when ovipositing eggs into aphids (Gehrer & Vorburger, 2012), or even through infected honeydew (Darby & Douglas, 2003).
Several recent reviews have summarised the protective effects of aphid facultative symbionts (Oliver et al., 2014; Guo et al., 2017), and their distribution among natural populations (Zytynska & Weisser, 2016). Here, we briefly describe the effects of these symbionts on aphids in relation to how these effects may alter aphid populations in natural systems. In general, symbionts protect aphids against more specialised natural enemies including entomopathogenic fungi and parasitoid wasps, yet once an aphid population is small, then generalist predators can help to reduce populations further. Thus, here we discuss the diversity of the whole natural enemy community as the combination of specialist parasitoid wasps, pathogenic fungi, and generalist predators.
Aphid symbionts have been found to confer resistance against parasitoid wasps, which are specialist natural enemies that lay an egg into an aphid, and as the larva develops it consumes the aphid, eventually emerging as an adult from the aphid mummy (hardened shell of a parasitised aphid). The level of protection that is afforded by symbionts varies across aphid species/genotypes and symbiont strains (Vorburger et al., 2010; Cayetano et al., 2015; Leclair et al., 2016), but in general leads to increased aphid survival with potential for extinction of the natural enemy (Sanders et al., 2016). Experimental work has confirmed the protective effects, against parasitoids, of the well-studied symbionts Hamiltonella defensa Moran, Russell, Koga & Fukatsu (reviewed in Oliver et al., 2014; Zytynska & Weisser, 2016; Guo et al., 2017), Regiella insecticola Moran, Russell, Koga & Fukatsu (Vorburger et al., 2010; Hansen et al., 2012), and X-type (PAXS) (Heyworth & Ferrari, 2015). It is now known that the mechanism by which Hamiltonella symbionts protect the aphid is via a bacteriophage in the bacterium itself, which releases a toxin that can kill the developing larva (Moran et al., 2005). Mechanisms of effect by the other symbionts are still to be fully determined.
Other protective effects against natural enemies include increased aphid survival when challenged by fungal pathogens for aphids hosting R. insecticola, Rickettsia, or Spiroplasma (Scarborough et al., 2005; Lukasik et al., 2013a,b). Another symbiont, Rickettsiella, has been implicated in altering the body colour of aphids, with subsequent effects on parasitism and predation rates across green and pink colour morphs of the pea aphid, Acyrthosiphon pisum (Harris) (Tsuchida et al., 2010, 2014). Variable infection rates of this symbiont across populations could alter the relative densities of each aphid colour morph, with cascading effects on the natural enemy populations (Tsuchida et al., 2014). Finally, symbionts can also mitigate abiotic stress factors. Serratia symbiotica Sabri et al. symbionts can protect an aphid host against heat shock (Chen et al., 2000; Russell & Moran, 2006), potentially enabling aphids to withstand high summer temperatures in more sun-exposed habitats or to withstand higher microclimatic temperatures within plant communities containing sparser vegetation cover.
The interaction between aphids and their symbionts has often been studied in a laboratory setting, with artificial curing or introduction of specific symbiont strains (as first described by Simon et al., 2007) determining the different effects that symbionts can have on their aphid hosts (reviewed in Guo et al., 2017). However, much of this work is focused on only a few aphid or symbiont species; for example, research is dominated by studies on the symbiont H. defensa in pea aphids (Oliver et al., 2014). Moreover, very few laboratory studies have introduced multiple symbionts (but see Oliver et al., 2006; Lukasik et al., 2013a; Tsuchida et al., 2014; Leclair et al., 2016, 2017; Doremus & Oliver, 2017; McLean et al., 2018), whereas field-collected aphids have been found to co-host up to four symbionts per individual (Ferrari et al., 2012; Russell et al., 2013; Smith et al., 2015; Zytynska et al., 2016). To date, more than 150 aphid species have been studied for bacterial symbionts from field-collected aphids and, although it is important to keep documenting symbiont infection rates, it is now time to go beyond descriptive field studies to further explore the role of these symbionts in the ecological community.
Aphid-symbiont interactions in natural food webs
Transferring what we know from controlled laboratory studies to understand field dynamics has proved complex. Controlled laboratory studies have generally compared populations of single aphid species (but see Sanders et al., 2016) of which every individual hosts a symbiont to populations that are totally uninfected by symbionts (i.e., with no variation in the frequency of infection). Yet, in natural systems, the diversity of (1) symbionts, (2) aphids, (3) natural enemies, and (4) host plants will all act together to influence the population and community dynamics of all these interacting species.
A trade-off between the protective benefits and fitness costs of hosting symbionts (Kwiatkowski & Vorburger, 2012) means that symbiont infection is rarely fixed in a population (i.e., often less than 100% of aphids will host any given symbiont within a population; Zytynska & Weisser, 2016). For example, co-hosting of symbionts Serratia and Hamiltonella conferred high protection against parasitoid wasps in the laboratory, but the prevalence of these ‘superinfected’ aphids was low in the field due to a strong fitness cost (Oliver et al., 2006). However, other studies have found strong positive associations in the field between these two symbionts across multiple aphid species (Leonardo & Muiru, 2003; Zytynska et al., 2016) suggesting that fitness costs can be variable across systems. Further, different strains of the same symbiont species can have variable effects on both the level of protection and the associated costs to the host (Vorburger et al., 2010; Cayetano et al., 2015; Leclair et al., 2016). The extent to which different symbiont strains are present within a single aphid population is unknown, but Hamiltonella strains vary among different pea aphid lineages (Leclair et al., 2016). Similarly, both the protection conferred and associated fitness costs of symbionts can also vary dependent on the genotype of the aphid and the genotype of the natural enemy (e.g., parasitoid wasps) (reviewed in Vorburger, 2014).
Natural communities are comprised of multiple aphid species, and competition between these aphids can be altered via variable symbiont infection rates across aphid species. At 100% infection rate, an experimental study showed that a protective symbiont could drive the extinction of unprotected aphid species and their specialist natural enemies (Sanders et al., 2016). However, with reduced symbiont infection rates across all aphid species, the community could be stabilised leading to increased potential for co-existence as we often see in the field (Zytynska et al., 2016; Zytynska & Venturino, 2018).
Studies have also shown that the occurrence of individual aphid bacterial symbionts can be influenced by the host-plant species on which an aphid feeds (Simon et al., 2003; Brady & White, 2013; Russell et al., 2013; Henry et al., 2015). For example, Hamiltonella had a high infection frequency in Aphis craccivora Koch aphids collected from alfalfa (Medicago sativa L.), but it was absent in all aphids collected from black locust (Robinia pseudoacacia L.) (Brady & White, 2013). In contrast, those aphids collected on black locust were found to be infected by Arsenophonus (Brady & White, 2013), which is now known to be involved in specialisation on this host-plant species (Wagner et al., 2015). Another well-studied example is pea aphids that have distinct genetically differentiated host races associated with different plant species. Secondary symbiont infection is thought to play a role in host-plant specialisation as different symbiont communities were found among different host races (Tsuchida et al., 2004; McLean et al., 2011; Russell et al., 2013; Oliver et al., 2014). In particular, pea aphids hosting Hamiltonella are more likely to be found on Lotus sp., Ononis sp., or Medicago sp. plants, and those with Regiella on Trifolium sp. (Ferrari et al., 2012; Russell et al., 2013).
Given these associations between individual host plants, aphid species, and symbionts within an aphid, host-plant diversity has strong potential to increase aphid-symbiont diversity through altering interactions between aphids and other trophic levels. In the following part of this review, we summarise known effects of plant diversity on insect communities, with a focus on aphids, and then explore this in relation to how plant diversity can mediate aphid-symbiont-natural enemy interactions. In particular, we examine how these interactions might influence pest regulation in agroecosystems and the implications of integrating plant diversity into biological control methods for regulating aphid pest populations.
Plant diversity effects
Plant diversity effect on insect communities
The global loss of species in recent centuries (Butchart et al., 2010) has raised questions about the functional importance of biodiversity (Schläpfer & Schmid, 1999). Over 20 years of research has demonstrated that biodiversity is of critical importance for ecosystem functioning, as a decline in biodiversity is typically associated with lower performance and greater temporal variability in performance in many ecosystem functions (e.g., Balvanera et al., 2006; Allan et al., 2013). Biodiversity experiments are a crucial tool for studying these species loss effects, where the diversity of (most often) plant communities is manipulated experimentally to study associated animal communities and ecosystem functions with plant species richness as the explanatory variable (Weisser et al., 2017). Such experiments have demonstrated that plant diversity affects the abundance and diversity of invertebrates (Scherber et al., 2010; Haddad et al., 2011; Borer et al., 2012; Hertzog et al., 2016b). These patterns have now been confirmed for many different invertebrate taxa across different years (Ebeling et al., 2018). Both herbivores and carnivores strongly benefit from an increase in plant species richness, with higher species richness and abundance of both trophic levels (Haddad et al., 2001; Vehviläinen et al., 2007; Borer et al., 2012; Ebeling et al., 2018). The ratio between herbivore and plant biomass (herbivore load) significantly decreased with plant species richness, whereas the ratio between predator and herbivore biomass (predator-prey ratio) did not show any significant change across the gradient of plant species richness (Ebeling et al., 2018). Whereas, herbivores were directly affected by plant species richness and not by plant biomass (Hertzog et al., 2016b), effects of plant diversity on predator communities (e.g., abundance and diversity) are likely driven by plant diversity-induced changes in the herbivore communities, which serve as a food resource for predators (Hertzog, 2017).
As a consequence of changes in the consumer community, plant diversity also affects ecosystem functions mediated by these consumers (Scherber et al., 2010; Ebeling et al., 2014). In grasslands, rates of herbivory (Meyer et al., 2017) and predation (Hertzog et al., 2017) increased with higher plant species richness, whereas parasitism rate of aphids by wasps showed a decrease with increasing plant diversity (Petermann et al., 2010b; Ebeling et al., 2012). Overall, this work has shown that multitrophic interactions can be stabilised by high plant diversity (Haddad et al., 2011; Ebeling et al., 2012).
Plant diversity effects on aphids
Aphids and their natural enemies have also been studied in biodiversity experiments with manipulated plant species richness. Petermann et al. (2010a,b) measured the densities and species richness of aphids and parasitic wasps (primary, secondary, and facultative tertiary parasitoids of aphids) that naturally colonised grassland plots, along experimental gradients of plant species richness. They found that the densities and richness of species at all trophic levels were influenced by changes in plant species richness. The effects were rarely direct but instead mediated by the abundance and species richness of aphid host plants and subsequent trophic levels. The herbivore and primary parasitoid levels were directly affected by changes in plant species richness, with the highest densities and species richness of insects occurring at intermediate species richness of plants (Petermann et al., 2010b). In another experiment, aphid abundance increased with a higher number of plant functional groups (Koricheva et al., 2000). In addition to densities and species richness of aphids and parasitic wasps, life-history traits of aphids (production of winged morphs) and their parasitoids (emergence rates) were also affected by plant species richness (Petermann et al., 2010a).
As for the consumers in general, changes in densities and species richness of aphids and their associated natural enemies translated into differences in ecosystem functions. Petermann et al. (2010b) calculated two ecosystem functions: aphid load (the number of aphid individuals per unit of host-plant biomass used as a proxy for herbivory) and parasitism rate. Aphid load was highest at intermediate plant species richness and negatively affected by both host-plant biomass and host-plant species richness. Parasitism rate was mostly affected indirectly via aphid density and overall weakly negatively related to plant species richness (Petermann et al., 2010b). Studying rates of predation, rather than parasitism, on aphids that were experimentally exposed by glueing individuals onto plastic labels at the soil surface of plots of differing plant species richness, Hertzog et al. (2017) showed higher rates of aphid predation at higher plant species richness. This increase in predation rates is likely explained by an increase in predator abundance and diversity, and reduced antagonistic interactions among predator species (Hertzog et al., 2017). In contrast, a microcosm study found decreased consumption of aphids at higher plant species richness when the number of predators was held constant (Aquilino et al., 2005). In the same study, there was an increased aphid consumption at higher diversity of natural enemies (Aquilino et al., 2005). As abundance and species richness of both aphids and predatory and parasitic arthropods increase with plant species richness in the field (see above), a combination of positive bottom-up and negative top-down effects on aphid survival determines the overall response of aphids to plant species richness (Petermann et al., 2010b). The small number of available studies currently prevents a quantitative synthesis of plant diversity effects on aphids. Yet, the above studies already illustrate that the overall effect of plant species richness on aphid populations is variable, likely depending on the study context. Plant diversity effects on aphids are likely also altered by the protective microbiome associated with the aphids.
Plant diversity effects on aphid-symbiont communities
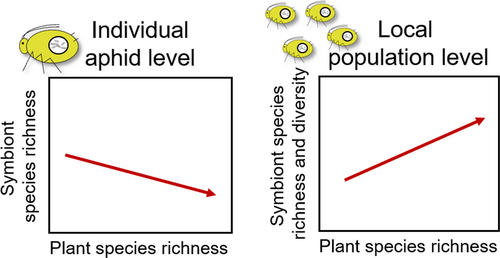
Plant diversity likely influences aphid symbionts given the documented effects on the aphid hosts, the natural enemies, and via the microclimate around aphid host plants. Yet, at present only a single study has investigated changes in aphid symbionts along a plant diversity gradient. In this study, three aphid species were collected along a gradient of plant species richness in a grassland biodiversity experiment. Each aphid species was collected from a different host plant and examined for the presence of common aphid symbionts (Zytynska et al., 2016). Aphids were documented to frequently co-host multiple symbionts. In all three distinct plant-aphid species combinations, plant diversity significantly affected the species richness of the hosted symbionts. However, the effect of plant diversity differed at the level of the individual aphid (i.e., the number of symbionts that one single aphid was hosting) and across the local population (i.e., the proportion of aphids hosting particular symbiont combinations) (Figure 1). Whereas, aphids tended to co-host more symbionts at lower plant diversity, the opposite was true across the population where a greater richness and diversity of symbionts were present at higher plant diversities (Zytynska et al., 2016). On average, the effect was to decrease/increase the symbiont species richness by one symbiont. Due to the survival impact of these symbionts, this can have strong ecological implications. However, this study was based on correlations between a plot's plant species richness and the symbionts hosted across the aphids, and was therefore not able to elucidate any mechanisms driving these effects. There are many potential ways in which plant diversity can drive changes in aphid-symbiont communities, and we expand on this to explore how these mechanisms can explain the observed patterns.
Potential mechanisms
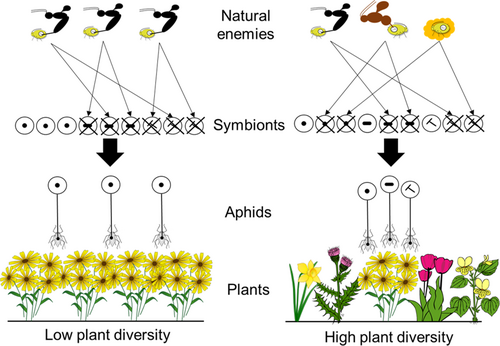
The mechanisms driving changes in the aphid-symbiont community could occur at either the level of the individual aphid or at the level of the whole aphid population. Individual aphids will experience the direct protective effects (increased survival) but also the associated costs (reduced fitness) of hosting symbionts. The frequency of different aphid-symbiont combinations across the population, along with the relative frequency of selection pressures (e.g., natural enemy abundances), will then drive effects at the population level. For example, a population with two aphid-symbiont combinations (one protected, one unprotected) and no natural enemy would favour the unprotected aphid due to higher growth rates. Whereas, if the natural enemy is present, the protected aphid would be favoured, despite reduced growth rates (survival is more important than high growth). The selection pressures for hosting symbionts and associated costs and benefits can change between plant communities of high and low diversity.
In a low-diversity plant system, there may be a restricted set of natural enemies with higher overall parasitism rate (Petermann et al., 2010b). Here, a particular combination of symbionts will provide greatest survival chances to individual aphids, and at the population level, if all aphids host this particular optimal combination of symbionts, there will be low symbiont diversity (Figure 2). Any impacts on aphid fitness will be shared by all aphids, reducing the effects of within-species competition through variable symbiont costs. In a stand with higher plant diversity and increased natural enemy diversity (Petermann et al., 2010b), the per-capita chance of an individual aphid being attacked by a specific natural enemy is low, but the chance of being attacked by at least one of the various types is high. Assuming there are high fitness costs to hosting all protective symbionts, one strategy for individual aphids is to host fewer symbionts and trade-off full protection with higher reproductive outputs. Therefore, in diverse systems where there is no single optimal symbiont community, the population will exhibit higher symbiont diversity (Figure 2). Aphid population structure can also then be further influenced by within-species competition via variable fitness effects of hosting the various symbiont communities, also dependent on the local natural enemy community composition.
When other interacting species are influenced by plant diversity, this can also have consequences for aphid-symbiont interactions. For example, aphids are often tended by ants that feed on the aphid honeydew and in return provide additional protection against natural enemies (Billick et al., 2007). This can be an obligatory relationship where the aphid requires the ant for survival, but often it is more opportunistic. When aphids were recurrently tended by ants, the proportion of aphids hosting Hamiltonella symbionts was reduced compared to aphids tended only occasionally by ants (Mandrioli et al., 2016). Ants can also help to maintain the health of an aphid colony by removing sticky honeydew that, if left, can encourage fungal growth (Buckley, 1987), and at least three aphid symbionts have been implicated in improving aphid resistance to fungi, including Regiella, Rickettsia, and Spiroplasma (Scarborough et al., 2005; Lukasik et al., 2013a,b). Although plant species richness had no direct effect on ant abundance along a diversity gradient, soil temperature had a positive effect on ant abundance, and plant cover had a negative effect (Hertzog et al., 2016a). As plant cover increases with plant species richness and soil temperature decreases with plant species richness, this suggests potential lower ant abundance and thus lower aphid attendance by ants at higher plant diversity. Reduced protection from natural enemies by ants at high plant diversity would further select for higher symbiont diversity in diverse plant-natural enemy communities.
The cost-benefit trade-off of hosting symbionts could also be mediated by plant diversity through changes in host-plant quality. For example, differences in growth stages or strategies of plants can alter phloem composition, with subsequent effects on aphid nutrition (Karley et al., 2002). This could influence the associated fitness costs of co-hosting symbionts, leading to the observed effect of higher proportions of aphids hosting multiple protective symbionts in monocultures. As the C:N ratio (and also the C:P ratio) of plant tissues tends to increase along the plant diversity gradient (Abbas et al., 2013), meaning that nutrient availability decreases, this could indicate lower phloem quality for aphids at higher plant species richness. For pollinators and non-aphid herbivores, it was already demonstrated that higher C:nutrient ratios in plants translate into increased C:nutrient ratios in animal tissues (Abbas et al., 2014). Similar effects might also occur in aphids. This is yet to be tested, but could partly explain the reduced occurrences of symbiont superinfections at higher plant diversities.
Higher plant diversity can also modify the local microclimate of a host-plant because of an increased density of the plant community (Lorentzen et al., 2008; Marquard et al., 2009) with consequent lower air temperature and increased air and top-soil humidity because of shading (Allan et al., 2013). These changes in microclimate could indirectly influence the protective effect of symbionts that help against heat shock (sun-exposed vs. shady patch) (Montllor et al., 2002), or entomopathogenic fungi (humid/shady vs. dry/exposed) (Millstein et al., 1982).
Implications for aphid biocontrol in agriculture
In agricultural systems, many natural ecological processes are disrupted as a consequence of management methods. By planting large areas with a single crop, herbivore populations can quickly increase their population sizes and avoid control by natural enemies. Aphids are an economically important pest species in agriculture where they can cause direct feeding damage and indirect damage through the transmission of devastating crop viruses which are typically controlled by the large-scale application of pesticides (van Emden & Harrington, 2017). Consequently, agricultural practices – including the widespread application of pesticides together with the destruction of natural habitats to create farming land – have been identified as an important driver of global species loss (Maxwell et al., 2016) likely contributing to the ongoing drastic insect decline (Hallmann et al., 2017).
One strategy for more sustainable agricultural production is (partially) replacing pesticide use by employing biological control agents (such as parasitoid wasps, generalist predators, and entomopathogenic fungi) against aphids. There is evidence that biological control of aphids can be highly successful in closed greenhouse environments (Powell & Pell, 2007; Messelink et al., 2014), but also that this can select for aphids hosting protective symbionts (Oliver et al., 2008; Sanders et al., 2016). The impact of aphid protective symbionts in closed systems is explored by Vorburger (2018), with suggestions to minimise the risk of selecting for symbiont-conferred resistance by deploying parasitoids early in the pest outbreak, or increasing parasitoid-to-aphid ratios. A further suggestion that is highly relevant to our proposal is to overcome the selection of resistant aphid/symbiont strains by increasing the diversity of the interacting community. This can be achieved by increasing the genetic diversity of a single parasitoid wasp species (Hafer & Vorburger, 2018) or by increasing species diversity of the natural enemies. In a greenhouse system such diversity can be achieved through release of specific natural enemy communities.
In low-diversity agricultural fields, the associated low diversity of natural enemies, resulting in low predation rates, can lead to increased symbiont-mediated resistance against the few natural enemies that are present across aphid populations similar to the greenhouse situation described above. However, in the field this resistance must be overcome through other more viable options than mass release of natural enemies. By increasing the diversity of plants in an agricultural landscape, the diversity of natural enemies can be increased as demonstrated in biodiversity experiments (see above). Whereas, diversifying plants within agricultural fields is hindered by constraints in farming methodology (planting and harvesting), increasing flowering plant diversity at field margins in the form of wildflower strips has been suggested as an appropriate method for diversification (Blaauw & Isaacs, 2012; Fabian et al., 2013; Balzan & Moonen, 2014). This has a two-step beneficial effect on pest regulation. First, a greater diversity of natural enemies means there is an increased number of ways to control the aphid populations and redundancy if one natural enemy is unable to establish a viable population. Second, increased diversity of natural enemies will reduce the effect of symbiont-mediated resistance and allow reduction of aphid pest populations by both specialised parasitoids and fungi, and subsequently more effective control by generalist predators that are more likely to drive smaller local populations to extinction (Senft et al., 2017). First field trials showed that increasing plant diversity through the planting of wildflower strips reduces aphids by 75% due to an increase in natural enemies (Tschumi et al., 2016).
There are some ways in which planting of wildflower strips adjacent to agricultural fields can increase the abundance of parasitoid wasps and generalist predators. Adult parasitoid wasps and many adults of generalist predators (e.g., lacewings or hoverflies) do not feed on the aphids themselves, but rather on flower nectar or even on the aphid honeydew (Lee et al., 2004). It is often the carnivorous larvae that consume aphids. Without additional sugar-based resources, adult wasps have limited lifespans of 2–3 days, yet when sugar is supplemented their lifespan can reach 2 weeks and host-searching behaviour is significantly increased (Russell, 2015), thereby increasing their potential to suppress aphid populations. Although changes in nectar resources with plant diversity have not been studied for parasitoid wasps, the frequency of pollinator visits and pollinator species richness increased with a higher amount and diversity of floral resources (Ebeling et al., 2008). Thus, positive effects of plant species richness can also be expected for parasitoid wasps and other generalist predators. Wildflower strips can also act as reservoirs for non-pest aphid species that benefit the establishment and maintenance of viable natural enemy communities. This occurs through the use of targeted plant species, but as yet an optimal set of plant species for common use has not been identified (Frank, 2010). Increasing plant diversity can be a suitable replacement, assuming that the diversity of plants will enable these services to be established (McLean & Godfray, 2016).
One caveat for the application of wildflower strips as a means to control aphids (and potentially also other herbivores) is the choice of the flowering plant community. It is a general practice for wildflower strips to be planted with various seed mixes of native plants, with an assumption that there is variation in flowering time and resources (Lu et al., 2014). However, it is essential that tailored flower strips are used to ensure the chosen flowering plants are suitable for maintaining populations of aphid-specific natural enemies, as not all plants are equally suitable (Russell, 2015).
Future research opportunities
In this review, we have explored the many ways in which plant diversity can mediate aphid-symbiont interactions. The abundance of literature on aphid-parasitoid interactions and the effect of plant diversity on herbivore natural enemies provide much support for the effect of plant diversity on aphid symbionts to be mediated by top-down selection pressures. Much of this is, however, based on a single study that looked at three plant-aphid combinations, and therefore we first suggest that these effects are explored further in other field systems.
The study of aphid-symbiont-natural enemy interactions in controlled model ecosystems often compares effects of parasitoid wasps on aphid populations that are all infected by symbionts, to those that are uninfected (Rothacher et al., 2016; Sanders et al., 2016). Yet, rarely do all aphids in a population host symbionts (Zytynska & Weisser, 2016), and the proportion of aphids hosting protective symbionts has been shown to be rather dynamic across the season in relation to the abundance of natural enemies (Smith et al., 2015). We therefore suggest a focus on developing experimental systems to study the protective effects of symbionts under more realistic conditions, for example, with variable starting proportions of symbiont-infected and uninfected aphids. Moreover, the temporal dynamics of natural enemy populations should also be addressed, as rarely will all natural enemies arrive and leave a field system at the same time.
Another currently vastly underexplored question regards the differences in the impacts of aphid-symbiont interactions in diverse field systems for aphids that are host-plant specialists compared to those that feed on multiple host plants within a single community. This is related to the impact of plant within-species variation that can drive variation in the distribution of aphids among host-plant individuals (Zytynska et al., 2014). The restriction of aphids to single host-plant species, or even genotypes within a host plant species, will likely exacerbate the effects of the surrounding plant diversity on aphid-symbiont interactions.
Lastly, in the agricultural context, the choice of plant species for wildflower strips must include sufficient resources for parasitoid wasps. Often, seed mixtures are chosen for their impact on pollinators, yet a vast amount of variation in the responses of parasitoid wasps to different flowering plants (Russell, 2015) highlights the need to produce seed mixes tailored towards natural enemy communities with ample research opportunities related to the selection of appropriate plant species.
Conclusions
There is a growing literature of research documenting the effect of plant diversity on herbivores and higher trophic levels. The expanding number of experimental field systems manipulating biodiversity is a unique platform for understanding how plant-invertebrate interactions can mediate other less-studied connections between species. The recent acknowledgement of the important role of an individual's microbiota for its health and fitness has boosted research on the role of bacterial symbionts in insects. How insect microbiome interactions are changed in response to the diversity of the food-web in which they are embedded is an emerging topic offering exciting future research opportunities and potential application in sustainable agriculture. Whereas, aphid symbionts have the potential to reduce biological control effectiveness through conferring protection for the host insect, increasing plant and natural enemy biodiversity can mitigate these effects. Beyond effects just on natural enemies of pests, increasing landscape complexity can have knock-on effects for other species such as bees, where the environmental landscape and available plant species can modify gut microbiota composition and through this influence their behaviour and fitness (Donkersley et al., 2018; Jones et al., 2018). By integrating plant diversity into agricultural systems, we can limit the impact of unfavourable species interactions, and use diversity to promote beneficial interactions for more sustainable pest control.
Acknowledgements
SEZ was supported by a British Ecological Society small research grant (SR16/1069) and the EU COST ACTION FA1405 on crop-arthropod-microbe interactions. STM was supported by the Deutsche Forschungsgemeinschaft (research unit ‘the Jena Experiment’ FOR 1451).




