Preference–performance in a specialist sawfly on congeneric host plants
Abstract
In herbivorous insects, the preference–performance hypothesis predicts that females will choose to oviposit on plants that maximize offspring development. Although there is evidence to support this hypothesis, several studies have shown that it does not always apply. Among the factors that may modulate the preference–performance relationship, diet breadth is a significant one, although no correlation has been found for monophagous insects. More case studies are needed, as more restricted diets are expected to require better discrimination ability. In this study, we explored the preference–performance linkage in a monophagous sawfly, Tequus schrottkyi (Konow) (Hymenoptera: Pergidae), which feeds as larva on the wild potato Solanum commersonii Dunal (Solanaceae). We used the cultivated potato, Solanum tuberosum L., as an alternative host plant for our experiments. Performance was evaluated from newly emerged larvae until adult emergence, raising individual larvae on potted plants or excised leaves. Female oviposition preference was studied in two-choice bioassays, in which individual females were offered whole plants of S. commersonii and S. tuberosum. The nutritional quality of the plants was assessed by measuring protein, non-structural carbohydrate, and water contents. The offspring performed better on their host plant, S. commersonii, resulting in more larvae completing their development to the adult stage. Moreover, the larvae developed faster and their cocoon weighed more when they were raised on their host plant. The females oviposited almost exclusively on S. commersonii, regardless of their previous food plant. The protein and non-structural carbohydrate contents did not differ between the plants, whereas water content was higher in the non-preferred S. tuberosum. The differences in water content and in secondary metabolites, which have been analyzed in a previous study, may play a role in female preference and offspring performance. The results are discussed within the framework of the preference–performance hypothesis and the chemical ecology of insect–plant interactions.
Introduction
The relationship between female oviposition choice and offspring performance is central to the study of plant–insect interactions (Thompson, 1988; Bernays & Chapman, 1994; Schoonhoven et al., 2005). In insect species with no parental care and slow-moving immature stages, female oviposition choice will largely determine the success of the newborn larvae. Therefore, choosing the right host plant may be an important element of fitness on which selection should play a relevant role. Consequently, it is expected that females have the ability to discriminate between plants and choose the plant that favors offspring performance (Jaenike, 1978; Thompson, 1988; Mayhew, 1997).
Plant chemistry influences the choice of oviposition substrates by female insects. Primary metabolites provide the nutrients needed for offspring development and reproduction, whereas secondary metabolites often function as defensive traits against herbivores, usually bearing negative effects on the feeding larvae or the female itself (Bernays & Chapman, 1994; Schoonhoven et al., 2005). In addition to plant chemistry, ecological factors, such as refuge from natural enemies, offspring competition for resources, or mutualistic interactions, may all have an impact on female oviposition choice and larval performance (Jaenike, 1978; Atsatt, 1981; Thompson, 1988; Ohsaki & Sato, 1994; Björkman et al., 1997; Mayhew, 1997; Wise & Weinberg, 2002; Gripenberg et al., 2010; Wilson & Leather, 2012).
This combination of factors makes preference–performance predictions hard to test. Although common knowledge supports the ‘mother knows best’ concept, several studies show no correlation between female preference and larval performance in a variety of insect species (for instance, Rausher, 1979; Valladares & Lawton, 1991; Underwood, 1994; Harris et al., 2001; Ferrier & Price, 2004; Digweed, 2006; Clark et al., 2011; Jiao et al., 2012; Potter et al., 2012). Various authors have attributed these contradictory results to variables such as offspring motility, adult diet, egg distribution, and larval diet breadth (Thompson, 1988; Mayhew, 1997; Foster & Howard, 1999; Scheirs et al., 2000; Gripenberg et al., 2010; Soler et al., 2012). However, according to a meta-analysis by Gripenberg et al. (2010), only diet breadth explains the lack of correlation between preference and performance: whereas oligophagous insects meet the expected correlation, monophagous and polyphagous insects do not.
As monophagy implies a more restricted diet, it seems counterintuitive that monophagous insects have a loose relationship between female preference and offspring performance. Both oligophagous and monophagous insects are specialist feeders that utilize plants of a single family or genus, respectively (Schoonhoven et al., 2005). Plant secondary chemicals are largely involved in determining specialization, and they are often used as cues by specialists to select host plants. As stated in the neuronal restriction hypothesis, specialized insects may find it easier to handle less complex stimuli associated with a more restricted diet breath, in comparison to generalists that evaluate a more complex set of stimuli associated with a greater diet breath (Levins & MacArthur, 1969; Bernays, 1998). All things considered, the process of host selection is expected to be more efficient in specialist insects, which in turn have a higher reliance upon their host plant for survival. Therefore, female preference of host plant for oviposition and the performance of their offspring are expected to correlate in monophagous species, at least as much as in oligophagous insects.
The lack of a correlation between preference and performance in monophagous insects may be due to the small number of studies on this group, in comparison with oligophagous and polyphagous species (see Appendix 3 in Gripenberg et al., 2010). In addition, some of these studies considered performance only during the larval stage (i.e., larval survival), without determining adult emergence (Åhman, 1984; Roininen & Tahvanainen, 1989; Kagata & Ohgushi, 2001). Finally, most preference–performance research has been carried out on lepidopteran species, possibly skewing the information (Gripenberg et al., 2010). Other groups of insects, such as the hymenopterans, have been infrequently studied in this regard, comprising only the families Tenthredinidae and Cephidae of the Symphyta suborder and showing inconsistent results in preference–performance correlations (Roininen & Tahvanainen, 1989; Price et al., 1999; Nagasaka & Ohsaki, 2002; Ferrier & Price, 2004; Digweed, 2006; Perez-Mendoza et al., 2006; Müller & Arand, 2007; Buteler et al., 2009; Braccini et al., 2013).
We add a new study of preference–performance in a monophagous species from a different hymenopteran family (Pergidae). This sawfly family is the third largest within the suborder Symphyta, currently including 12 subfamilies, 60 genera, and 441 species, with scarce information about their biology and host plants (Schmidt & Smith, 2006, 2016). Within the Pergidae, we studied female oviposition preference and offspring performance in the sawfly Tequus schrottkyi (Konow). The genus Tequus occurs in the Neotropical region, as does most of the family, and includes 14 species with little or no information on their natural history (Schmidt & Smith, 2016). They have always been found on plants of the genus Solanum (Solanaceae). In particular, three species – namely Tequus munroi (Smith), Tequus willei (Smith), and Tequus ducra (Smith) – have been reported to feed on Solanum tuberosum L., causing economically relevant damage to potato crops in Bolivia and Peru (Munro, 1954; Carrasco, 1967; Aréstegui, 1976; Ormachea & Galindo, 1994). Studies on the biology exist only for two species, T. ducra (Carrasco, 1967; Ormachea & Galindo, 1994) and an undetermined species from Peru (García-Sinche & Catalán-Bazán, 2011). They have at least three generations per year, with a diapausing period as a pre-pupa for up to 6 months. This period appears to be synchronized with the absence of the host plant and the dry season. The eggs are laid into the leaf tissues and the larvae feed on the leaves, building a silk cocoon to pupate into the soil. The adults are sexually dimorphic, females being larger than males (García-Sinche & Catalán-Bazán, 2011). We have observed these same characteristics in our study species, T. schrottkyi. In the laboratory, the mature larvae form a silk cocoon in the soil, in which they pupate, with female pupae roughly twice as large as male pupae. The adults are also sexually dimorphic, with females being larger than males and differently colored. The females lay their eggs individually into the leaf tissues, on the leaf margins (Altesor et al., 2016).
Tequus schrottkyi was first described in Paraguay, without any information about its biology and host plant. We have found larvae of this species feeding on plants of Solanum commersonii Dunal in southern Uruguay, with several generations occurring between March and July (Altesor et al., 2016). This Solanum species has its distribution center in Uruguay and is the southernmost species in the genus in South America (Spooner & Hijmans, 2001). As S. commersonii foliage is less available during spring and summer, it is assumed that T. schrottkyi enters diapause and/or moves to another host plant during the rest of the year.
Our previous studies with local populations of T. schrottkyi have shown that the larvae are found almost exclusively on S. commersonii, even in the presence of S. tuberosum. In choice laboratory bioassays, the larvae preferred to feed on S. commersonii, but they also fed on the cultivated potato (Altesor et al., 2014). Therefore, S. tuberosum may also be a suitable host, as is the case for other Tequus species, making the wild and cultivated Solanum species a potential system for testing preference–performance correlations. Here, we evaluated T. schrottkyi larval performance and female oviposition preference on S. commersonii and S. tuberosum. We also compared the nutritional value of both Solanum plants, in order to correlate preference and performance with plant chemistry. The assessment of nutritional parameters adds to our previous comparative study of glycolalkaloid contents (Altesor et al., 2014), the main defensive metabolites produced by Solanum species. Specifically, in this study we tested (1) the performance of immature stages of T. schrottkyi on both plants in a non-choice setup, (2) the preference of female T. schrottkyi for laying eggs on S. commersonii when offered a dual choice with S. tuberosum, and (3) differences in the contents of non-structural proteins, carbohydrates, and water in both Solanum species.
Materials and methods
Plants and insects
We used the same plant germplasm employed in previous studies (Altesor et al., 2014), namely S. commersonii (accession 05.02-6) and S. tuberosum var. Iporá, a variety that is well adapted to local environmental conditions and presents virus resistance (Vilaró et al., 2004). Clones were obtained from a Solanum germplasm bank located at the National Institute of Agricultural Research of Uruguay (INIA-Las Brujas Experimental Station, Canelones, Uruguay) and were propagated under laminar flow from internodal stem cuts bearing a single bud. They were maintained in vitro for 15 days on enriched agar containing salts, vitamins, and sucrose (Murashige & Skoog, 1962; Staba, 1969), under controlled conditions of light (3 000 lux), 21 ± 2 °C, and L16:D8 h photoperiod. Once four nodes were developed, the plants were transferred to soil seedbeds and maintained in a greenhouse for 15 days. Afterward, they were transferred to plastic pots (6 cm diameter, 7 cm high) and maintained in the greenhouse for 15–30 additional days before taking them to the laboratory for experimentation or maintenance of T. schrottkyi. All experiments were carried out with plants in their vegetative stage, carrying 8–16 developed leaves with 5–7 leaflets per leaf.
The sawflies (T. schrottkyi) were collected as eggs and larvae from wild patches of S. commersonii located at INIA-Las Brujas Experimental Station, between March and July in 2014 and 2015. They were maintained on potted S. commersonii plants under laboratory-controlled conditions (5 000 lux, 21 ± 3 °C, 50 ± 10% r.h., L14:D10 h photoperiod), until they were used for experiments, either as larvae or adults, on the same generation of field collection.
Performance
The performance study was conducted using two setups, one with whole plants in order to achieve more natural experimental conditions and one with excised leaves for a more detailed control of larval development. For the whole-plant experimental setup, newly emerged first-instar T. schrottkyi (24–48 h after egg eclosion) were transferred individually to a single plant of S. commersonii or S. tuberosum (n = 35 and 36, respectively) and placed on an intermediate leaf. The insects were not transferred to the plants as eggs because the eggs are laid underneath the leaf surface. The plants were of the same age, and they were enclosed separately in plastic containers (20 × 20 cm cross section, 24 cm high) closed with voile, under the same environmental conditions described for insect maintenance. Every 2 days for 30 days, the larvae were checked for survival and developmental stage, cocoon formation and weight (balance readability: 0.01 mg), adult emergence, and sex ratio. Since the cocoon forms in the soil, it was not always possible to find the pupa without risking a disruption of the experimental conditions. Hence, some replicates lack the transition time from larva to cocoon and the weight and survival of the cocoons. However, all replicates include data for adult emergence.
For the experimental setup with excised leaves, larvae were used during the first 24 h after egg eclosion. The larvae (n = 50 per plant treatment) were individually transferred to single leaflets of S. commersonii or S. tuberosum, enclosed in Petri dishes (5.5 cm diameter, 1.2 cm high) and maintained in an incubator at 20 ± 1 °C, 60 ± 10% r.h., and L14:D10 h photoperiod. The leaflets were obtained from fully developed leaves of plants of the same age. The leaflets were wrapped at the petiole with wet cotton to maintain moisture, and they were replaced when they had been consumed, or when they started showing signs of dryness. Observations were conducted daily to record larval survival and development, the transition to prepupa, cocoon, and adult, and the weight of the cocoon. As the females that emerged from this experiment were used for the oviposition preference test (see below), the number and viability of eggs laid by the females was also evaluated as an additional performance variable.
Oviposition preference
Female preference for oviposition substrate was also evaluated with two experimental setups, using T. schrottkyi virgin females. Virgin females lay eggs that hatch into haploid males (arrhenotokous parthenogenesis), whereas fertilized eggs hatch into diploid females. Hence, virgin females are also expected to search for suitable oviposition substrates. In the first experiment, all females were raised on their host plant, S. commersonii. One female (24–48 h after adult emergence) was placed at the bottom of a plastic container closed with voile (20 × 20 cm cross section, 24 cm high), between two potted plants of S. commersonii and S. tuberosum of the same age (n = 23 females). The containers were placed in a room at 21 ± 1 °C, 60 ± 10% r.h., and L14:D10 h photoperiod. Each container included a filter paper square with 10% honey solution for female feeding. Oviposition preference was evaluated by examining the plants every day for 8 days, after which no females remained alive and no new larvae emerged. As the eggs are laid within the plant tissue and may not be readily visible without excessive manipulation of the plant, the number of eggs was obtained from the sum of hatched larvae, which were easily observed, plus the number of unhatched eggs, which was recorded under a magnifying glass when the experiment concluded. It is important to note that T. schrottkyi larvae are rather immobile during the first stages and remain feeding on the same leaf area in which the eggs were laid (P Altesor, pers. obs.). Therefore, the daily checking ensured that the recorded larvae hatched from eggs laid on the same leaf and did not move between plants.
A second oviposition preference test was performed to evaluate the effect of the rearing plant on female oviposition preference, using females raised on S. commersonii or S. tuberosum. The females were obtained from the performance test; they were raised individually from their first instar until adult emergence in Petri dishes containing one excised leaf of either S. commersonii or S. tuberosum. Within the first 24 h after adult emergence, oviposition preference was assessed using the same protocol as described above, except that the emerged larvae were removed as they were found to prevent cannibalism of unhatched eggs.
Plant nutritional quality
Protein, non-structural carbohydrate, and water contents were measured for both Solanum species to assess nutritional quality (n = 5 and 4 plants for S. commersonii and S. tuberosum, respectively). The aerial parts of the plants were dried in a stove at 50 °C until no weight loss was observed. The plant tissue was then ground, divided for the various analytical procedures, and further dried in a stove at 105 °C for weight percentage calculations. Total nitrogen percentage (Kjeldahl method) and non-protein nitrogen percentage were measured to obtain the protein percentage (defined as 6.25 × protein nitrogen percentage) (Helrich, 1990). The non-structural carbohydrate percentage, composed of sugar and starch, was determined as 100 minus the sum of the percentages of crude proteins, structural carbohydrates, inorganic matter, and lipids. These were determined as follows: crude proteins were calculated as the total nitrogen × 6.25; structural carbohydrates – including cellulose, hemicellulose, and lignin – were measured by the neutral detergent fiber procedure, using a Fiber Analyzer 200 (Ankom Technology, Fairport, NY, USA); inorganic matter was determined from the total ashes obtained at 600 °C; and lipids were measured by the ethereal extract procedure (Helrich, 1990; Van Soest et al., 1991).
Statistical analysis
Survival in the performance bioassays, considering all life stages of the insect, was compared by the Kaplan–Meier survival analysis. Survival for each stage was compared by the χ2 test, using Yates correction when expected frequencies were below 5. All other comparisons were done by the Mann–Whitney U-test (α = 0.05). Data are expressed as median and interquartile 25–75% range throughout.
Results
Performance
In both experiments, whether raised on whole plants or on excised leaflets, T. schrottkyi performed better on their host plant S. commersonii. In the whole-plant experiment, a higher number of adults emerged from the larvae that fed on S. commersonii (overall proportion: 0.74, n = 35) than from the larvae raised on S. tuberosum (0.39, n = 36) (Kaplan–Meier survival analysis: χ2 = 9.61, d.f. = 1, P = 0.002). No significant differences were observed in larval survival [proportions: 0.97 (n = 35) and 0.83 (n = 36) for S. commersonii and S. tuberosum, respectively; χ2 test with Yates correction: χ2 = 2.41, d.f. = 1, P = 0.12]. Instead, survival rates on S. commersonii were higher during the pre-pupal and/or cocoon stages, with an emerging proportion of 0.77 (n = 34) for larvae raised on S. commersonii compared to 0.47 (n = 30) for those raised on S. tuberosum (χ2 = 6.04, d.f. = 1, P = 0.01). There was no sex bias in the survival rates, as equal numbers of female and male adults emerged from both food plants (χ2 = 0.05, d.f. = 1, P = 0.82).
As with the experiment on whole plants, the overall survival of T. schrottkyi on excised leaflets was better on S. commersonii than on S. tuberosum (overall proportion: 0.5 vs. 0.16, both n = 50; Kaplan–Meier survival analysis: χ2 = 15.02, d.f. = 1, P<0.001) (Figure 1). The excised leaflets enabled a more detailed observation of the various developmental stages. Survival was the same in the larval and cocoon stages for S. commersonii vs. S. tuberosum, respectively [larvae, proportion: 0.94 vs. 0.86, both n = 50; χ2 = 1.78, d.f. = 1, P = 0.18; cocoon: 0.78 (n = 32) vs. 0.67 (n = 12); χ2 test with Yates correction: χ2 = 0.15, d.f. = 1, P = 0.7]. Survival in the pre-pupal stage, before the formation of the cocoon, was higher for S. commersonii-reared than for S. tuberosum-reared insects [0.68 (n = 47) vs. 0.28 (n = 43); χ2 = 14.51, d.f. = 1, P<0.001] (Figure 1). Again, no differences were found in the sex ratio of emerged adults (15 females and 10 males on S. commersonii vs. 7 females and 1 male on S. tuberosum; χ2 test with Yates correction: χ2 = 1.01, d.f. = 1, P = 0.32).
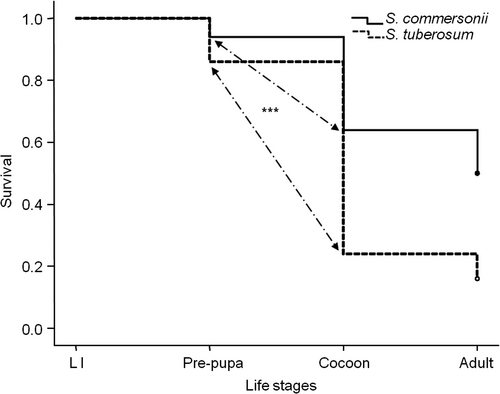
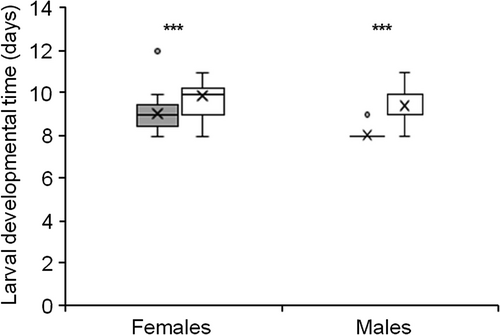
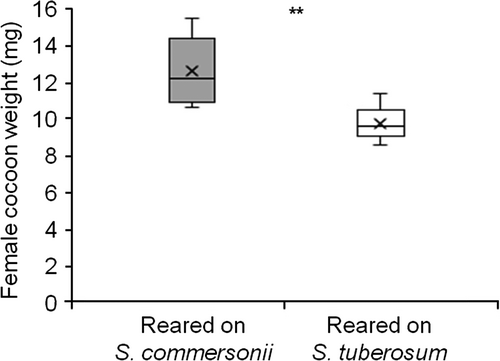
Both female and male larvae developed faster when reared on S. commersonii than when reared on S. tuberosum (Mann–Whitney U-test: W = 629 and 313, respectively, both P<0.001) (Figure 2). Like in other sawfly species, T. schrottkyi females have one more instar (VI) than males (V), hence usually require more time to develop (Esperk et al., 2007). Female larvae (n = 29) took longer to develop than male larvae (n = 19) on S. commersonii (Mann–Whitney U-Test: W = 278.5, P<0.0001), but when raised on S. tuberosum, the difference was not significant (W = 229.5, P = 0.1; n = 30 females and 13 males) (Figure 2). The developmental times of the pre-pupal and cocoon stages did not differ between the two food plants [median (interquartile range): 10 (9–11), both for females raised on S. commersonii (n = 15) or S. tuberosum (n = 7); W = 88, P = 0.58]. Finally, cocoons were heavier for females reared on S. commersonii (W = 20, P = 0.003) (Figure 3). The sample size of males emerging from S. tuberosum was too small (n = 1) for any statistical comparison with males emerging from S. commersonii (n = 10).
Oviposition preference
Females (n = 23) of T. schrottkyi raised on their host plant, S. commersonii, oviposited exclusively on S. commersonii. No hatched larvae or unhatched eggs were observed on S. tuberosum. The median number of viable eggs laid by a female on S. commersonii was 27 (interquartile range: 15–33). Hatched larvae were found between days 4 and 7 after the onset of the experiment, with a peak on day 5. Four days into the experiment all females had already died.
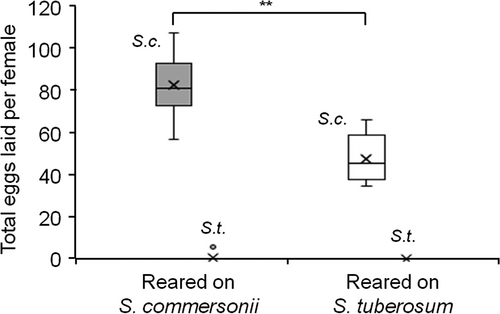
Females reared on S. commersonii (n = 9) or S. tuberosum (n = 5) displayed the same oviposition preference; eggs were laid almost exclusively on S. commersonii, regardless of the plant on which they were raised (Figure 4). Of the six eggs laid on S. tuberosum only three hatched, all laid by a single female reared on S. commersonii. The rearing plant did not affect the survival of the females [median (interquartile range): 5 (4.5–6) vs. 5 (4.5–5) days for females reared on S. commersonii vs. S. tuberosum, respectively; Mann–Whitney U-test, W = 34, P = 0.9], but it did influence the number of eggs laid, with more eggs laid by females reared on S. commersonii (W = 16, P = 0.002) (Figure 4). The viability of the eggs laid on S. commersonii was high and did not differ between females reared on either plant [median (interquartile range): 96.5 (95.6–97.6) vs. 96.1% (85.7–98.6) hatching rate for females reared on S. commersonii vs. S. tuberosum, respectively; W = 33.5, P = 0.63]. For both rearing conditions, egg hatching started on the 4th day after the females were enclosed with the choice of plants and peaked on day 5, as in the first preference experiment. Egg hatching lasted until day 11 vs. 12 for females reared on S. commersonii vs. S. tuberosum, respectively. Removing the hatched larvae to prevent cannibalism resulted in a larger number of hatched eggs and longer hatching period compared to the first experiment.
Nutritional quality of plants
No significant differences were found in protein and non-structural carbohydrates levels, whereas water content was significantly higher in S. tuberosum (Table 1).
| S. commersonii | S. tuberosum | Mann–Whitney U-test | ||
|---|---|---|---|---|
| W | P | |||
| Proteins | 24.2 (22.4–24.7) | 21.8 (21.0–22.5) | 13 | 0.11 |
| Non-structural carbohydrates | 29.8 (26.0–30.5) | 24.8 (24.0–25.2) | 13 | 0.11 |
| Water | 92.1 (91.8–93.0) | 94.0 (93.9–94.4) | 30 | 0.02 |
Discussion
Our results demonstrate a positive correlation between female oviposition preference and offspring performance of the specialist phytophagous sawfly T. schrottkyi on two closely related host plants. When offered a choice between their usual host plant, the wild potato S. commersonii, and the cultivated potato S. tuberosum, the females laid eggs almost exclusively on the former. The larvae, in turn, in non-choice performance assays, were more likely to complete their development on the species chosen by the females.
Although the performance difference on the two Solanum plants was clear, the non-preferred S. tuberosum was not unsuitable for the development of T. schrottkyi. This came as no surprise, as the genus Tequus is associated with various Solanum species, and some Tequus species are considered occasional pests of cultivated potato in other regions of South America (Carrasco, 1967; García-Sinche & Catalán-Bazán, 2011). Indeed, larval survival did not differ between the two Solanum species, and the performance difference became evident only on larval developmental time and cocoon weight and was most evident in the survival of the pre-pupal stage. This result indicates the importance of completing performance assays beyond the larval stage, especially for holometabolous insects in which the remarkable changes that take place during pupation may be affected by sublethal factors to which the larvae are exposed. Another performance parameter affected by the food plant was the number of eggs laid by the females, although the lower egg-laying capacity may also be the result of the smaller body weight of females raised on S. tuberosum. Worth of note, the potential performance bias from using larvae reared only on S. commersonii was minimized by using newly emerged first instars, 24–48 h after egg eclosion.
Tequus schrottkyi females displayed a strong preference for ovipositing on S. commersonii, revealing a high degree of specialization. This preference was not the result of experience, as shown by the second oviposition preference test, in which the females were raised on either of both food plants. Experience includes aspects of the pre-imaginal environment, such as food plant odor and other cues, that may have an effect in determining patterns of host use during the adult stage (Barron, 2001). The females clearly preferred to oviposit on S. commersonii even if they had been raised on S. tuberosum from the first instar. This preference is in line with our previous studies using contiguously planted field plots of both Solanum species. After 6 weeks of insect surveillance, we found the larvae of T. schrottkyi almost exclusively on S. commersonii (154 out of 155 larvae), even though both Solanum plants were readily available in close proximity (Altesor et al., 2014). These larvae most likely originated from eggs laid by females that arrived naturally to the field site. Our current study was conducted under controlled conditions, with experimental plants of similar age, maintained in identical conditions, and (to the best of our knowledge) free of disease and natural enemies. Therefore, the females must be basing their decision on physical and/or chemical characteristics of the plants.
Plant primary and secondary metabolites may have a strong influence on the performance of herbivorous insects. We assessed the contents of proteins, carbohydrates, and water of both plants, which are regarded as the most relevant factors in determining the performance of chewing insects (Bernays & Chapman, 1994; Schoonhoven et al., 2005). Closely related plant species may be expected to yield similar nutritional value to a feeding herbivore; yet, a cultivated and a wild species may differ due to decades of artificial selection on the former. We concluded that proteins and non-structural carbohydrates do not explain the better performance on S. commersonii, because both plants displayed similar contents. Water content, however, was higher in the non-preferred S. tuberosum. The relevance of this difference, both in larval performance and female preference, should be further evaluated. High water contents have been shown to negatively affect egg viability in the wheat stem sawfly, Cephus cinctus Norton, although low contents also reduced larval survival (Varella et al., 2016).
The secondary chemistry of the same germplasm material as used in this study was investigated recently (Altesor et al., 2014). The main toxic compounds in Solanum species are steroidal glycoalkaloids (GAs), which have been shown to confer resistance against herbivory (Tingey, 1984; Flanders et al., 1992; Fragoyiannis et al., 1998; Kowalski et al., 1999; Yencho et al., 2000; Friedman, 2002; Jansky et al., 2009; Nenaah, 2011; Altesor et al., 2014). GAs are twice more concentrated in S. commersonii than in S. tuberosum, and the plants also differ in the type of GA that they produce (Altesor et al., 2014). GAs may have a positive effect on performance if they are incorporated by the larvae during feeding, thus protecting either the larvae or the pupae against predators and/or soil pathogens. Although our performance experiments were done in a simplified bitrophic system, soil microorganisms will have been present in the experiment with potted plants, and they may have influenced pupation success. However, the performance experiment on excised leaflets yielded the same result, with no possibility of soil microorganisms affecting performance. This suggests that the performance difference, if resulting from secondary metabolites such as GAs, is most likely due to a better adaptation to the host plant defensive GAs. Given the different GA composition of both plants, it is conceivable that T. schrottkyi is better adapted to the GAs from its host plant, than to those from S. tuberosum.
It may be argued that host preference by the ovipositing females is actually the result of adaptive avoidance of a toxic, non-suitable host. However, we have previously shown that T. schrottkyi larvae choose to feed on S. tuberosum if GAs from S. commersonii are added to the leaf substrate (Altesor et al., 2014). The larvae thus display a positive preference for the GAs of their host plant, rather than rejection of GAs of the non-host plant. Similarly, the adults may show preference based on chemical cues, rather than adaptive avoidance of non-host chemicals. Clearly, the effect of qualitative and quantitative differences in GAs on the performance and preference of T. schrottkyi deserve further investigation, in a context of more complex tritrophic or multitrophic systems. Remarkably, most experimental studies concerning the link of preference and performance have been conducted in the absence of natural enemies (Gripenberg et al., 2010).
Whether GAs exert a positive or a negative influence on oviposition choices, the females need to contact these plant compounds, as is generally the case with oviposition-mediating secondary metabolites. The relevance of secondary metabolites on oviposition decisions by specialists is not surprising; whereas generalist insects are regarded to estimate the presence and balance of particular nutrients and/or secondary metabolites, specialists would be selecting specific contact secondary metabolites that characterize their host plant taxa (Bernays & Chapman, 1994; Renwick & Chew, 1994; Honda, 1995; Bernays, 1998; Schoonhoven et al., 2005). Depending on their polarity and solubility, these compounds may move from the plant tissues to the epicuticle, or they can be contacted through wounds in the plant tissue (Müller & Riederer, 2005). GAs are not likely to translocate into the cuticle, however—sawflies penetrate the leaf tissue with their serrated ovipositor (Smith, 1972; Weltz & Vilhelmsen, 2014); hence, they likely come into contact with GAs in the plant tissue. Other sawfly species use secondary metabolites in the selection of their host plant, such as Euura amerinae (L.) and Euura lasiolepis Smith, monophagous species that recognize and select their Salix host plants based on characteristic phenolic glycosides (Kolehmainen, 1994; Roininen et al., 1999). Host plant parameters not considered in the present study – such as leaf hardness, leaf surface, and plant volatiles – may also affect sawfly oviposition preferences (Buteler & Weaver, 2012; Braccini et al., 2013; Varella et al., 2016). They deserve further investigation in our study system.
Our study adds a new study on monophagous species to the preference–perfomance correlation literature, one in which this correlation stands. In their meta-analysis, Gripenberg et al. (2010) found that, among the factors that may modulate this correlation, diet breath is significant. They also found the rather counterintuitive result that a positive correlation was displayed only in insects regarded as oligophagous. The authors argued that the lack of correlation in monophagous species may be an artifact caused by the experimental approach. Preference in monophagous species is usually evaluated with host plants of the same genus, or even with populations of a single species, whereas oligophagous insects are mostly tested with plants of different genera. It can then be argued that this experimental approach imposes a ‘harder task’ on monophagous species, as they have to discriminate more similar plants. However, given that monophagous insects only utilize very similar hosts, there is no other way to test the hypothesis than using closely related host plants. We agree that more studies on monophagous species are needed, and argue that given their high degree of specialization, they are expected to discriminate between closely related plants, on which they heavily rely for development.
In summary, in this study we report that females of the monophagous sawfly T. schrottkyi clearly preferred to oviposit on their host plant S. commersonii over the congener S. tuberosum. In addition, T. schrottkyi performed better when raised on the preferred plant. The performance difference was small for the larvae but large for the pre-pupal stage, resulting in considerable differences in adult emergence rates. Future studies will focus on the role of water content and GAs, the main secondary metabolites of Solanum plants, in the preference and performance of T. schrottkyi sawfly.
Acknowledgements
The authors acknowledge technical assistance by personnel of the National Institute for Agricultural Research (INIA) for processing germplasm material. The Graduated Academic Committee (CAP) granted fellowships to P. Altesor. The National Agency for Research and Innovation (ANII) funded part of the research (FCE-2013-100788). Support by the graduate program in Biological Sciences PEDECIBA is also acknowledged.




