Tomato-mediated interactions between root herbivores and aphids: insights into plant defence signalling
Abstract
Reactions of plants to insect pests include the activation of a local and systemic defence response. This response is based on transcriptional changes that are mainly controlled and coordinated by phytohormones. The above- and belowground part of plants can be challenged by different insects and therefore, the defence response to one attacker can influence other insects. The study of plant-mediated interaction between pests that are physically or temporally separated yielded a variety of outcomes, with positive, negative, and neutral effects described in the literature. In this study, we examined possible plant-mediated interactions between above- and belowground insect pests with different feeding guilds in tomato, Solanum lycopersicum L. (Solanaceae). Root feeding by Agriotes lineatus (L.) (Coleoptera: Elateridae) larvae caused a decrease in the development rate, fertility, and weight in Myzus persicae (Sulzer) (Hemiptera: Aphididae). To gain insights into the plant systemic signalling mechanisms, we also performed a time-course expression analysis of defence-related phytohormone marker genes. The result indicated a dynamic systemic response in leaves following root herbivory, which comprises the activation of genes dependent on different molecular pathways involved in plant stress response. Our work demonstrated that root herbivory increased aphid resistance in tomato and that a combination of signals enables the communication between below- and aboveground pests with different feeding guilds.
Introduction
Plants have evolved multifaceted defence mechanisms to respond to a variety of biotic stresses, which are usually categorised in constitutive (i.e., present irrespectively of the harmful organism) or inducible (i.e., enhanced following attack) (Howe & Jander, 2008). Following pest recognition, plants activate a local response that is often accompanied by a systemic activation of defence pathways in distal, undamaged tissues (Howe & Jander, 2008). Systemic signalling involves a complex network of transduction and amplification mechanisms (Bonaventure, 2014). Phytohormones also mediate plant defence responses to virtually any biotic stress, such as insects, bacteria, viruses, and fungal pathogens. Phytohormones are important signalling molecules for both local and systemic resistance, and for within- and between-plants communication (Robert-Seilaniantz et al., 2011). There is a general consensus that the jasmonic (JA) and salicylic acid (SA) are main regulators of the induced defence signalling network in plants (Erb et al., 2012; Pieterse et al., 2012). The contribution to signalling of other phytohormones, such as ethylene (ET) and abscisic acid (ABA), has also been proved (Erb et al., 2012; Pieterse et al., 2012).
In their lifetime, plants deal with multiple attacks, which may affect different organs or parts. Therefore, plants need to prioritise and balance various defence responses. For example, various species of insect pests can attack the same plant simultaneously or sequentially. In these instances, the plant reaction to one attack may also affect the growth, development, or survival of the other insect species feeding on the same host (van der Putten et al., 2001; Poveda et al., 2003; Soler et al., 2005). This interaction occurs also between insect species that have different feeding habits and/or that sequentially attack the plant (Mouttet et al., 2013). For this reason, plants act as mediators in the interaction between pests that are spatially or temporally separated (Soler et al., 2008).
The study of these multitrophic interactions has focused traditionally on aboveground organisms, mainly folivores (Soler et al., 2012). More recently, attention has also been given to the interaction between above- and belowground organisms, because these two communities almost never come into direct contact and their interaction is typically mediated only by the shared plant (Bezemer et al., 2003). Whereas many studies have focused on root and foliar herbivores, comparatively little information is available on the plant-mediated interaction between insects with different feeding guilds, such as phloem feeders and chewers (Bezemer et al., 2003). The induced defence response in plants has a degree of specificity (Walling, 2000). Spatio-temporal distribution and feeding guild of the attackers are considered main determinants of the outcome of the plant-mediated interactions because of their influence on phytohormone signalling (Mouttet et al., 2013). For example, different gene-expression patterns are typically observed with insects having different feeding guilds (Howe & Jander, 2008), making it difficult to predict the outcome of plant-mediated pest interactions. It is widely accepted that root feeders affect aboveground plant-based communities, yet positive interactions have been described (Gange & Brown, 1989; Masters et al., 2001; Poveda et al., 2005), as well as negative (Birch et al., 1992; Tindall & Stout, 2001; Bezemer et al., 2004) and neutral interactions (Moran & Whitham, 1990; Salt et al., 1996; Hunt-Joshi & Blossey, 2005), underlining that specific combinations of biotic stresses are crucial to determine not only plant responses but also potential cross-resistance.
In this work, we examined the possible presence of plant-mediated interaction between a root herbivore [Agriotes lineatus (L.) (Coleoptera: Elateridae)] and a phloem feeder [Myzus persicae (Sulzer) (Hemiptera: Aphididae)] in tomato [Solanum lycopersium L. (Solanaceae)]. Wireworms are polyphagous larvae that feed exclusively on roots. These pests are common on Solanaceae and can produce significant damage, especially on young plants. Belowground herbivory can cause severe crop decline, especially because of its frequent association with other stresses (Blossey & Hunt-Joshi, 2003). It is becoming increasingly clear that plant response to root herbivores can shape crop resistance and resilience to other biotic stresses (McKenzie et al., 2016 and references therein).
Aphids are among the most destructive pests for horticultural crops in temperate climates. Among them, M. persicae is a polyphagous, worldwide pest for tomato and other Solanaceae, being also an important virus vector. We focused on tomato not only because it is arguably the most important vegetable in the world, but also because of the molecular knowledge about its response to stress and phytohormone signalling. In order to shed light on the mechanisms that are involved in the interaction between root herbivores and phloem feeders, we also carried out an expression profile of genes involved in the main phytohormone signalling pathways.
Materials and methods
Biological material
The study was carried out on tomato plants (S. lycopersicum) cv. ‘Microtom’. Seeds were sterilised and sown on wet filter paper in Petri dishes. Six days following germination, plants were transplanted to pots and grown in a greenhouse at 21 ± 3 °C (day), 16 ± 3 °C (night), L16:D8 photoperiod, and 70 ± 10% r.h. We used 5- to 6-week-old plants. Agriotes lineatus larvae were provided by Koppert Biological Systems (Berkel en Rodenrijs, The Netherlands). Until use, larvae were placed inside a moist sandy soil in a climatic chamber at 10 ± 1 °C in the dark. Myzus persicae was obtained from a population grown on Brassica rapa (L.) at the Laboratory of Entomology, Wageningen University (Wageningen, The Netherlands). A clonal population was subsequently raised on tomato in a growth chamber at a 22 ± 1 °C, 50 ± 5% r.h., and L16:D8 photoperiod. Apterous adults were transferred to uninfested tomato plants for 24 h to allow for reproduction and 1st-day nymphs were used for bioassays.
Bioassays
To evaluate the influence of the root herbivore on aphid performance, M. persicae were transferred onto leaves of tomato plants either previously infested by A. lineatus or uninfested. Before bioassay, the A. lineatus larvae were left for 1 day at room temperature to increase hunger. Three larvae per pot were released on the soil in the proximity of the plant's stem. After 10 min, every plant was checked to ensure that the three larvae had moved into the soil. Eight days after the root-herbivore infestation, three 1st-day nymphs were transferred onto the third and three onto the fourth fully expanded true leaf (leaves were numbered from the bottom of the plant). Specifically, we placed one nymph per leaflet and aphids were confined to the leaflet using a ca. 1–2 cm long polyester sleeve on the petiolule. First-day nymphs were obtained from apterous adults of M. persicae left for 24 h on leaves of different tomato plants. Plants were distributed randomly in various mesh-cages and relocated to avoid positional effects. From the 1st day when each aphid had become an adult (i.e., appearance of the first nymph), the number of nymphs produced daily by aphids was recorded for five consecutive days and then each nymph was removed. Subsequently, the fresh and dry weights of each adult aphid were measured using an MT-5 microbalance (Mettler Toledo, Columbus, OH, USA). Each aphid was then dried at a 45 °C for 24 h. At the end of the experiments, we checked for the presence and activity of the larvae and then the fresh and dry weights of roots and shoots of each plant were measured. Samples were dried at 70 °C for at least 48 h.
We tested all experimental combinations at the same time and bioassays were replicated 3× for a total of 10 independent plant replicates for each experimental condition. Statistical analysis (two-tailed t-test for independent samples or ANOVA followed by Duncan post-hoc test for multiple comparisons) was performed with SPSS v.20 software (IBM-SPSS, Chicago, IL, USA).
Transcriptional analysis
Because of the destructive sampling required for time-course gene-expression analysis, plant tissues were harvested from separate sets of plants, growing at the same time and infested as described above. Leaves were harvested 1, 2, and 8 days following infestation with aphids, the root herbivore, or their combination. Control leaves from uninfested plants were also harvested at each time point. Plant tissues were immediately frozen in liquid nitrogen and stored at −80 °C. RNA isolation, DNAse I treatment, and first-strand cDNA synthesis were performed as described before (Coppola et al., 2015). To test cDNA synthesis, samples were amplified with the primers StbEF Fw and LeEF (Table S1). These primers span an intron of the Elongation Factor 1-α (EF1-α) gene, allowing to detect the presence of contaminant genomic DNA. Real-time PCRs were carried out in a final volume of 12.5 μl, containing 6.5 μl of 2X QuantiFast SYBR Green PCR Master Mix (Qiagen, Hilden, Germany), the primer pairs (300 nM), and the cDNA template. Amplifications were performed as reported (Corrado et al., 2012). Primers and their temperature of annealing are presented in Table S1. Real-time RT-PCR was performed in three biological replicates. Reactions were carried out in three technical replicates in an ABI 7900 HT thermocycler (Applied Biosystems, Foster City, CA, USA). For quantification of gene expression, we employed the ΔΔCT method (Livak & Schmittgen, 2001), using the EF1-α as reference gene and the control (uninfested) plants as calibrator genotype for each time points. Fold changes are expressed in relative quantities (RQ) compared to the calibrator condition, set to 1.
Results
Aboveground–belowground interference
To investigate the impact of a root herbivore (A. lineatus) on a phloem-feeder pest (M. persicae), we studied the performance of the aphid population form nymph to adult stage. First-day nymphs of M. persicae were transferred onto leaves of control tomato plants and plants previously attacked by A. lineatus. The presence of the root herbivore significantly reduced the performance of M. persicae (Figure 1). The average development time (i.e., the number of days to reach the adult stage and reproduce) for aphids feeding on infested plants was ca. 3 days longer than for control aphids. The number of nymphs was significantly reduced by ca. 17%. A negative effect on growth was also expressed in aphid fresh weight.
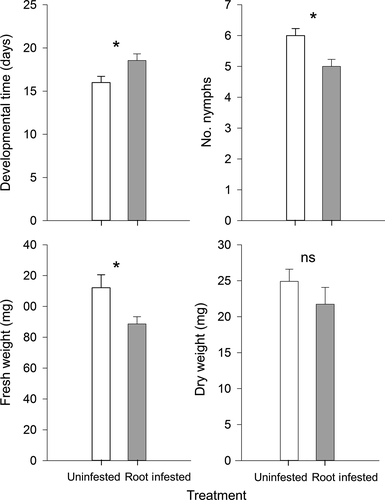
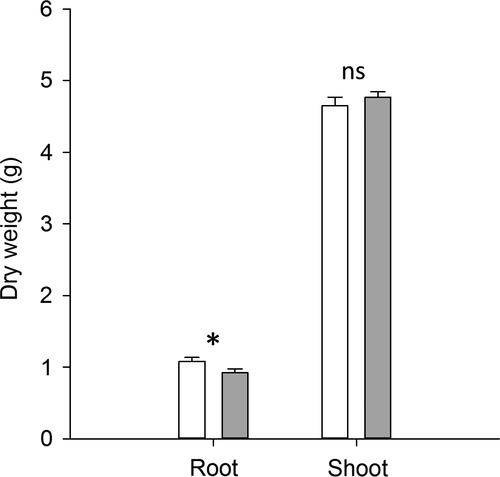
To test whether the differences between aphids grown on control vs. infested plants are related to a possible alteration of plant biomass, we compared the dry weight of roots and shoots of the control plants to those attacked only by the soil insects. Infested tomato plants had a similar shoot biomass compared to the untreated control and the root-herbivore attack decreased the root biomass (Figure 2).
Gene-expression analysis of the systemic response
To investigate the defence responses induced following the damage caused by the two biotic stresses under investigation and their combination, we analysed the systemic response in tomato leaves. We examined five genes considering their active role in plant resistance to biotic stress and association with phytohormone signalling. Specifically, we studied the expression of the 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene, positively correlated with ET biosynthesis (Ruduś et al., 2013); the ABA-dependent dehydrin (DEH) gene (Borovskii et al., 2002); the pathogenesis-related 1 (PR1) gene, which depends upon SA-signalling (Zhang et al., 1999); and the wound-induced proteinase inhibitor II (Inh II) and the threonine deaminase (TD) genes, activated in tomato by the JA-pathway (Zhang et al., 1999; Wasternack et al., 2006). Distant undamaged leaves were harvested after 1, 2, and 8 days from uninfested control plants and from plants that were infested by the root herbivore, the aphids, or both.
The systemic response to the controlled aphid infection was minor. None of the selected genes showed a significant alteration in comparison with undamaged control plants (Figure 3A). The systemic response in leaves of the plants attacked by the root herbivore indicated the presence of a multifaceted response (Figure 3B). The expression of genes such as ACO, PR1, and TD reached a peak 48 h following infestation and declined at 8 days post-infestation. The expression of the dehydirn was not significantly altered after 1 and 2 days of infestation and was clearly higher after 8 days. Also, the expression of the late-defence gene InhII was significantly different 8 days following infestation, although the magnitude of the expression in the undamaged systemic leaves is much smaller than the systemic response to a folivorous pest (data not shown).
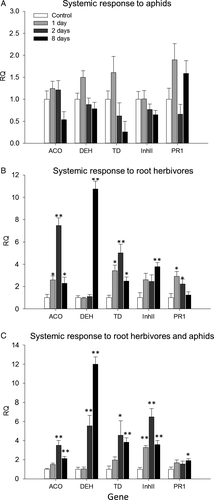
The systemic response to the attack of aphids and root herbivores also indicated the activation of different defence pathways (Figure 3C). The early response genes ACO and TD had a maximum expression level 48 h following attack. However, in comparison with the systemic response to the root herbivore only, the extent of ACO genes was lower. Also, the response of PR1 was weaker. Another difference was that InhII reached its maximum expression at 48 h. Similarly, the expression of the DEH gene was different at 48 h, and as for the systemic response to the RHs, reached its peak at 8 days.
Discussion
Plants are exposed to multiple attacks not only in natural but also in agricultural ecosystems. Plant-mediated interactions are important determinants of community structure (Dicke & Hilker, 2003; Bruce & Pickett, 2007) yet, the study of crops can provide information useful to evaluate the interaction of different responses under field conditions. In tomato, M. persicae performance was negatively affected when aphids grew on plants previously damaged by the root herbivore, as indicated by significant decrease in development rate, fertility, and body weight. The study of the influence of a phytophagous pest on phloem-feeder performance has provided different outcomes. The M. persicae offspring was significantly reduced in Plantago lanceolata L. attacked by root-feeding nematodes [Pratylenchus penetrans (Cobb) Filipjev & Schuurmans Stekhoven)] (Wurst & van der Putten, 2007). Interspecific competition was detected between the root aphid and shoot aphid populations in Cardamine pratensis L. (Salt et al., 1996). On the other hand, Aphis fabae Scopoli performance was improved when aphids grew on Capsella bursa-pastoris (L.) Medik. plants attacked by larvae of the root herbivore Phyllopertha horticola (L.) (Gange & Brown, 1989). In that study, the combination low water stress regime and root damage improved the quality of the areal part of the plant, as measured by total soluble nitrogen. In wild mustard (Sinapis arvensis L.) naturally colonised by aphids, root herbivory associated with an increased aphid population (Poveda et al., 2005). In barley, a reciprocal positive interaction was observed between Rhopalosiphum padi (L.) and Agriotes spp. (Johnson et al., 2009).
Such a range of results can be explained considering that two main mechanisms have been proposed to describe the plant-mediated interactions between above- and belowground insects. The first assumes that the root damage causes a significant reduction of the root biomass, which in turn determines a compensatory effect in leaves. The reallocation of amino acids and carbohydrates in leaves should increase the performance of the herbivorous insects that feed on the aerial part (Gange & Brown, 1989; Masters et al., 1993). A second mechanism is based on the activation of plant endogenous defences in response to ground-dwelling. The higher production or a different distribution of defence compounds in leaves is expected to negatively affect the performance of aboveground insects (Bezemer et al., 2003; van Dam et al., 2003). Our data indicated that the A. lineatus attack did not change the biomass of the aerial part of the tomato plant, in agreement with other experimental systems (Dunn & Frommelt, 1998; Bezemer et al., 2003). Agriotes lineatus damage should have influenced aphids mainly through the activation of a systemic response. To shed light on the signalling network of this below- and aboveground interaction, we analysed genes that are transcriptional markers of main hormone-dependent defence pathways. Root herbivory activated a systemic response in leaves of both JA-, SA-, and Et-dependent genes, such as ACO, TD, and PR1, which all reached the maximum expression level 48 h following attack. The late JA-responsive gene InhII reached its maximum at the latest time point investigated. The strongest overexpression was observed for the ABA-DEH gene. The high level of expression later in time could be explained by a water stress-related activation associated to ABA accumulation (Erb et al., 2011). In maize, the corn rootworm (Diabrotica virgifera virgifera LeConte) activated dehydrins and induced a local and systemic accumulation of ABA (Erb et al., 2009).
On the other hand, the presence of aphid nymphs in leaves did not associate with a noticeable systemic response. Aphids did not affect plant biomass (data not shown) – low aphid densities rarely affect plant biomass (Wurst & van der Putten, 2007). To monitor fertility, we daily removed new-born nymphs and it is likely that aphid damage was not sufficient to elicit a significant systemic response. Similarly, when tomato plants previously attacked with A. lineatus were infested with M. persicae, the expression levels of the analysed genes showed a comparable expression pattern of the systemic response to A. lineatus. Genes associated to the JA- and Et-pathways reached the higher expression at 48 h. The dehydrin displayed an early activation, whereas the overexpression of the SA-dependent PR1 gene was milder. Perhaps the stronger activation of JA (i.e., TD and InhII)- and Et (i.e., ACO)-dependent genes and the milder induction of the SA-dependent PR1 gene may reflect the antagonistic relation between these two defence pathways in relation to pests with different feeding guilds (Pieterse et al., 2012; Thaler et al., 2012; Coppola et al., 2013). Overall, the time-course expression analysis indicated a dynamic systemic transcriptional response in tomato following root herbivory, which comprises the activation of genes dependent on pathways with a role in plant resistant to biotic stress (Ryan, 1990; Mohase & van der Westhuizen, 2002; Chen et al., 2005; Cooper & Goggin, 2005; Zhu-Salzman et al., 2008).
In conclusion, the gene-expression analysis indicated that a combination of signals enables plant-mediated communication between below- and aboveground pests in tomato. Our work revealed leaf-systemic interactions based on the combination of multiple defence mechanisms (Stout et al., 1999). The integration was evident when biotic stressors were spatially and temporally separated, with root herbivory inducing pathways that are expected to help tomato against a biotic stressor with a different feeding style (Bruce & Pickett, 2007). Future studies may also address the soil chemical and physical features that influence plant response to root herbivores and the induction of systemic resistance to stress (Mody et al., 2009; Han et al., 2014).



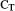 method
method
