The impact of immature granulocytes on the outcome of patients with decompensated cirrhosis
Abstract
Background
Higher immature granulocyte levels have a predictive role in several clinical conditions, although data concerning cirrhosis are scarce. Reduced muscle mass is a known factor affecting the outcome of these patients. The aim of the study was to evaluate the association of immature granulocytes with muscle mass and their role in predicting the outcome (survival, death or liver transplantation) in patients with stable decompensated cirrhosis.
Methods
We prospectively studied 210 patients with decompensated cirrhosis awaiting liver transplantation. Their clinical and laboratory characteristics were recorded, including complete blood count with immature granulocyte count and immature granulocyte percentage. The severity of liver disease was evaluated by estimating the Child-Turcotte-Pugh and MELD-sodium scores. Dual energy X-ray absorptiometry was used to quantify the total and regional lean mass, while mid-arm muscle circumference was used for the evaluation of upper limb muscle mass.
Results
Immature granulocyte percentage was proved to be the only factor independently associated with transplant-free survival (Hazard Ratio: 1.98, 95% confidence interval [1.03–3.81], p = .04). Stratification of our cohort based on the best discriminative cut-off values of immature granulocyte count and percentage revealed significant differences in the outcome based on Kaplan–Meier curves, while immature granulocyte count and percentage were significantly associated with parameters of body composition.
Conclusions
Higher immature granulocyte count and percentage have a significant prognostic role and are associated with worse outcome in patients with stable decompensated cirrhosis.
1 INTRODUCTION
Decompensated cirrhosis (DC) is characterized by the development of portal hypertension-related complications, and it is associated with high mortality rates without liver transplantation (LT).1 The Child-Turcotte-Pugh (CΤP) classification is widely used in daily clinical practice for the assessment of prognosis of cirrhotic patients and is based on objective (serum albumin, serum bilirubin and prothrombin time) and subjective (ascites and encephalopathy) variables.2, 3 More recently, the Model for End-Stage Liver Disease (MELD)-Sodium (MELD-Na) score based only on objective serum laboratory variables [creatinine, bilirubin, international normalized ratio (INR) and sodium (Na)] is associated with short and intermediate term outcomes with satisfactory accuracy.4, 5 Nevertheless, many studies have attempted to evaluate parameters with prognostic value in order to estimate the probability of death in patients with DC thus the need for LT. However, the large number of relevant studies indicates the need of prognostic markers that predict more accurately the outcome of patients with DC irrespectively of aetiology and severity of underlying liver disease.6 In this setting, muscle mass reduction has been associated with increased risk for liver-related complications and adverse outcome7 being a promising parameter for a more accurate evaluation of patients with cirrhosis.
DC considered as a systematic disease is characterized by the presence of increased cardiac output and low systemic vascular resistance, which have been attributed to several pathogenetic factors including the increased endothelial nitric oxide synthase activity and nitric oxide production and the enhanced bacterial translocation from the intestine.8 The latter leads to sub-clinical endotoxemia and the development of systemic inflammatory response and immune system dysfunction.8 This clinical condition may be associated with chronic overproduction of proinflammatory cytokines leading to stimulation of bone marrow granulocytes, which are the most common types of white blood cells.9 As a result, a ‘left shift’ of the leukocyte lineage is observed, that is, the recruitment in the systemic circulation of precursors and less differentiated forms of granulocytes (namely immature granulocytes, IGs).10
IGs include metamyelocytes, myelocytes and promyelocytes but not neutrophils or myeloblasts, and they have been evaluated in patients with inflammatory conditions, as well as bacterial and viral infections including SARS-CoV-2 infection.10-12 Interestingly, previous studies have shown that IGs are associated with the presence of bacteremia or sepsis.13 In the setting of chronic liver disease, only one recently published study including 76 patients with alcohol liver disease and 11 patients with alcoholic hepatitis,14 evaluated the prognostic impact of IGs. IG count was found significantly higher in patients with alcoholic hepatitis compared to those with alcohol liver disease, while the percentage of IGs in the peripheral blood was associated with mortality.14 However, no study has evaluated the predictive value of IGs in patients with liver diseases of other etiologies or cirrhosis.
Thus, the aim of the present prospective study was to investigate, for the first time in the literature, the association of IGs with muscle mass reduction and their predictive role in the outcome (survival, death or LT) of patients with clinically stable DC of various etiologies and decompensating events.
2 MATERIALS AND METHODS
2.1 Study population
We conducted a prospective single-center study including all consecutive adult patients assessed as outpatients to our Department with clinically stable DC between January 2021 and December 2023. Their outcome (alive, death or LT) was evaluated after a follow up of at least 6 months (unless death or LT occurred earlier). Exclusion criteria were (a) age < 18 years, (b) acute liver failure, (c) need for combined kidney and liver transplantation and (d) previous LT. Diagnosis of cirrhosis was based on biopsy or imaging (ultrasound, computer tomography or magnetic resonance imaging) together with cirrhosis-related findings such as ascites, thrombocytopenia or gastro-oesophageal varices. DC was defined as a history of ascites, variceal bleeding, encephalopathy or nonobstructive jaundice in patients with cirrhosis. Patients were clinically stable regarding their cirrhosis, that is, they had no variceal bleeding, encephalopathy, infection, such as spontaneous bacterial peritonitis, at baseline and during the last month before their admission. The presence of infection was evaluated, whenever necessary, with detailed clinical examination, laboratory measurements [C-reactive protein, procalcitonin, ascitic fluid paracentesis, urine examination, blood and urine cultures] and radiological methods (chest x-ray, abdominal ultrasound).
On baseline, for every patient the following demographic and clinical characteristics were recorded: age, sex, cause of the underlying liver disease and previous history of cirrhosis-related complications [i.e. variceal bleeding, encephalopathy, spontaneous bacterial peritonitis and hepatorenal syndrome], medication regarding the liver disease (duration and dosage), concomitant extra-hepatic diseases (e.g. diabetes mellitus and coronary artery disease) and vital signs (arterial blood pressure and pulse rate). Several laboratory variables were also evaluated including aminotransferases (aspartate and alanine), alkaline phosphatase, γ-glutamyl transpeptidase, urea, creatinine, electrolytes (Na, potassium, magnesium, calcium and phosphate), lactate dehydrogenase, protein, albumin, γ-globulins, bilirubin (total and direct), inflammatory indexes (C-reactive protein, ferritin) as well as clotting profile (prothrombin time, INR, activated partial thromboplastin time and fibrinogen). In addition, whole peripheral blood samples were collected and used for the measurement of haematocrit, white blood cells, platelet count, neutrophil-lymphocyte ratio, as well as IG count and IG percentage (i.e. the proportion of the total white blood cells that are IGs) using an automated Sysmex ΧΕ 2100 hematology analyzer (TOA Medical Electronics). The severity of liver disease and the prognosis of the included patients was evaluated by estimating the CTP15 and MELD-Na score.4 The presence of hepatocellular carcinoma was also recorded. In addition, anthropometric parameters, including triceps skinfold (TSF) thickness, mid-arm circumference (MAC) and mid-arm muscle circumference (MAMC) [calculated by the following formula: MAMC = MAC– (3.14 × TSF thickness)]16 were evaluated. Finally, dual energy X-ray absorptiometry (DEXA) was used to quantify the body composition (Hologic Horizon W). The Lean Mass to height ratio [LM/h2] for measurement of the total body muscle mass and appendicular lean mass to height ratio [ALM/h2] for measurement of the amount of lean mass in the arms and legs relative to height, were evaluated.
We followed the included patients prospectively and analysed their clinical course. Primarily, we evaluated their transplant-free survival (alive without LT) from the baseline (on admission) and its correlation with IG count and IG percentage. The study protocol was approved by our Institutional Review Board and conformed to the ethical guidelines of the 1995 Declaration of Helsinki. Written informed consent was obtained from all patients.
2.2 Statistical analysis
Continuous variables were presented as mean ± standard deviation (normally distributed) or median with interquartile range (nonnormally distributed). Categorical variables were expressed as frequencies or percentages. Comparisons of parameters between patients were performed using Student's t or Mann–Whitney U tests, as appropriate, for continuous variables and chi-square test for categorical variables, while multivariable logistic regression analysis was used to identify the independent variables. Univariate and multivariate analyses were performed in the context of competing risks, with death as the primary outcome and liver transplantation as the competing event. These analyses were conducted using the Fine-Gray sub-distribution hazards model to estimate the effect of covariates on the cumulative incidence of death while accounting for the competing risk of liver transplantation.17 Factors with p < .05 in the univariate cox regression analysis were entered into the multivariate model. Survival rates were estimated by the Kaplan–Meier method and the differences were compared using the log–rank test. To assess the discriminative ability to predict the outcome of patients with DC, time-dependent ROC analysis was performed, and time-dependent area under the curve (AUC) values were calculated at specified time points.18 To determine the best cut-off point for the time-dependent ROC analysis, Youden's Index that optimized the balance between sensitivity and specificity was calculated as the sum of sensitivity and specificity minus one. The threshold corresponding to the maximum Youden's Index was selected as the best cut-off point.
Additionally, Harrell's C-statistic was computed for each Cox regression model to evaluate the overall discriminative performance across the entire follow-up period.19 p value <.05 was considered statistically significant. Statistical analysis was performed using SPSS (version 29.0, IBM) and appropriate R packages within R software (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria).
3 RESULTS
Α total of 224 patients with DC were evaluated, but 14 patients were excluded due to unavailable data regarding IGs. Thus, 210 patients were included in the final cohort [137 (65.2%) males, mean age 55.2 ± 10.9 years] and their baseline characteristics are shown in Table 1. The mean values of MELD-Na and CTP scores were 14 ± 6 (range: 7–33) and 8 ± 2 (range: 5–14), respectively. The most common aetiology of cirrhosis were alcohol liver disease in 67 (32%) and viral hepatitis in 57 (27%) of patients. Previous history of variceal bleeding, hepatic encephalopathy, spontaneous bacterial peritonitis and hepatocellular carcinoma was recorded in 19%, 23%, 12% and 10% of patients, respectively. The median values of IG count and IG percentage were .02 (range: 0–.56) ×109/L and .4% (range: 0%–3%), respectively (Table 1). The median follow-up time was 12 (range: 6–48) months: 57 (27%) patients died (all due to liver-related complications), and 28 (13%) patients underwent LT during the follow-up period, while 125 (60%) were still alive at the end of follow-up.
| Variable | Patients, n = 210 |
|---|---|
| Age, (mean ± SD, years) | 55.2 ± 10.9 |
| Sex, male n, (%) | 137 (65.2) |
| Aetiology of cirrhosis, n, (%) | |
| Alcohol | 67 (32) |
| Viral hepatitis | 57 (27) |
| MASLD | 48 (23) |
| Other | 38 (18) |
| History of complications, n, (%) | |
| GI bleeding | 40 (19) |
| Encephalopathy | 48 (23) |
| SBP | 37 (12) |
| HCC, n, (%) | 21 (10) |
| Total bilirubin, (median, range, mg/dL) | 1.7 (.21–18) |
| Albumin, (median, range, g/dL) | 3.4 (2.1–5.1) |
| INR, (mean ± SD) | 1.49 ± .4 |
| Sodium, (mean ± SD, mEq/L) | 137 ± 4 |
| Creatinine, (median, range, mg/dL) | .77 (.32–5.4) |
| WBC, (mean ± SD, 109/L) | 4.5 ± 1.2 |
| Neutrophils cells, (mean ± SD, 109/L) | 3.1 ± .8 |
| NLR, (median, range) | 2.7 (.83–32) |
| Fibrinogen, (mean ± SD, mg/dL) | 292 ± 114 |
| Platelets, (median, range, 103/μL) | 116 (20–535) |
| Ferritin, (median, range, ng/mL) | 276 (5–3374) |
| CRP, (mean ± SD, mg/L) | 10 ± 4 |
| Immature granulocytes, count (median, range, ×109/L) | .02 (0–.56) |
| Percentage of immature granulocytes (median, range, %) | .4 (0–3) |
| MAMC, cm (mean ± SD) | 20 ± 9 |
| Appendicular/height ratio (kg/m2) (mean ± SD) | 6.4 ± 3 |
| Lean mass/height ratio (kg/m2) (mean ± SD) | 16.5 ± 7 |
| CTP score, (mean ± SD) | 8 ± 2 |
| MELD-Sodium score, (mean ± SD) | 14 ± 6 |
- Abbreviations: CRP, C-reactive protein; CTP, Child-Turcotte-Pugh; GI bleeding, gastrointestinal bleeding; HCC, hepatocellular carcinoma; INR, international normalized ratio; MAMC, mid-arm muscle circumference; MASLD, metabolic dysfunction- associated steatotic liver disease; NLR, neutrophil-lymphocyte ratio; SBP, spontaneous bacterial peritonitis; SD, standard deviation; WBC, white blood count.
3.1 Characteristics of patients when divided according to best discriminative cut-off for IG count and percentage
Our cohort was divided into two groups, based on the best discriminative cut off of IG count (.03 × 109/L). Patients (n = 152) with IGs equal to or less than .03 × 109/L, compared to those (n = 58) with IGs greater than .03 × 109/L, had significantly higher MAMC (21 ± 7 vs. 18 ± 8 cm, p = .015), LM/h2 (16.7 ± 5 vs. 15.3 ± 3 kg/m2, p = .022) and ALM/h2 (6.6 ± 3 vs. 6.1 ± 2 kg/m2, p = .027) (Table 2). The results of multivariate analysis are shown in Table S1A. The Kaplan–Meier curve detected a significant different outcome between the two groups (Figure 1A) (log rank test: 5.29, p = .021).
| Variables | Patients with IG ≤.03 × 109/L (n = 152, 72%) | Patients with IG >.03 × 109/L (n = 58, 28%) | p Value |
|---|---|---|---|
| Age, (mean ± SD, years) | 57 ± 10 | 54 ± 12 | .18 |
| Sex, male (n, %) | 98 (65) | 39 (67) | .65 |
| Previous history of SBP (n, %) | 23 (15) | 14 (24) | .03 |
| Albumin (mean ± SD, g/dL) | 3.6 ± .6 | 3.2 ± .6 | <.001 |
| Bilirubin (median, range, mg/dL) | 1.3 (.21–18) | 2.4 (.30–11.4) | <.001 |
| INR, (mean ± SD) | 1.4 ± .4 | 1.6 ± .6 | .04 |
| Sodium, (mean ± SD, mEq/L) | 138 ± 4 | 135 ± 5 | <.001 |
| Fibrinogen, (mean ± SD, mg/dL) | 288 ± 102 | 299 ± 130 | .007 |
| Platelets (median, range, 103/μL) | 120 (35–535) | 85 (20–340) | <.001 |
| Ferritin (median, range, ng/mL) | 109 (5–1644) | 427 (5–3374) | <.001 |
| CRP, (mean ± SD, mg/L) | 11 ± 4 | 10 ± 4 | .23 |
| MAMC, cm (mean ± SD) | 21 ± 7 | 18 ± 8 | .015 |
| Appendicular/height ratio (kg/m2) (mean ± SD) | 6.6 ± 3 | 6.1 ± 2 | .027 |
| Lean mass/height ratio (kg/m2) (mean ± SD) | 16.7 ± 5 | 15.3 ± 3 | .022 |
| CTP score (mean ± SD) | 7 ± 2 | 9 ± 3 | .013 |
| MELD-sodium score (mean ± SD) | 13 ± 5 | 16 ± 7 | <.001 |
- Abbreviations: CRP, C-reactive protein; CTP, Child-Turcotte-Pugh; IG, immature granulocyte; INR, international normalized ratio; MAMC, mid-arm muscle circumference; SBP, spontaneous bacterial peritonitis; SD, standard deviation.
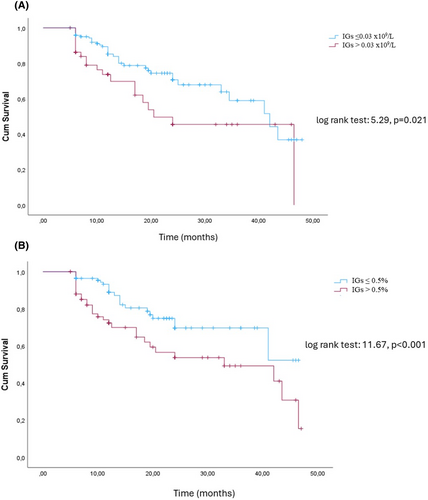
Then, our cohort was divided into two groups based on the best discriminative cut-off of IG percentage (.5% of total white blood cells). Patients (n = 156) with IG percentage equal to or less than .5%, compared to those (n = 54) with IG percentage more than .5% of the total white blood cells, had significantly higher MAMC (22 ± 7 vs. 17 ± 8, p = .015), LM/h2 (16.6 ± 5 vs. 15.6 ± 3 kg/m2, p = .022) and ALM/h2 (6.8 ± 3 vs. 6.2 ± 2 kg/m2, p = .027) (Table 3). The results of multivariate analysis are shown in Table S1B. Based on the Kaplan–Meier curve, the former group had a superior outcome compared to those with abnormal IG percentage (log rank test: 11.67, p < .001) (Figure 1B).
| Variables | Patients with IG ≤.5% of the total white blood cells (n = 156, 74%) | Patients with IG >.5% of the total white blood cells (n = 54, 26%) | p Value |
|---|---|---|---|
| Age, (mean ± SD, years) | 57 ± 10 | 54 ± 12 | .18 |
| Sex, male (n, %) | 95 (61) | 42 (78) | .86 |
| Previous history of SBP (n, %) | 24 (15.5) | 13 (24) | .03 |
| Albumin (mean ± SD, g/dL) | 3.6 ± .6 | 3.2 ± .6 | <.001 |
| Bilirubin (median, range, mg/dL) | 1.3 (.21–18) | 2.4 (.30–11.4) | <.001 |
| International normalized ratio (INR), (mean ± SD) | 1.4 ± .4 | 1.6 ± .6 | .04 |
| Sodium, (mean ± SD, mEq/L) | 138 ± 4 | 135 ± 5 | <.001 |
| Fibrinogen, (mean ± SD, mg/dL) | 281 ± 101 | 298 ± 122 | .04 |
| Platelets (median, range, 103/μL) | 122 (21–535) | 87 (20–309) | .02 |
| Ferritin (median, range, ng/mL) | 187 (9–1644) | 381 (5–3374) | <.001 |
| CRP, (mean ± SD, mg/L) | 11 ± 4 | 10 ± 3 | .27 |
| MAMC, (mean ± SD, cm) | 22 ± 7 | 17 ± 8 | .015 |
| Appendicular/height ratio (kg/m2) (mean ± SD) | 6.8 ± 3 | 6.2 ± 2 | .027 |
| Lean mass/height ratio (kg/m2) (mean ± SD) | 16.6 ± 5 | 15.6 ± 3 | .022 |
| CTP score (mean ± SD) | 8 ± 2 | 8 ± 2 | .98 |
| MELD-sodium score (mean ± SD) | 13 ± 5 | 16 ± 7 | <.001 |
- Abbreviations: CRP, C-reactive protein; CTP, Child-Turcotte-Pugh; IG, immature granulocyte; INR, international normalized ratio; MAMC, mid-arm muscle circumference; SBP, spontaneous bacterial peritonitis; SD, standard deviation.
3.2 Characteristics of patients who survived or died/underwent LT
The patients who were alive [n = 125 (60%)], compared to those who died or underwent LT [n = 85 (40%)] at the end of follow up, had significantly less frequently a previous history of encephalopathy (16% vs. 33%, p = .02) and spontaneous bacterial peritonitis (13% vs. 25%, p = .015), as well as lower baseline bilirubin [1.2 (range: .21–18) vs. 2.5 (range: .30–11.4) mg/dL, p < .001], INR (1.4 ± .5 vs. 1.6 ± .5, p = .037), CTP score (7 ± 2 vs. 9 ± 2, p < .001) and MELD-Na score (13 ± 5 vs. 15 ± 6, p = .007), neutrophil-lymphocyte ratio [2.5 (.94–10.3) vs. 3.3 (.83–32), p = .036], C-reactive protein (9 ± 3 vs. 12 ± 5 mg/L, p = .03), IG count [.01 (0–.16) vs. .02 (0–.56) ×109/L, p = .016] and IG percentage [.3 (0–2.6) vs. .45 (0–3)%, p < .001]. In addition, patients who survived (not transplanted) had significantly higher albumin (3.7 ± .7 vs. 3.2 ± .5 g/dL, p < .001), Na (138 ± 3 vs. 136 ± 4 mEq/L, p < .001) and fibrinogen (313 ± 110 vs. 261 ± 113 mg/dL, p = .001) (Table 4).
| Variables | Patients who survived (n = 125, 60%) | Patients who died or underwent LT (n = 85, 40%) | p Value |
|---|---|---|---|
| Age, (mean ± SD, years) | 54 ± 9 | 56 ± 11 | .67 |
| Sex, male (n, %) | 76 (61) | 61 (72) | .11 |
| History of complications, n, (%) | |||
| GI bleeding | 30 (24) | 10 (12) | .23 |
| Encephalopathy | 20 (16) | 28 (33) | .02 |
| SBP | 16 (13) | 21 (25) | .015 |
| Albumin (mean ± SD, g/dL) | 3.7 ± .7 | 3.2 ± .5 | <.001 |
| Bilirubin (median, range, mg/dL) | 1.2 (.21–18) | 2.5 (.30–11.4) | <.001 |
| INR, (mean ± SD) | 1.4 ± .5 | 1.6 ± .5 | .037 |
| Sodium, (mean ± SD, mEq/L) | 138 ± 3 | 136 ± 4 | <.001 |
| Creatinine (median, range, mg/dL) | .75 (.32–2.2) | .81 (.46–5.4) | .62 |
| WBC, (mean ± SD, 109/L) | 4.3 ± .9 | 4.6 ± 1.3 | .56 |
| Neutrophils cells, (mean ± SD, 109/L) | 2.9 ± .7 | 3.2 ± .8 | .94 |
| Fibrinogen, (mean ± SD, mg/dL) | 313 ± 110 | 261 ± 113 | .001 |
| NLR, (median, range) | 2.5 (.94–10.3) | 3.3 (.83–32) | .036 |
| Platelets (median, range, 103/μL) | 120 (40–535) | 111 (20–310) | .15 |
| Ferritin (median, range, ng/mL) | 271 (5–1200) | 290 (10–3374) | .27 |
| CRP, (mean ± SD, mg/L) | 9 ± 3 | 12 ± 5 | .03 |
| Immature granulocytes, count (median, range, ×109/L) | .01 (0–.16) | .02 (0–.56) | .016 |
| Percentage of immature granulocytes, (median, range, %) | .3 (0–2.6) | .45 (0–3) | <.001 |
| MAMC, cm, (mean ± SD) | 20 ± 9 | 20 ± 8 | .95 |
| Appendicular/height ratio (kg/m2) (mean ± SD) | 6.5 ± 3 | 6.3 ± 2 | .55 |
| Lean mass/height ratio (kg/m2) (mean ± SD) | 16.4 ± 7 | 16.9 ± 7 | .32 |
| CTP score (mean ± SD) | 7 ± 2 | 9 ± 2 | <.001 |
| MELD-sodium score (mean ± SD) | 13 ± 5 | 15 ± 6 | .007 |
- Abbreviations: CRP, C-reactive protein, CTP, Child-Turcotte-Pugh; GI bleeding, gastrointestinal bleeding; INR, international normalized ratio; LT, liver transplantation; MAMC, mid-arm muscle circumference; NLR, neutrophil-lymphocyte ratio; SBP, spontaneous bacterial peritonitis; SD, standard deviation; WBC, white blood count.
3.3 Factors associated with the outcome: univariate and multivariate analysis
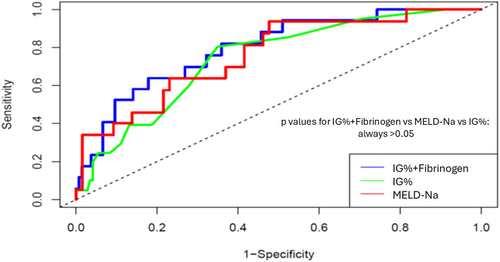
Univariate Cox regression competing analysis showed that INR (HR: 2.42, 95% CI [1.34–4.38], p = .003), total bilirubin (HR: 1.12, 95% CI [1.02–1.23], p = .016), creatinine (HR: 1.54, 95% CI [1.02–2.32], p = .041), ferritin (HR: 1.0, 95% CI [1.0–1.001], p = .04), C-reactive protein (HR: 1.01, 95% CI [1.001–1.014], p = .0017), MELD-Na score (HR: 1.08, 95% CI [1.02–1.14], p = .007), Na (HR: .89, 95% CI [.84–.96], p = .002), albumin (HR: .94, 95% CI [.89–.98], p = .0063) and fibrinogen (HR: .997, 95% CI [.993–.999], p = .025) were significant factors associated with our patients' outcome (death or LT) (Table 5). In addition, IG count (HR: 172, 95% CI [65.3–5973], p < .001) and IG percentage (HR: 2.47, 95% CI [1.62–3.75], p < .001) had significant prognostic role. In multivariate Cox regression competing analysis including the MELD-Na score but excluding its component (i.e. bilirubin, creatinine, INR and Na), it was found that IG percentage (HR: 1.98, 95% CI [1.03–3.81], p = .04) was the only factor independently associated with transplant-free survival. Finally, excluding MELD-Na score, but including the components of MELD-Na score, it was showed that IG percentage (HR: 1.84, 95% CI [1.014–3.36], p = .04) and fibrinogen (HR: .97, 95% CI [.96–.99], p = .044) were independently associated with transplant-free survival. Interestingly, based on time-dependent AUC values, the IG percentage had good performance with AUC values of .74, .68 and .59 at time points 10, 20 and 30 months, respectively, In addition, the model based on IG percentage and fibrinogen demonstrated good discriminative ability for predicting the outcome, with AUC values of .79, .70 and .63 at time points 10, 20 and 30 months, respectively. Its performance was higher, compared to that of MELD-Na score (AUC: .75, .67 and .61, respectively), but the difference was not significant (p = .17) (Figure 2). The Harrell's C-statistic was .510, .550 and .514 for IG percentage, the new model and MELD-Na, respectively.
| Variables | Hazard ratio | p Value | 95% confidence interval (lower-upper) |
|---|---|---|---|
| Age (mean ± SD, years) | .97 | .90 | .96–1.02 |
| Sex (n, %) | .74 | .21 | .36–1.55 |
| Hepatocellular carcinoma (n, %) | 1.15 | .33 | .97–1.24 |
| Albumin (mean ± SD, g/dL) | .94 | .0063 | .89–.98 |
| Bilirubin (median, range, mg/dL) | 1.12 | .016 | 1.02–1.23 |
| INR, (mean ± SD) | 2.42 | .003 | 1.34–4.38 |
| Sodium, (mean ± SD, mEq/L) | .89 | .002 | .84–.96 |
| Creatinine (median, range, mg/dL) | 1.54 | .041 | 1.02–2.32 |
| WBC, (mean ± SD, 109/L) | 1.0 | .48 | 1.0–1.0 |
| Neutrophils cells, (mean ± SD, 109/L) | 1.0 | .44 | 1.0–1.0 |
| Fibrinogen, (mean ± SD, mg/dL) | .997 | .025 | .993–.999 |
| Platelets (median, range, 103/μL) | 1.0 | .089 | 1.0–1.0 |
| Ferritin (median, range, ng/mL) | 1.0 | .04 | 1.0–1.001 |
| CRP, (mean ± SD, mg/L) | 1.01 | .0017 | 1.001–1.014 |
| NLR, (median, range) | 1.11 | .29 | .95–1.21 |
| Immature granulocytes, count (median, range, ×109/L) | 172 | <.001 | 65.3–5973 |
| Percentage of immature granulocytes (median, range, %) | 2.47 | <.001 | 1.62–3.75 |
| MAMC, (mean ± SD, cm) | .96 | .07 | .92–1.1 |
| Appendicular/height ratio (kg/m2) (mean ± SD) | .94 | .55 | .78–1.14 |
| Lean mass/height ratio (kg/m2) (mean ± SD) | .98 | .49 | .96–1.02 |
| CTP score (mean ± SD) | 1.15 | .15 | .99–1.22 |
| MELD-sodium score (mean ± SD) | 1.08 | .007 | 1.02–1.14 |
- Abbreviations: CRP, C-reactive protein; CTP, Child-Turcotte-Pugh; INR, international normalized ratio; MAMC, mid-arm muscle circumference; NLR, neutrophil-lymphocyte ratio; SD, standard deviation; WBC, white blood count.
4 DISCUSSION
In our study, we evaluated for the first time the impact of IG count and IG percentage on the outcome of 210 patients with clinically stable DC. The prognostic role of both markers was assessed in a large heterogeneous cohort of patients with different etiologies of cirrhosis and severity of liver dysfunction based on CTP and MELD scores. Interestingly, IG count and IG percentage were associated with worse outcomes (death or LT) indicating their predictive value in candidates for LT. A significant correlation with muscle mass, which was evaluated through LM/h2, ALM/h2 and MAMC measurements, was also found, although no association with the outcome was established for these indexes.
DC is considered a clinical condition with increased morbidity and mortality, since it is characterized by the development of several life-threatening complications.1 Although MELD and CTP scores are well-established prognostic tools used in daily clinical practice for the assessment of the prognosis of patients with cirrhosis within a given time interval, literature data have shown that their diagnostic accuracy is not optimal, indicating the need for developing new prognostic scores.20 The latter should be based on objective laboratory variables and be able to assess accurately the prognosis of patients with cirrhosis.
At the same time, several studies have shown that IGs (IG count and/or IG percentage) may play a predictive role in many clinical conditions allowing earlier therapeutic interventions, as well as greater possibilities of response and survival. For example, IGs have been evaluated with promising results as screening markers for early detection of bacteremia regardless of the site of infection and the affected organ.21 In addition, IGs can be used as specific indicators for the exclusion of sepsis.10 IGs may also be useful as discriminatory factors for the presence of ventilator-associated pneumonia22 and the need for intubation12 during SARS-CoV-2 infection, facilitating the optimal management of bacterial infections with early and/or appropriate initiation of antibiotic therapy. Furthermore, IGs have been independently associated with the outcome23 as well as the development of acute respiratory distress syndrome in patients with acute pancreatitis.11 Interestingly, a previous study has shown that IG percentage was a strong predictor of in-hospital mortality in patients with acute upper gastrointestinal bleeding.24
In the past, quantification of IGs was traditionally done through manual microscopy, which was a time-consuming and labor-intensive process with many inter- and intra-observer variabilities. Nowadays, IGs can be reliably identified and quantified through automated modern analyzers. The latter can provide very rapidly the automated IG count and IG percentage measurements, making these potential markers widely available in daily clinical practice.
Interestingly, literature data regarding the predictive impact of IGs on liver diseases are very scarce. In fact, there is only one recently published small cohort study which evaluated the role of IGs in patients with alcohol liver disease and alcoholic hepatitis.14 It was found that IGs were significantly elevated in patients with alcoholic hepatitis, compared to those with chronic alcohol liver disease. In addition, the percentage of IGs was associated with short- and long-term mortality. The authors attributed their findings to the immunosuppressive, phagocytic and metabolic functions of IGs. Nevertheless, this study was based on a relatively small number of patients focused only on alcohol-related liver disease.14
In our study, the predictive role of IG count and IG percentage was evaluated for the first time in a large heterogeneous cohort of patients with stable DC waiting for LT, and we were able to confirm their prognostic impact on the outcome. Indeed, in the multivariate Cox regression competing analysis and excluding MELD-Na score, IG percentage and fibrinogen (which is considered a marker of inflammation, while it is inversely associated with the severity of liver disease25) were independently associated with the outcome. In addition, these results were confirmed when the MELD-Na score was included in the multivariate analysis, where IG percentage was the only independent factor of transplant-free survival. In addition, in our cohort, we found that both IG count and IG percentage were associated with survival when we divided our cohort based on the optimal cut-off values (Figure 1A,B). However, IG count was not independently associated with the outcome, indicating that IG count may have a less important predictive role compared to IG percentage. This finding could be explained by the fact that patients with DC have already low white blood cells due to splenomegaly, and thus the relative proportion of IGs (i.e. IG percentage) may have a higher predictive impact compared to the absolute number of IGs.
It should be mentioned that our cohort included patients without complications of cirrhosis during the last month and with no evidence of active infection at baseline. Thus, it could be postulated that the predictive role of IGs was associated with the presence of increased translocation of bacterial products through the intestine leading to the development of sub-clinical endotoxemia and systemic inflammation.8, 26 Nevertheless, no other inflammatory indexes such as C-reactive protein, ferritin and neutrophil-lymphocyte ratio were independently associated with the outcome.
Interestingly, we found that elevated IG count and IG percentage were associated with LM/h2 and ALM/h2, as well as independently with MAMC. In the context of cirrhosis, reduced skeletal muscle mass is known to be a highly prevalent trait among patients affecting about one third of them in a recent meta-analysis while it is associated with a higher risk of mortality.7 In end stage liver disease, several methods have been used for the evaluation of muscle mass depletion including ultrasound, cross-sectional imaging and DEXA.27 The latter is a well-studied method used for the estimation of skeletal mass although its results are influenced by the presence of ascites. MAMC, on the other hand, has been used for the evaluation of upper extremity skeletal muscle mass, and it is associated with short-term mortality in patients with DC.28 Nevertheless, in our study, no significant association was found between muscle mass measurements with outcome. This finding could be attributed to the methods used for muscle mass quantification (MAMC and ALM/h2), since most of the studies evaluating muscle mass in patients with cirrhosis are based on quantification through computed tomography scan.7, 27 In fact, measurement of L3 vertebrae psoas muscle with computed tomography scan has been validated for the evaluation of reduced skeletal muscle in end stage liver disease27 and is considered as more objective compared to MAMC, while it is not influenced from fluid retention compared to measurements from DEXA-scan.29 Although the exact mechanisms implicated in the pathogenesis of muscle mass loss in cirrhosis have not been clarified, it has been suggested that the presence of chronic inflammation contributes to muscle reduction.30 Thus, it could be hypothesized that IGs are related with muscle mass and MAMC reflecting the hyper-inflammatory status of patients with DC.
We acknowledge that there are some limitations, including that our study is a single-centre study, while no markers associated with intestinal permeability and bacterial toxin translocation, such as lipopolysaccharides, were evaluated. Furthermore, as mentioned above, regarding the evaluation of muscle mass, in our study it was measured with DEXA scan instead of L3 vertebrae psoas muscle measure with computed tomography scan. However, for the first time in the literature, the prognostic impact of IGs was assessed in the setting of cirrhosis, while our study was prospective, including patients of various aetiology and severity of liver disease. Importantly, IGs can be easily determined using automated analyzers and could provide additional important information regarding the outcome of patients with DC in daily clinical practice. Nevertheless, further studies are needed to elucidate the impact of IGs in other relevant settings, such as those with acute liver failure, acute on chronic liver failure and spontaneous bacterial peritonitis. In addition, the changes of IGs over time need further evaluation.
In conclusion, our study showed for the first time strong evidence that IG count and IG percentage can be used as objective and easily available markers of prognosis in patients with clinically stable DC. Nevertheless, further studies are needed to validate these findings.
AUTHOR CONTRIBUTIONS
Magdalini Adamantou analysed data and wrote the paper. Dimitrios Glaros, Evangelinos Michelis, Apostolos Papageorgiou, Eleni Adamopoulou, Antonia Alevizou, Menelaos Athanasiadis, Eleni Pergantina and Vasiliki E. Georgakopoulou collected data. Vasileios Lekakis analysed data. Evangelos Cholongitas designed the study, analysed data and wrote the paper. All authors have approved the final draft of the manuscript for publication.
ACKNOWLEDGEMENTS
No external funding was received for this study.
FUNDING INFORMATION
The research did not receive a specific grant from any funding agency in the public, commercial or not-for-profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest related to the study design or its results.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Citations are included in the reference list.




